INTRODUCTION
Full-thickness macular hole (MH) is a full layers defect of the neutral retina in the foveal region from the internal limiting membrane (ILM) to the retinal pigment epithelium(RPE). Some epidemiological data showed that in the cases of patient with full-thickness MH, the proportion of idiopathic macular hole (IMH) patients accounts for 87.1%[1]. IMH leads to central visual loss and it is widely accepted that its formation is caused by the tangential contraction on the fovea area from the premacular cortical vitreous[2-3]. Some supporters believe that full-thickness MH is primarily caused by vitreomacular traction (VMT) or it as a direct result of pathologic characteristics other than VMT[4]. However not all patients with VMT will suffer from MH and MH can also develop in eyes where there is no possibility of VMT[5-6].Therefore, we propose that there may be some other reasons which play parts in the formation of IMH.
Some studies point out that risk factors for IMH include age, vitreofoveal traction, axial length, hormonal influences,intrinsic pigment epithelium diseases, and so on[1,7-10]. It has been shown that the macular thickness decreases with increasing age which should make the retina more susceptible to MH in relatively aged individuals[11]. Meanwhile, epidemiologic studies show that bilateral IMHs occurred in 11.7% of MH patients[9]and the estimate risk of the fellow eyes developing an MH is 12.0% at 5y and 16.9% at 10y[12]. The increased incidence of fellow eyes reveals that it is likely that unilateral MH patients are more susceptible to develop bilateral MH. We suspect that some general factors lead to disease susceptibility.According to prior researches, the choroidal thickness in unilateral IMH eyes decreased dramatically compared with that of normal people[13-14]and the authors suspected that the decreased choroidal thickness might be associated with the blood flow and perfusion of the choriocapillaries in IMH patients. Aras et al[15]discovered that the foveolar choroidal blood flow of IMH patients was significantly lower than that of normal individuals and suggested that quantitative measurement of foveolar choroidal blood flow may be helpful to identify the subjects who have increased risk of development of IMH. Ahn et al[16]found that vascular density of choriocapillaris in surgically closed MH was lower than that of normal controls and suggested that the variation of choriocapillaris blood flow was involved in the pathogenesis of MH. This begs the question of whether the hypoperfusion compared with the normal people is a partial or a systematic indicator and whether there is hypoperfusion phenomenon on optic nerve head (ONH).
To explore microvascular alterations related to systemic and ocular diseases, noninvasive techniques for ocular blood flow assessment are of crucial importance. The recent development of optical coherence tomography angiography (OCTA) with split-spectrum amplitude decorrelation angiography (SSADA)offers a good basis for quantitative angiography of the ONH microcirculation and shows good reliability for the observation of retinal blood flow density in the region of the macula[17-18].As a non-invasive technology, OCTA can readily visualize all layers of the retinal vasculature without dye injection[19].
In our study, by using OCTA we first aimed to explore the perfusion status of the ONH in IMH patients and compare the differences of the ONH blood flow status in both eyes of unilateral IMH with normal control eyes and then investigate its correlationship with the macular perfusion by using OCTA.
SUBJECTS AND METHODS
Study Population This study was conducted in the Beijing Tongren Hospital, Capital Medical University. The research protocols were approved by the Institutional Review Board at Beijing Tongren Hospital, Capital Medical University and carried out in accordance with the tenets of the Declaration of Helsinki. Written informed consent was obtained from all testing participants of the study. Patients with one eye diagnosed as having idiopathic full-thickness MH and unaffected fellow eyes were recruited. The diagnosis was confirmed by using the spectral-domain optical coherence tomography (SD-OCT)system (Cirrus, Carl Zeiss Meditec AG, Jena, Germany), as well as intraoperative observation. A healthy age-matched subject was enrolled as control. All participants underwent routine eye examination including best-corrected visual acuities (BCVA), color fundus photography, OCT, axial length(Carl Zeiss Meditec AG, Jena, Germany), and intraocular pressure (IOP) (Full Auto Tonometer TX-F Canon).Inclusion criteria for normal eyes were as follows: 1) BCVA better than 0.9; 2) IOP less than 22 mm Hg; 3) axial length less than 26 mm; 4) no ocular diseases history (excepting mild ocular surface diseases such as conjunctivitis); 5) no systemic diseases; 6) symmetric ONH between left and right eyes.
Exclusion criteria for all eyes were as follows: 1) axial length more than 26 mm; 2) age younger than 30y or older than 80y;3) refractive error greater than -6.00 diopter (D) IOP more than 22 mm Hg; 4) other eye diseases, including glaucoma, uveitis,retinal disease history, retina surgery or laser treatment, ocular trauma or tumor, poor image quality due to media opacity, or unstable eye fixation; 5) one eye from each participant was imaged and analyzed.
