INTRODUCTION
Retinoblastoma (RB) is still the most common pediatric intraocular tumor[1]. Over the past decade, systemic chemotherapy has replaced radiotherapy as the preferred eye preserving primary method for treatment of RB. This approach avoids the known toxicity of external beam radiotherapy (EBR)with similar rates of ocular retention. However, curing RB with vitreous involvement remains the most challenging[2-4].
Vitreous seeding is typically seen in advanced intraocular RB and represents a major determinant for eye grouping at presentation and this type is known as the primary type[5]. It may also appear during the treatment course in eyes devoid of it at diagnosis and this is known as secondary vitreous seeds or vitreous relapse. The administration of diode laser hyperthermia is a possible iatrogenic cause to increase this risk of secondary vitreous seeding[2]. Another cause is the sudden vitreous dispersion of large tumors shortly after the initiation of chemotherapy due to a necrotic disruption of the internal limiting membrane[6].
RB with vitreous seeds accounts for approximately 30% of all patients with the disease[3]. Some cases of vitreous seeding are amenable to conventional treatment with systemic intravenous chemotherapy and EBR. However, others can be resistant to these measures with unfavorable prognosis leading to enucleation[3,7-8].
Novel routes of drug administration such as intra-arterial[9-11],intravitreal[12-14]and periocular chemotherapy[15-17]have improved the ocular delivery compared with systemic chemotherapy[5].
Intra-arterial chemotherapy involves super-selective drug administration through the ophthalmic artery, resulting in better penetration of ocular structures and lower systemic toxicity[18].It was proved to be effective asfirst-line of treatment in Group C and Group D eyes, according to international intraocular classification of RB[19]. Also, Group E salvaged using this modality in some reports[4]. It was used as rescue therapy in recurrent RB after failure of other treatment modalities[18].
Despite the dramatic increase in ocular salvage with intraarterial chemotherapy, vitreous seeding is still one of the main reasons for subsequent enucleation in treated eyes[20]. More than 50 years ago, Ericson and Rosengren[21]reported on the intravitreal delivery of chemotherapeutical drugs targeting vitreous seeds. However, this method was not employed in routine use due to the concern on possible extraocular spread of tumor cells and inconsistent successes[22]. Half a century later, several groups revisited the chemotherapeutical drugs and intravitreal drug delivery methods for treating vitreous seeds.They stated that intravitreal chemotherapy with melphalan is an effective and safe modality for eliminating vitreous seeds from RB[23-25].
More than 10y ago, experimental work demonstrated that periocular carboplatin quickly entered the vitreous cavity in high concentrations and it has been incorporated in the Children’s Oncology Group for management of Group C and Group D eyes[16].
The aim of this study was to evaluate safety and efficacy of posterior sub-Tenon’s carboplatin injection compared to intravitreal melphalan injection for management of RB with secondary vitreous seeds. The outcome measures were vitreous seeds regression, need for other treatment modalities to achieve ocular salvage and possible side effects.
SUBJECTS AND METHODS
Study Design and Settings A prospective interventional comparative non randomized study was conducted at the Ocular Oncology Unit, Ophthalmology Department and Oncology and Radiation Medicine Department, Ain Shams University Hospital started from September 2012 and ended in December 2016. All RB patients presented during their follow up period with secondary vitreous seeds after favorable response to primary treatments were included in the present study.
All treatment procedures including possible side effects were explained to the parents and they provided us with an informed written consent. This study was adhered to the tenets of the Declaration of Helsinki and was approved by Faculty of Medicine, Ain Shams University Research Ethical Committee.The medical records of these patients were reviewed including:age at time of presentation, complaint (its onset, course,and duration) as reported by the parents, family history and consanguinity, any previous investigations or lines of treatment. Reports of ophthalmological examination of the children at presentation and at the time of primary treatment were reviewed including fundus examination and photography with documentation of tumor size, location, number and the presence of subretinal fluid, subretinal seeds and vitreous seeds. Staging was recorded according to international intraocular classification of RB[19].
The study excluded resistant cases to primary treatments and advanced disease (Group E) who was candidate for enucleation. Anterior segment assessment, intraocular pressure and indirect ophthalmoscopy were performed. The new findings at the diagnosis of secondary vitreous seeds were documented using fundus photography (Genesis D;Kowa Medicals, Tokyo, Japan). B-scan ultrasonography was performed to determine tumor dimensions.