Peripapillary and Parafoveal Perfusion Measurements Using Optical Coherence Tomography Angiography OCTA scans were obtained by the spectral domain system RTVue-XR Avanti (Optovue, Inc., software V.2015.100.0.33).This device had an A-scan rate of 70 kHz per second, and B-san frame rate of 200 per second using a 840 nm light source with a bandwidth of 45 nm. A 3 mm×3 mm scanning area centered on the parafoveal area and a 4.5 mm×4.5 mm scanning area centered on the optic disc. The volumetric scans were processed by the split-spectrum amplitudedecorrelation angiography (SSADA) algorithma[20]. SSADA analyzed the decorrelation of signal amplitude and created a contrast between static and non-static tissue, so that the blood flow could be visualized. The blood flow and vessel density were analyzed by using the built-in Optovue software(Optovue, Inc., software V.2015.100.0.33). The fluctuation amplitude caused by blood flow was distinguished from the static signal which was defined as a positive pixel. The vessel density (blood volume per unit of time) was calculated by the proportion of pixel over the threshold of a specific region.
The peripapillary region was defined as a 700 µm wide elliptical annulus expanding from the optic disc boundary.Overall size of the peripapillary region and the optic disc named as whole en face. The peripapillary region once again was divided into six parts: nasal, inferior nasal, inferior temporal, superior nasal, superior temporal and temporal(Figure 1A). The vessel density was defined as the percentage area occupied by the large vessels and microvasculature in the radial peripapillary capillary (RPC) region from ILM to nervefibers layers (NFL), ranged from ILM to 100 µm below(Figure 1B). The parafoveal perfusion was measured by a masking procedure. The masking overlay consisted of an annulus, defined by an inner radius of 0.3 mm and an outer radius of 1.25 mm (Figure 1C). The superficial layer of the parafoveal area was defined as being from the ILM with a downward offset of 3 µm to the inner plexiform layer (IPL)with a downward offset of 15 µm (Figure 1D). The deep layer of the parafoveal area was defined as being from the IPL with a downward offset of 15-70 µm and the retina capillary network was seated in this layer (Figure 1E). The outer layer of the parafoveal area was defined as being from the IPL with a downward offset of 70 µm to the RPE with a downward offset of 30 µm (Figure 1F). The choroid part of the parafoveal area was defined as being from the RPE reference with a downward offset of 30-60 µm and the choriocapillaris was seated in this layer (Figure 1G). The peripapillary vessel density was defined as the proportion of the total area occupied by vessels. All images were taken by the same trained examiner and poorquality images with a signal strength index less than 40 were excluded from the analysis.
Statistical Analysis Statistical analysis was performed using a statistical software program (SPSS for Mac, version 22;IBM/SPSS, Chicago, Illinois, USA). Data was shown as mean±SD. Statistical comparisons on baseline, the parafoveal purfusion and optic disc perfusion, were performed using the nonparametric Mann-Whitney U test to analyze the differences between normal group and IMH group. All blood flow densityrelated parameters were being adjusted by Friedman tests compared between IMH eyes and unaffected eyes. All the parameters between the three groups were used by Kruskal-Wallis test. Spearman’s rank correlations were using to evaluate the vessel density between ONH and parafoveal. All the tests had a significance level of 5%.
RESULTS
Study Population Peripapillary and parafoveal retinal perfusion were studied in 24 normal eyes and 19 IMH eyes that met the inclusion and exclusion criteria. The mean age and standard deviation was 62.11±4.64y for IMH patients and 59.79±4.06y for healthy controls. There was no statistically significant difference of gender, age, IOP, or axial length in the normal and IMH patients (Table 1).
Comparison of Optic Nerve Head and Parafoveal Blood Flow Density Between Idiopathic Macular Hole Eyes,Unaffected Fellow Eyes, and Normal Control The comparison of ONH flow density between IMH eyes, unaffected fellow eyes, and normal control eyes was shown in Table 2. The mean value of the whole en face flow density was 56.55±1.83 in healthy controls, 55.81±3.79 in the IMH group and 54.40±3.44 in the unaffected fellow group. The peripapillary flow density was 63.10±2.29 in healthy controls, 61.82±3.75 in the IMH group and 60.86±4.21 in the unaffected fellow group. The data presented that normal control >IMH > unaffected fellow eyes.A statistical difference was found among the three groups in the regions of temporal (P=0.007). Between IMH unaffected fellow eyes and normal control eyes, the region of temporal(P=0.002) represented the magnificent statistical difference.
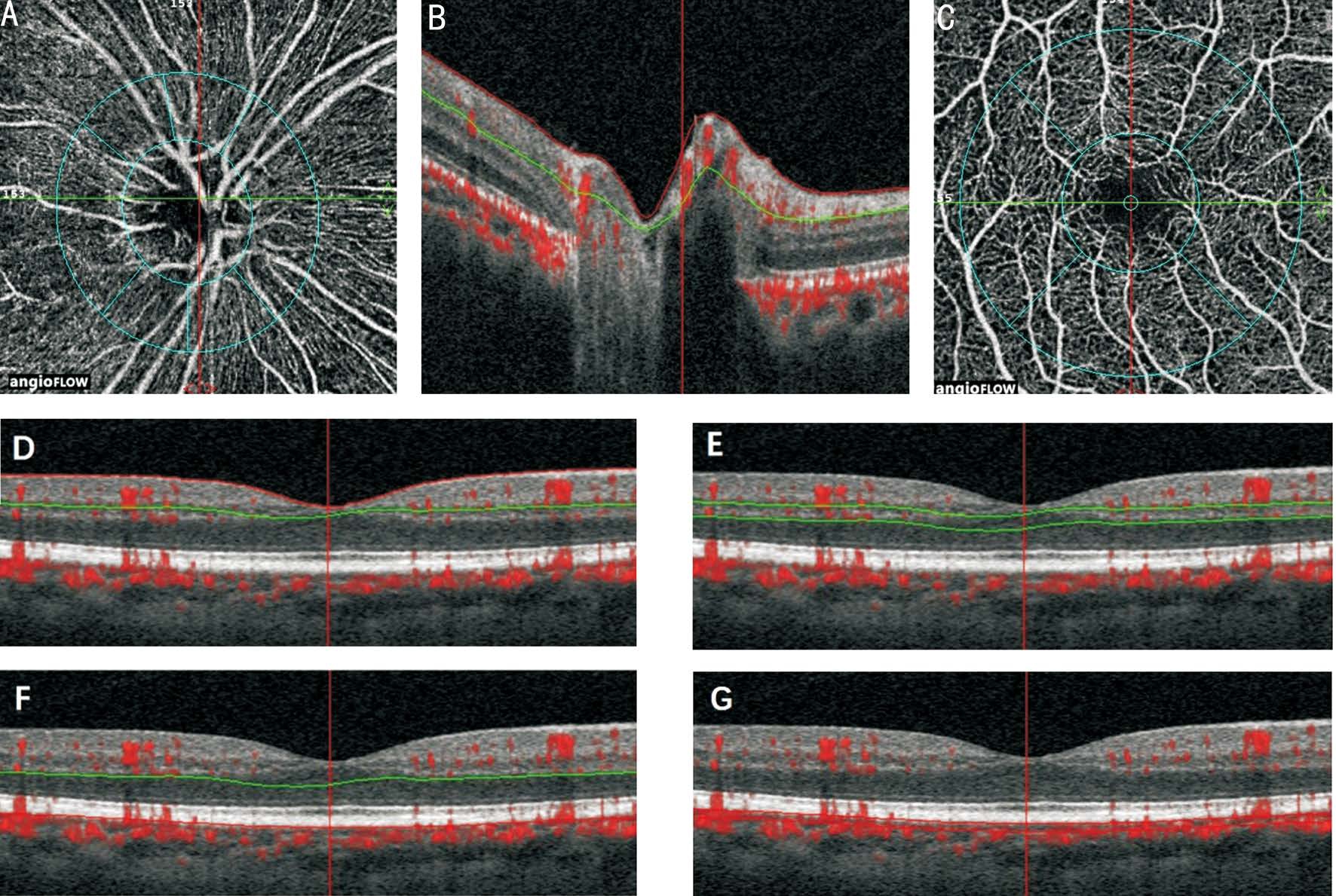
Figure 1 ONH and parafoveal perfusion was measured by OCTA of the emmetropic eye A: The peripapillary region was defined as a 700 µm wide elliptical annulus expanding from the optic disc boundary. Overall size of the peripapillary region and the optic disc named as whole en face and the peripapillary region was divided into six parts; B: The boundaries used for segmentation of the RPC region were indicated by the red and blue lines (from ILM to NFL, ranged from ILM to 100 µm below); C: The parafoveal region (by an inner radius of 0.3 mm and an outer radius of 1.25 mm); D: The superficial layer in macular and parafoveal region (from the ILM with a downward offset of 3 µm to the IPL with a downward offset of 15 µm); E: The deep layer in macular and parafoveal region (from the IPL with a downward offset of 15-70 µm); F: The outer layer in macular and parafoveal region (from the IPL with a downward offset of 70 µm to the RPE with a downward offset of 30 µm); G:The choroid layer in macular and parafoveal region (from the RPE reference with a downward offset of 30-60 µm).
Table 1 Characteristics of normal and IMH subjects
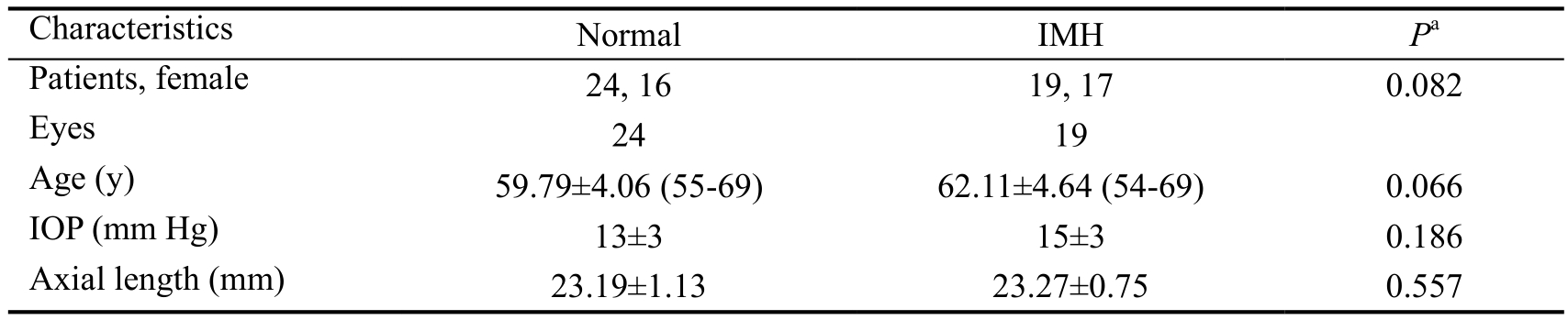
Numbers displayed are mean±population standard deviation;aMann-Whitney U test.