All patients previously started treatment based on staging of the disease guided by Shields et al[26]. For Group A, focal treatments in the form of transpupillary thermotherapy or cryotherapy were performed until complete tumor regression.For Groups B, C, D, six cycles of systemic chemotherapeutic agents were administered by oncologist including the following protocol; in the 1stweek, on day 1: carboplatin (560 mg/m2),on day 3: etoposide (200 mg/m2or 5 mg/kg for patients with age less than 36mo) and vincristine (1.5 mg/m2or 0.05 mg/kg for patients with age less than 36mo) was given on day 1 of 3rdweek. This cycle was repeated every 28d. This was in conjunction with focal treatments that were administered with systemic chemotherapy to achieve complete tumor regression.Thermotherapy was performed according to Shields et al[27]. A semiconductor diode laser delivered with a 1300 μm large spot indirect ophthalmoscope delivery system [IRIDEX OcuLight SLx (California, USA)] was used. The infrared laser was set at a continuous mode and the power was adjusted at 300-400 mW and was applied for 5-10min in each session of treatment.Cryotherapy was performed according to Shields et al[28]. The nitrous oxide cryotherapy probe indented the globe to localize the tumor and to elevate it on the tip. Freezing was done and the ice ball was watched to ensure that it involved the entire mass. Tumor destruction was usually achieved with one or two session of triple freeze thaw cryotherapy applied at 4wk interval.
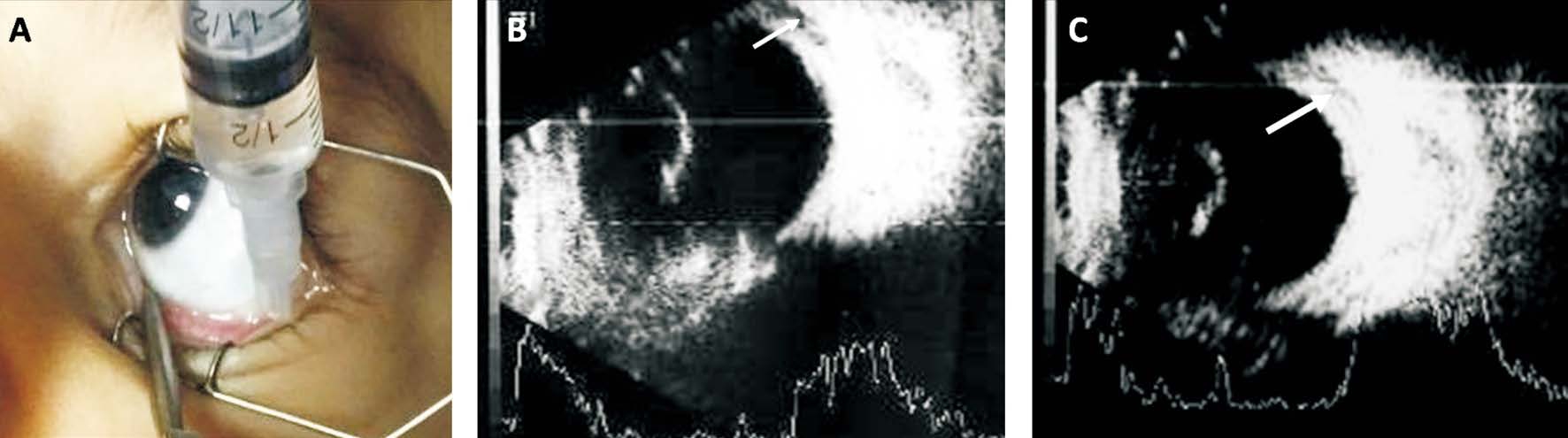
Figure 1 Technique of posterior sub-Tenon’s carboplatin injection A: The drug (20 mg/2 mL) was delivered using 25 gauge needle without a conjunctival cut down; B, C: Ocular ultrasound show the drug (black area pointed to by the white arrows) was in sub-Tenon’s space.