Table 2 ONH and parafoveal blood flow density of the three groups (normal group, IMH group, and unaffected fellow group) mean±SD
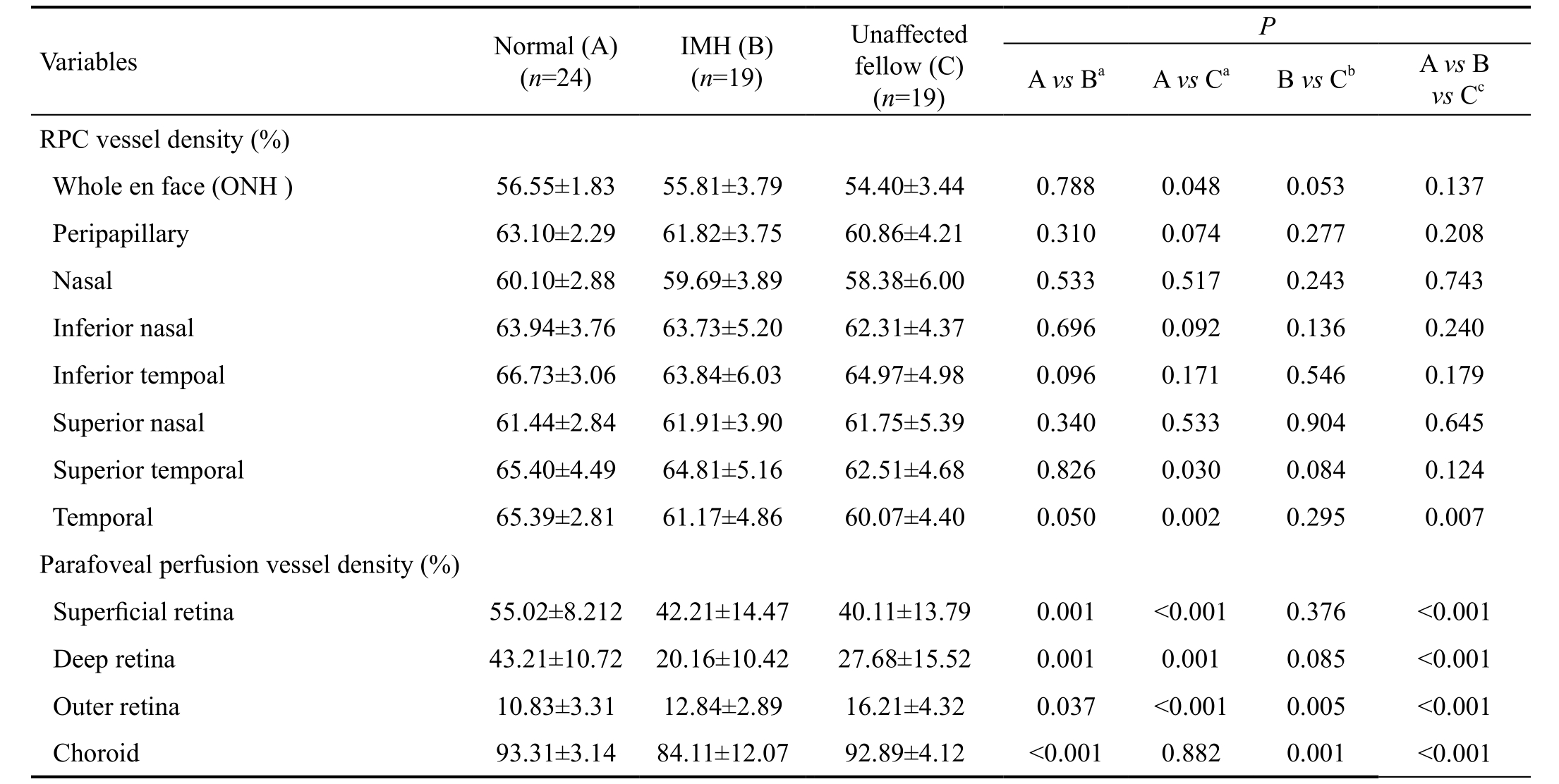
aCalculated by Mann-Whitney U test;bCalculated by Friedman test;cCalculated by Kruskal-Wallis test.
Typical examples of parafoveal and macular retinal angiograms for IMH group showed notable decreased blood flow density except the layer of outer retina. The mean parafoveal vessel density in IMH group was significantly lower than the normal control group except for the layer of outer retina (superficial retina P=0.001, deep retina P=0.001 and choroid P<0.001).The mean parafoveal vessel density in unaffected fellow eyes was significantly lower than the normal control group except for the layer of outer retina (superficial retina P<0.001,deep retina P=0.001). Although there was no significant statistical discrepancy in the layer of choroid between IMH and unaffected fellow groups, we could also see the choroid retina both in IMH and unaffected fellow eyes were lower than healthy controls. A statistical difference was found among three groups in all the layers (P<0.001) (Table 2).
Correlation Between Optic Nerve Head and Parafoveal In IMH eyes, univariate regression analysis using the Spearman’s rank test was used to analyze the correlation between the regions of blood flow density measurements on ONH and on the different layers of parafoveal. The correlation coefficient showed that whole en face and peripapillary flow density were correlated with parafoveal choriocapillary blood flow density(whole en face: r=0.528, P=0.020 and peripapillary: r=0.525,P=0.021 respectively). Meanwhile the whole en face and peripapillary flow density also were correlated with deep retina perfusion (whole en face: r=0.569, P=0.011 and peripapillary:r=0.504, P=0.028 respectively) (Figure 2; Table 3).
DISCUSSION
In spite of the theory by Gass[2]that the formation of MH is caught by proliferation of Müller cells above the fovea and VMT, some studies suggest the possibility that a focal retinal degenerative process might also play a role in the pathogenesison of IMH in the apparent absence of cortical vitreous[5-6]. In addition, some studies lay attention to the choroidal thickness and blood flow of choroid beneath macular in MH patients[13-14,16]. In this study, we reported thefirst use of OCTA to quantify ONH blood in IMH. We found that the mean values presented that normal control >IMH >unaffected fellow eyes in whole en face and peripapillary regions. A statistical difference also was found among three groups in the temporal region of ONH (P=0.007). Both eyes’ perfusion of IMH patients on the layers of superficial retina, deep retina and choroid were lower than that of normal control. As there was little blood flow on the layer of outer retina (from the IPL with a downward offset of 70 µm to the RPE with a downward offset of 30 µm) and the measurement results were relatively small, we did not focus on the layer of outer retina.Furthermore, there was a correlationship of the ONH flow density with the retina capillary network and choriocapillaris blood flow density in parafoveal.
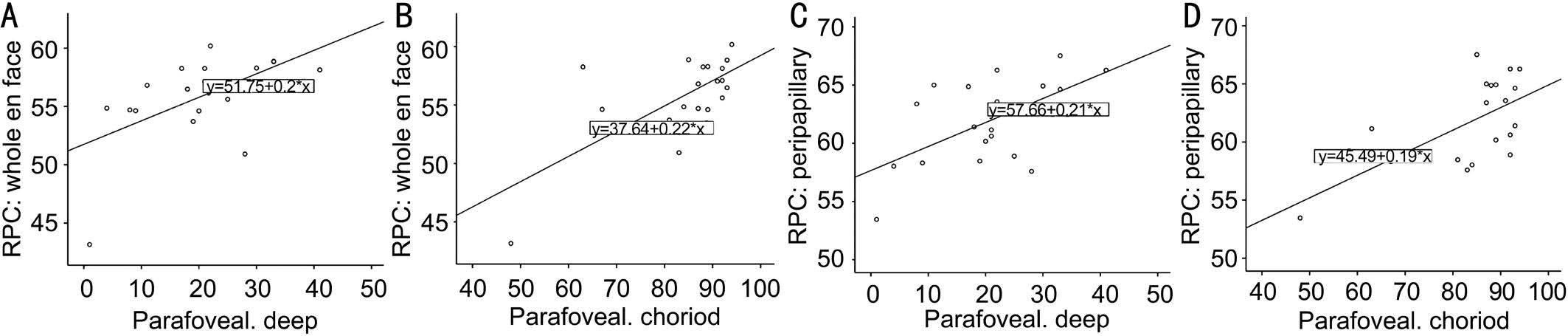
Figure 2 Correlation of ONH vessel density with parafoveal perfusion vessel density (using Spearman’s rank correlations P<0.05) A:Whole en face and parafoveal deep: r=0.569, P=0.011; B: Whole en face and parafoveal choriod: r=0.528, P=0.020; C: Peripapillary and parafoveal deep: r=0.504, P =0.028; D: Peripapillary and parafoveal choriod: r=0.525, P=0.021.