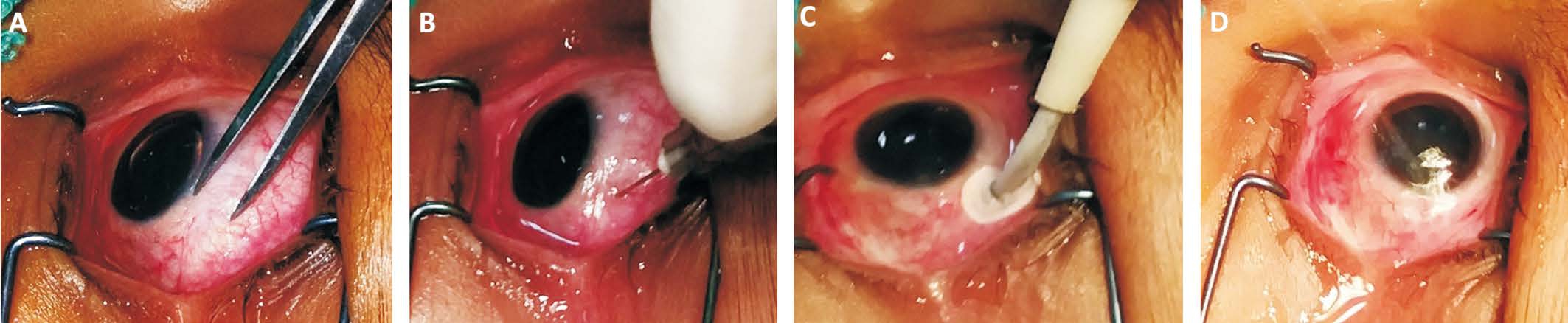
Figure 2 Technique of intravitreal melphalan injection A, B: A dose of 20 μg/0.1 mL melphalan was injected via a pars plana approach using 30 gauge needle; C: Cryoprobe was applied to the injection site while the needle was withdrawn (triple freeze-thaw technique); D: Copious irrigation of the injection site by distilled water for 3min.
Unresponsive disease was defined as persistence of retinal tumors, vitreous seeds or subretinal seeds following the end of the six cycles of systemic chemotherapy, with no appreciable signs of regression. Recurrence was defined as the regrowth of retinal tumors, vitreous or subretinal seeds after an initial favorable response and regression and those were the candidates of the present study.
Cases with secondary vitreous seeding that were developed after chemoreduction and focal therapies were subdivided into two groups after explanation of local injection techniques and all the possible side effects to the parents: study group I: posterior sub-Tenon’s carboplatin injection was added to systemic chemotherapy ± focal treatments; study group II: intravitreal melphalan injection was added to systemic chemotherapy ± focal treatments.
Local Chemotherapy Injection Techniques
Posterior sub-Tenon’s carboplatin injection A uniform concentration of 10 mg/1 mL was used for posterior sub-Tenon’s carboplatin injection. The concentration was chosen as the highest stable concentration recommended by the manufacturer. A total of 2 mL of carboplatin solution was given per injection and repeated every 4wk. Initially one injection/month was given for 3 successive months. Then every month according to response, injections were discontinued if there was a progression of the disease or complete clinical response.All injections were given under general anesthesia prior to administration of systemic chemotherapy on the same day and were administrated posteriorly in sub-Tenon’s space without a conjunctival cut down (Figure 1A), guided by ultrasonography(Figure 1B, 1C) using 25 gauge needle to deliver the chemotherapy over the quadrant where seeds were present.Cryotherapy as an adjuvant therapy was administered to the periphery of the injected eye at the same sessions in some patients in an attempt to increase drug delivery. Topical antibiotic/corticosteroid combination ointment was prescribed four times daily after the procedure for one week with cold fomentation.
Intravitreal melphalan injection Eligibility criteria for intravitreal melphalan and the injection technique were according to Munier et al[12]. This was performed under general anesthesia at the operative theatre under complete sterile conditions. By using 30 gauge needle, a dose of (20 μg/0.1 mL)melphalan was injected via a pars plana approach (Figure 2A,2B). It was injected under umbrella of systemic chemotherapy.Upon needle withdrawal, the injection site was sealed and sterilized with cryotherapy (triple freeze-thaw technique)(Figure 2C) followed by copious irrigation of the injection site by distilled water (Figure 2D) for 3min. Topical antibiotic/corticosteroid combination ointment was prescribed following the procedure. Intravitreal injection of melphalan was given on response and on a weekly schedule.
EBR was used in resistant cases and was administered by oncologist in a dose ranging from 35.0 to 45.0 Grey using the lateral lens sparing technique[29]. Enucleation was performed in cases of failure of treatment with no hope of useful vision.
The patients were followed up under anesthesia by fundus examination and photography every 4wk during treatment period until complete response. It was established if the seedswere completely disappear (vitreous seeds regression type 0),refringent and/or calcified residues (type I), amorphous often non spherical inactive residues (type II), or a combination of the latter two (type III)[24]. Follow up was carried out every 3mo after complete response. Episodes of tumor and or seeds recurrences, new mass development, side effects of treatments and rate of eye salvage were documented along a period of 12mo from end of secondary treatments.
Table 1 Demographic data of the study groups n (%)
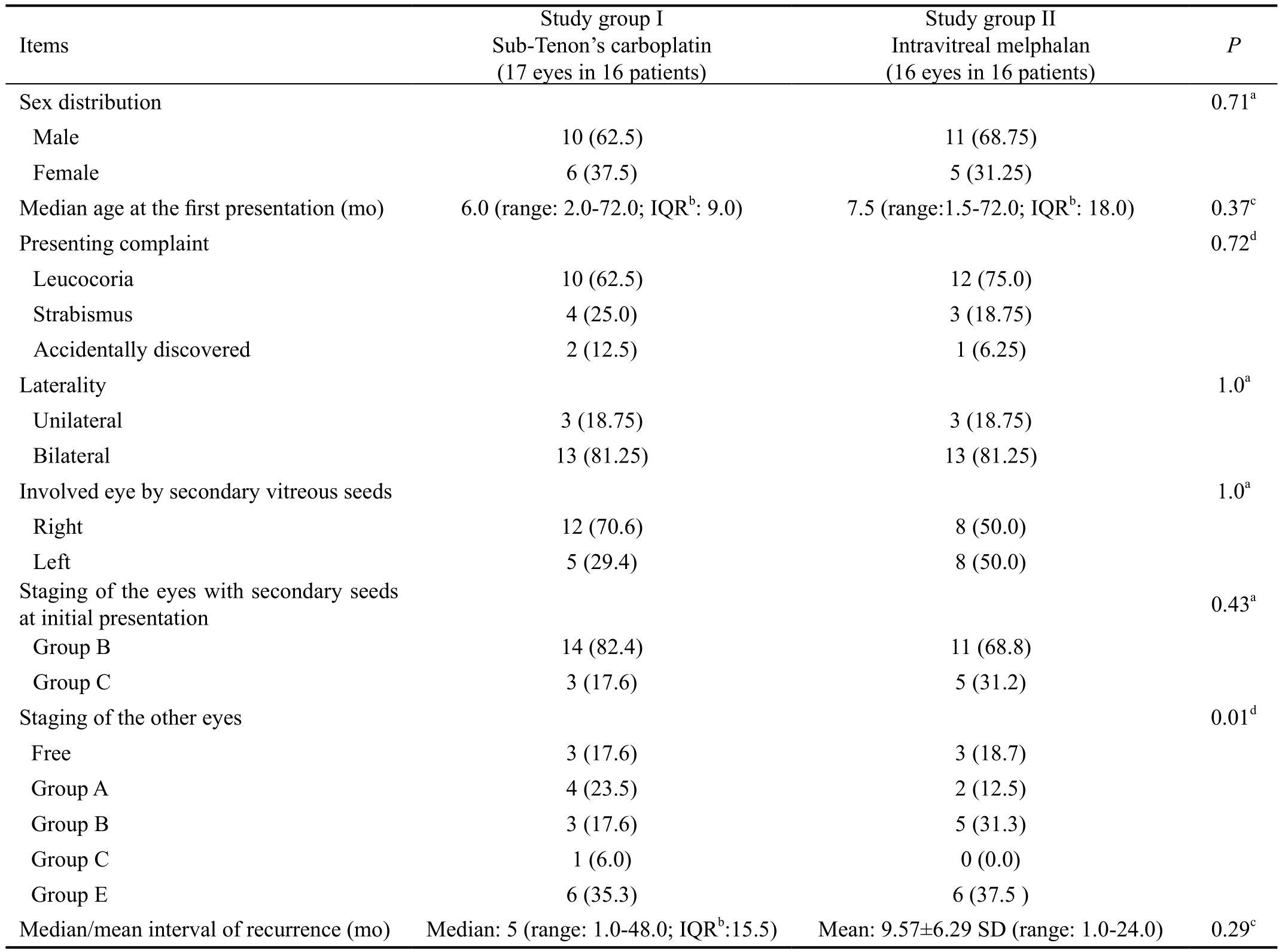
aFisher’s exact test;bIQR: Interquartile range;cMann-Whitney U test;dChi-square test. P-value set at ≤0.05 was statistically significant.