Table 3 Spearman correlation coefficients matrix on ONH vessel density and parafoveal perfusion vessel density
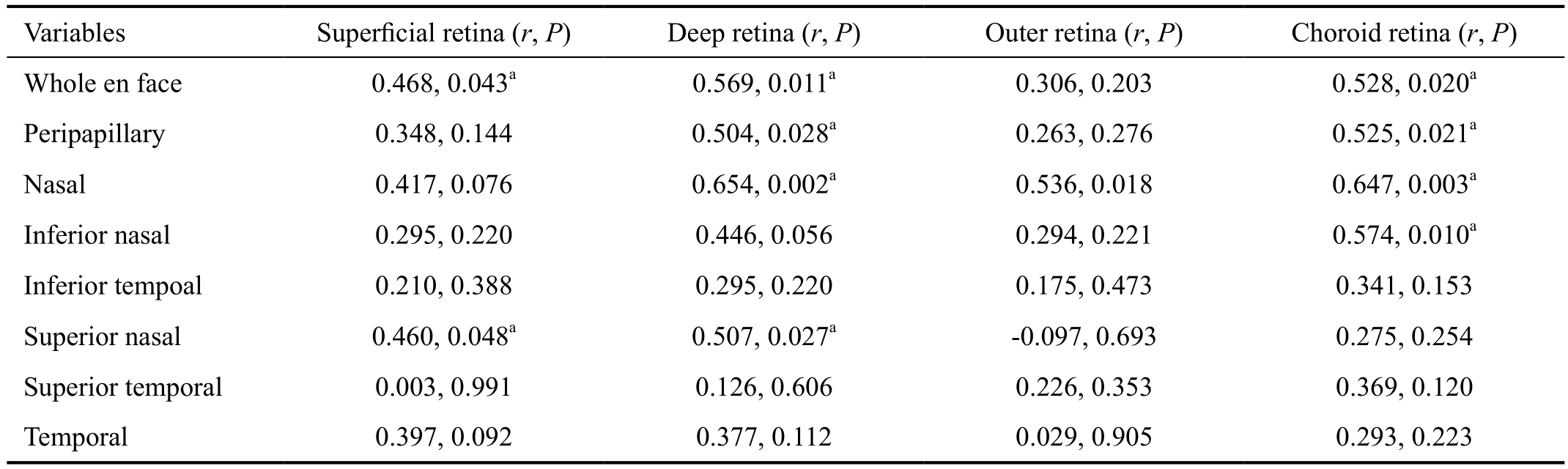
aStatistically significant correlation (P<0.05).
The main source of blood supply to the orbit is the ophthalmic artery, and its major branches is the central retinal artery which comes from the posterior ciliary arteries. There are three main source of blood flow supply the ONH: RPC in the superficial layer of the ONH (nervefiber layer on the surface of the optic disc) by the central retinal artery circulation which shares many characteristics with the retinal circulation; deeper layers (prelaminar tissue) by the peripapillary choroid or short posterior ciliary arteries; and lamina cribrosa by the posterior ciliary artery[21-23]. The posterior pole of the eye is nourished by two independent vascular beds. The inner retina and middle layer of the retina including the retinal ganglion cells are supplied by the retinal circulation with oxygen and nutrients.The outer retina including the photoreceptors was supplied by the choroidal circulation. As our previous study reported that compared with the normal eyes the choroidal perfusion beneath the macular and parafoveal significantly decreased,while no difference was found between unaffected fellow eyes and the healthy control eyes by using examination technique of OCTA[24]. We also found that typical examples of parafoveal and macular retinal angiograms for IMH group showed notable decreasing perfusion except the layer of outer retina in this study. Meanwhile, we found that the perfusion in the layers of deep retina and choroid in both eyes of IMH patients were lower than those of normal people. Some studies have found that in the parafoveal region, eyes after MH surgery have a tendency to have lower superficial and deep capillary plexuses’density than in the control group[25-26]. Our study found that this state already had existed before the operation.
Many studies have shown that blood flow of ONH were reduced in patients with glaucoma, myopia, central retinal artery occlusion, multiple sclerosis[27-35]. In IMH patients,we also found there was a decreasing of ONH blood flow density which was in the both eyes of IMH patients, especially in the unaffected eyes. Because of the limited sample size and resulting limited power, the deficiency of statistical significance might be caused by a type II error. However,rather than relying on a P value, it was important to consider the magnitude of the association. We speculated that the blood flow in IMH eyes were lower than those of the normal people and the hypoperfusion of the retina might lead to the susceptibility to the VMT. Since retinal microvasculature provided a window for detecting changes in microvasculature relating to the development of cardiovascular diseases such as arterial hypertension or coronary heart disease[36], the decreased blood flow on ONH and parafovea might re flect the ocular hypoperfusion, and possibly even indicate the condition of ocular flow and systemic circulation .
A statistical difference was found between IMH unaffected fellow eyes with normal controlled eyes in the temporal region of ONH. As the posterior pole retina mainly nourished by the temporal branch of central retinal artery, it indirectly demonstrated the decreased blood flow in the retina, especially in the region of macular in the unaffected eyes of the MH patients.The perfusion of ONH in IMH eyes slightly elevated compared with those of the unaffected eyes. In some investigations of retinal hemodynamics, the regulation of retinal blood flow acts in response to the change of physiology[37-38]. Some studies showed that by increasing capacitance of blood vessels, ocular blood flow on ONH could be efficiently improved, as a result to potentially decrease the resistance of increased IOP[39].Along with the broken of the harmonious condition in IMH eyes, we speculated that the autoregulation play a role in IMH eyes. But further studies are needed on the exact pathological process.
Wang et al[40]found that in normal people higher density of retina capillary network was associated with younger age and choriocapillaries density with examination technique of OCTA. We could deduce that compared with young people,older persons may have lower density of retina capillary and choriocapillaries. In our study, compared with the agematched normal quinquagenarian and elderly people (the age of 55-69y), the decreasing density of the ONH and parafoveal in MH patients might indicate that the older persons are more vulnerable to MH, simultaneously with the hypoperfusion of the eyes. We also discovered that the decresing ONH blood flow density was positively correlated with the deep retina and parafoveal choroidal blood flow density in MH patients. This might further reflect the ocular hypoperfusion in MH eyes.Due to the patients all got full-thickness MH, we did not know whether the blood flow played an important decisive factor in the formation of MH played an important decisive factor aside from VMT or just physiological changes followed by retinal defect and traction in the development of disease, but we still regard pushing the improvement of ocular blood flow might be a significantly effective intervention in the process of IMH.