Statistical Analysis Data were analyzed using SPSS 13 software (SPSS Inc., Chicago, Illinois, USA). Kolmogorov-Smirnov test was used to assess normality of data. Non-parametric quantitative data was expressed as median and interquartile range(IQR), 95% confidence interval. Comparison between variables of two groups was performed using the Mann-Whitney U test. Parametric continuous variables were expressed as mean and standard deviation. Independent sample t-test was used to compare between quantitative variables of two groups.Description of qualitative variables was in the form of numbers and percentages. Chi-square and Fisher’s exact tests were used to compare between qualitative data. Spearman’s correlation coefficient was used to correlate between variables. The level of significance was set at P≤0.05.
RESULTS
The study included 21 males (65.6%) and 11 females (34.4%).Median age at thefirst presentation and primary therapy was 7.5mo (range: 1.5-72.0; IQR: 18.0). Leucocoria was the most common presentation in 22 patients (68.8%), strabismus in 7 patients (21.9%) and accidentally discovered RB during routine fundus examination in 3 patients (9.4%). Family history was positive in 5 patients (15.6%) and with positive consanguinity among 4 patients (12.5%). RB was bilateral in 26 patients (81.3%) with the other eye was enucleated in 12 patients (37.5%). Thirty-three eyes of 32 RB patients developed secondary vitreous seeds. Study group I included 17 eyes (16 patients) and study group II included 16 eyes (16 patients). Staging of these eyes at presentation was: Group B in 25 eyes (75.8%), Group C in 8 eyes (24.2%). One patient(3.1%) had RB with bilateral recurrent vitreous seeds,both eyes staged as Group B at the initial presentation and treatment. This patient was included in study group I. The median/mean intervals till recurrence of vitreous seeds were 5.0mo and 9.75mo in study group I and study group II respectively. Table 1 demonstrated demographic data of each of study groups.
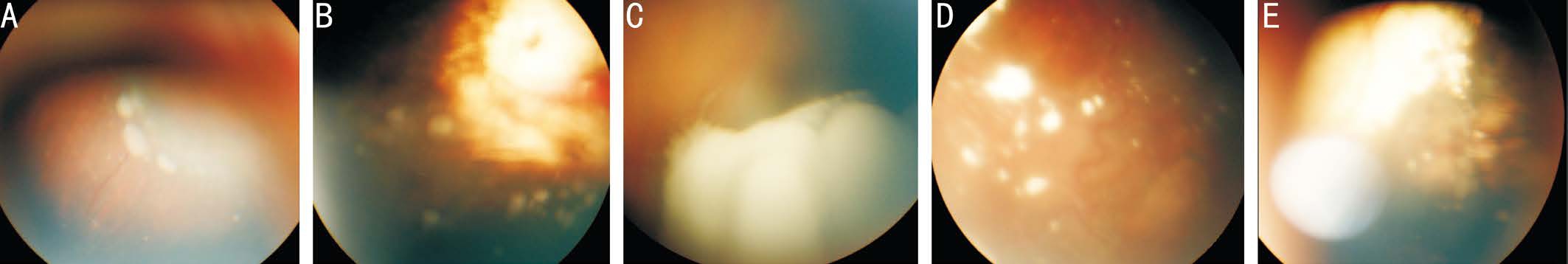
Figure 3 Vitreous seeds A, B: Sphere active type; C: Dust active type on surface of tumor; D, E: Calcified vitreous seeds following intravitreal melphalan and posterior sub-Tenon’s carboplatin injections respectively.
From the 17 eyes received posterior sub-Tenon’s injections:14 eyes (82.4%) were in Group B and 3 eyes (17.6%) were in Group C. From the 16 eyes received intravitreal injections:11 eyes (68.8%) were in Group (B) and 5 eyes (31.2%) were in Group C. As regards initial tumor characteristics: median number of tumors/eye was 2.0 (range: 1-4; IQR: 1.0). Sixteen eyes (48.5%) had tumors located <3 mm from the fovea and 17 eyes (51.5%) had tumors located ≥3 mm from foveal center in both study groups. There was no statistically significant difference between study groups as regards tumor location(P=0.17). The mean tumor base diameter was 4.67±1.79 SD(range: 1.0-8.0 mm). Eighteen eyes (54.5%) had tumors <5 mm in base diameter and 15 eyes (45.5%) had tumors of ≥5 mm in base diameter. There was a statistically significant difference between study groups as regards frequency of eyes had mean tumor base diameter ≥5 mm, being higher in study group II,(P=0.01). The mean tumor thickness was 4.31±1.42 SD (1.5-7.0 mm). The tumor thickness was <5 mm in 9 eyes (27.3%)and ≥5 mm in 24 eyes (72.7%). At presentation localized subretinal fluid was present in 3 eyes (9.1%) and subretinal seeds in 4 eyes (12.1%).