Our study has its limitations, including its small sample size and the limitation of accurate value of the algorithm to analyze blood vessel parameters with OCTA. Therefore, further studies are needed to help elucidate the relationship between the ocular blood flow parameters and pathology of IMH. As the technology of OCTA improves the accuracy of blood flow measurement, we expect to expand the sample size for further research.
In conclusion, our study revealed the decreased blood flow density of ONH in IMH and its positive correlation with parafoveal decreased blood flow density by using OCTA. Our study further revealed the relationship between the ocular blood flow and the pathology of IMH. The reduction of vessel densities may indicate the hypoperfusion in IMH eyes.
ACKNOWLEDGEMENTS
Conflicts of Interest:Liu XX, None; Teng YF, None; Gao M, None; Liang XD, None; Yu YP, None; Liu W, None.
REFERENCES
1 Darian-Smith E, Howie AR, Allen PL, Vote BJ. Tasmanian macular hole study: whole population-based incidence of full thickness macular hole.Clin Exp Ophthalmol 2016;44(9):812-816.
2 Gass JD. Idiopathic senile macular hole. Its early stages and pathogenesis.Arch Ophthalmol 1988;106(5):629-639.
3 Johnson RN, Gass JD. Idiopathic macular holes. Observations, stages of formation, and implications for surgical intervention. Ophthalmology 1988;95(7):917-924.
4 Duker JS, Kaiser PK, Binder S, de Smet MD, Gaudric A, Reichel E,Sadda SR, Sebag J, Spaide RF, Stalmans P. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction,and macular hole. Ophthalmology 2013;120(12):2611-2619.
5 Smiddy WE, Flynn HW Jr. Pathogenesis of macular holes and therapeutic implications. Am J Ophthalmol 2004;137(3):525-537.
6 Smiddy WE. Macular hole formation without vitreofoveal traction. Arch Ophthalmol 2008;126(5):737-738.
7 Risk factors for idiopathic macular holes. The Eye Disease Case-Control Study Group. Am J Ophthalmol 1994;118(6):754-761.
8 Sen P, Bhargava A, Vijaya L, George R. Prevalence of idiopathic macular hole in adult rural and urban south Indian population. Clin Exp Ophthalmol 2008;36(3):257-260.
9 McCannel CA, Ensminger JL, Diehl NN, Hodge DN. Population-based incidence of macular holes. Ophthalmology 2009;116(7):1366-1369.
10 Singh AJ, Muqit MM, Woon WH. Is axial length a risk factor for idiopathic macular hole formation? Int Ophthalmol 2012;32(4):393-396.
11 Song WK, Lee SC, Lee ES, Kim CY, Kim SS. Macular thickness variations with sex, age, and axial length in healthy subjects: a spectral domain-optical coherence tomography study. Invest Ophthalmol Vis Sci 2010;51(8):3913-3918.
12 Kumagai K, Ogino N, Hangai M, Larson E. Percentage of fellow eyes that develop full-thickness macular hole in patients with unilateral macular hole. Arch Ophthalmol 2012;130(3):393-394.
13 Reibaldi M, Boscia F, Avitabile T, Uva MG, Russo V, Zagari M,Bonfiglio V, Reibaldi A, Longo A. Enhanced depth imaging optical coherence tomography of the choroid in idiopathic macular hole: A crosssectional prospective study. Am J Ophthalmol 2011;151(1):112-117.
14 Zhang P, Zhou M, Wu Y, Lu B, Li T, Zhao J, Wang F, Sun X. Choroidal thickness in unilateral idiopathic macular hole: a cross-sectional study and Meta-analysis. Retina 2017;37(1):60-69.
15 Aras C, Ocakoglu O, Akova N. Foveolar choroidal blood flow in idiopathic macular hole. Int Ophthalmol 2004;25(4):225-231.
16 Ahn J, Yoo G, Kim JT, Kim SW, Oh J. Choriocapillaris layer imaging with swept-source optical coherence tomography angiography in lamellar and full-thickness macular hole. Graefes Arch Clin Exp Ophthalmol 2018;256(1):11-21.
17 Jia Y, Morrison JC, Tokayer J, Tan O, Lombardi L, Baumann B, Lu CD, Choi W, Fujimoto JG, Huang D. Quantitative OCT angiography of optic nerve head blood flow. Biomed Opt Express 2012;3(12):3127-3137.
18 Wei E, Jia Y, Tan O, Potsaid B, Liu JJ, Choi W, Fujimoto JG, Huang D.Parafoveal retinal vascular response to pattern visual stimulation assessed with OCT angiography. PLoS One 2013;8(12):e81343.
19 Spaide RF, Klancnik JJ, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography.JAMA Ophthalmol 2015;133(1):45-50.
20 Jia Y, Tan O, Tokayer J, Potsaid B, Wang Y, Liu JJ, Kraus MF, Subhash H, Fujimoto JG, Hornegger J, Huang D. Split-spectrum amplitudedecorrelation angiography with optical coherence tomography. Opt Express 2012;20(4):4710-4725.
21 Hayreh SS. Blood supply of the optic nerve head and its role in optic atrophy, glaucoma, and oedema of the optic disc. Br J Ophthalmol 1969;53(11):721-748.
22 Lieberman MF, Maumenee AE, Green WR. Histologic studies of the vasculature of the anterior optic nerve. Am J Ophthalmol 1976;82(3):405-423.
23 Onda E, CioffiGA, Bacon DR, Van Buskirk EM. Microvasculature of the human optic nerve. Am J Ophthalmol 1995;120(1):92-102.
24 Teng Y, Yu M, Wang Y, Liu X, You Q, Liu W. OCT angiography quantifying choriocapillary circulation in idiopathic macular hole before and after surgery. Graefes Arch Clin Exp Ophthalmol 2017;255(5):893-902.
25 Kim YJ, Jo J, Lee JY, Yoon YH, Kim JG. Macular capillary plexuses after macular hole surgery: An optical coherence tomography angiography study. Br J Ophthalmol 2017.
26 Demirel S, Değirmenci MFK, Bilici S, Yanik Ö, Batıoğlu F, Özmert E, Alp N. The recovery of microvascular status evaluated by optical coherence tomography angiography in patients after successful macular hole surgery. Ophthalmic Res 2017.
27 Wang X, Kong X, Jiang C, Li M, Yu J, Sun X. Is the peripapillary retinal perfusion related to myopia in healthy eyes? A prospective comparative study. BMJ Open 2016;6(3):e010791.
28 Bojikian KD, Chen CL, Wen JC, Zhang Q, Xin C, Gupta D, Mudumbai RC, Johnstone MA, Wang RK, Chen PP. Optic disc perfusion in primary open angle and normal tension glaucoma eyes using optical coherence tomography-based microangiography. PLoS One 2016;11(5):e0154691.
29 Chen CL, Bojikian KD, Gupta D, Wen JC, Zhang Q, Xin C, Kono R,Mudumbai RC, Johnstone MA, Chen PP, Wang RK. Optic nerve head perfusion in normal eyes and eyes with glaucoma using optical coherence tomography-based microangiography. Quant Imaging Med Surg 2016;6(2):125-133.
30 Akagi T, Iida Y, Nakanishi H, Terada N, Morooka S, Yamada H,Hasegawa T, Yokota S, Yoshikawa M, Yoshimura N. Microvascular density in glaucomatous eyes with hemifield visual field defects: an optical coherence tomography angiography study. Am J Ophthalmol 2016;168:237-249.
31 Damento G, Chen MH, Leng T. Spectral-domain optical coherence tomography angiography of central retinal artery occlusion. Ophthalmic Surg Lasers Imaging Retina 2016;47(5):467-470.
32 Wang X, Jiang C, Ko T, Kong X, Yu X, Min W, Shi G, Sun X. Correlation between optic disc perfusion and glaucomatous severity in patients with open-angle glaucoma: an optical coherence tomography angiography study. Graefes Arch Clin Exp Ophthalmol 2015;253(9):1557-1564.
33 Liu L, Jia Y, Takusagawa HL, Pechauer AD, Edmunds B, Lombardi L, Davis E, Morrison JC, Huang D. Optical coherence tomography angiography of the peripapillary retina in glaucoma. JAMA Ophthalmol 2015;133(9):1045-1052.
34 Jia Y, Wei E, Wang X, Zhang X, Morrison JC, Parikh M, Lombardi LH, Gattey DM, Armour RL, Edmunds B, Kraus MF, Fujimoto JG,Huang D. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology 2014;121(7):1322-1332.
35 Wang X, Jia Y, Spain R, Potsaid B, Liu JJ, Baumann B, Hornegger J, Fujimoto JG, Wu Q, Huang D. Optical coherence tomography angiography of optic nerve head and parafovea in multiple sclerosis. Br J Ophthalmol 2014;98(10):1368-1373.
36 Liew G, Wang JJ. Retinal vascular signs: A window to the heart?. Rev Esp Cardiol 2011;64(6):515-521.
37 Pournaras CJ, Riva CE. Retinal blood flow evaluation. Ophthalmologica 2013;229(2):61-74.
38 Newman EA. Functional hyperemia and mechanisms of neurovascular coupling in the retinal vasculature. J Cereb Blood Flow Metab 2013;33(11):1685-1695.
39 Riva CE, Hero M, Titze P, Petrig B. Autoregulation of human optic nerve head blood flow in response to acute changes in ocular perfusion pressure. Graefes Arch Clin Exp Ophthalmol 1997;235(10):618-626.
40 Wang Q, Chan S, Yang JY, You B, Wang YX, Jonas JB, Wei WB.Vascular density in retina and choriocapillaris as measured by optical coherence tomography angiography. Am J Ophthalmol 2016;168:95-109.