Focal therapies in conjunction with chemoreduction were used in the form of cryotherapy in 4 eyes (12.1%) and transpupillary thermotherapy in 20 eyes (60.6%). Median of laser sessions was 3.0 (range: 2.0-6.0; IQR: 1.0). There was a statistically significant higher number of laser sessions in study group I compared study group II (P=0.001). Following treatment using chemoreduction ± focal therapies, complete regression of the RB tumors occurred in 22 eyes (66.7%), resistance in 4 eyes(12.1%) and recurrence of the tumor developed after initial improvement in 7 eyes (21.2%). New masses developed in 5 eyes (15.1%). Secondary vitreous seeds were focal (<3 mm from main tumor margin) in 15 eyes (45.5%) and diffuse in 18 eyes(54.5%). According to the shape of vitreous seeds, spheres(Figure 3A, 3B) were present in 12 eyes (36.4%), dusts (Figure 3C) in 14 eyes (42.4%), and clouds in 7 eyes (21.2%) with no statistically significant difference between the study groups(P=0.69).
Posterior sub-Tenon’s injection of carboplatin was used in 17 eyes (51.5%). The average number of injections was 3 times/eye. Intravitreal melphalan injection was performed in 16 eyes(48.5%). Injections ranged from 1-10 times (median: 3, IQR:
3) with 2-4wk interval. Table 2 demonstrated the clinical characteristics and treatment outcomes among the study groups.EBR was added in 18 eyes (54.5%) due to failure of local chemotherapy in causing regression of vitreous seeds. It was given to 10/17 eyes (58.8%) in study group I, 4/10 eyes were salvaged following its administration. Eight /16 eyes (50.0%)received EBR in study group II, 6/8 eyes were salvaged.Eight eyes were enucleated following failure of EBR (6 eyes belonged to study group I and 2 eyes belonged to study group II).
In the remaining 15 eyes (45.5%), EBR was avoided in 10 eyes, (4 eyes belonged to study group I and 6 eyes belonged to study group II) and those eyes were completely salvaged following local chemotherapies. Five eyes was enucleated without use of EBR due to advanced disease (3 eyes belonged to study group I and 2 eyes belonged to study group II).The mean/median duration of follow up following local chemotherapy injections were 2.0y and 2.37y in study group I and study group II respectively.
The total number of salvaged eyes was 20 eyes (60.6%), 8 eyes in study group I and 12 eyes in study group II. Six eyes(42.8%) and 2 eyes (66.7%) belonged to Groups B and C respectively were salvaged in study group I, 7 eyes (63.6%)and 5 eyes (100%) belonged to Groups B and C respectively were salvaged in study group II at the end of follow up.
Ocular salvage rates were 23.5% and 37.5% following posterior sub-Tenon’s carboplatin injection and intravitreal melphalan injection without EBR respectively raised to 47.1%and 75% with addition of EBR (Table 2) with no statistically significant difference between study groups (P=0.16). Table 3 summarized the ocular salvage rates among the study groups based on international classification of RB and the use of different treatment modalities.
Regarding type of regression of vitreous seeds in salvaged eyes, it was type (0) in 6 eyes, type I in 3 eyes (Figure 3D,3E), type II in 8 eyes and type III in 3 eyes (Table 2). There was no statistically significant difference between the study groups regarding type of regression of vitreous seeds (P=0.11).There was a statistically significant difference between the study groups regarding type of regression of vitreous seeds and ocular salvage rate (P=0.001). It was found that 10/14 eyes, 10/12 eyes and 0/7 eyes harbor dust, spheres and clouds
respectively were salvaged. A statistically significant moderate positive correlation was found between ocular salvage rate and type of vitreous seeds either dust, spheres and clouds(Spearman’s correlation coefficient was 0.42, P=0.015).
Table 2 Clinical characteristics and treatment outcomes among the study groups n (%)
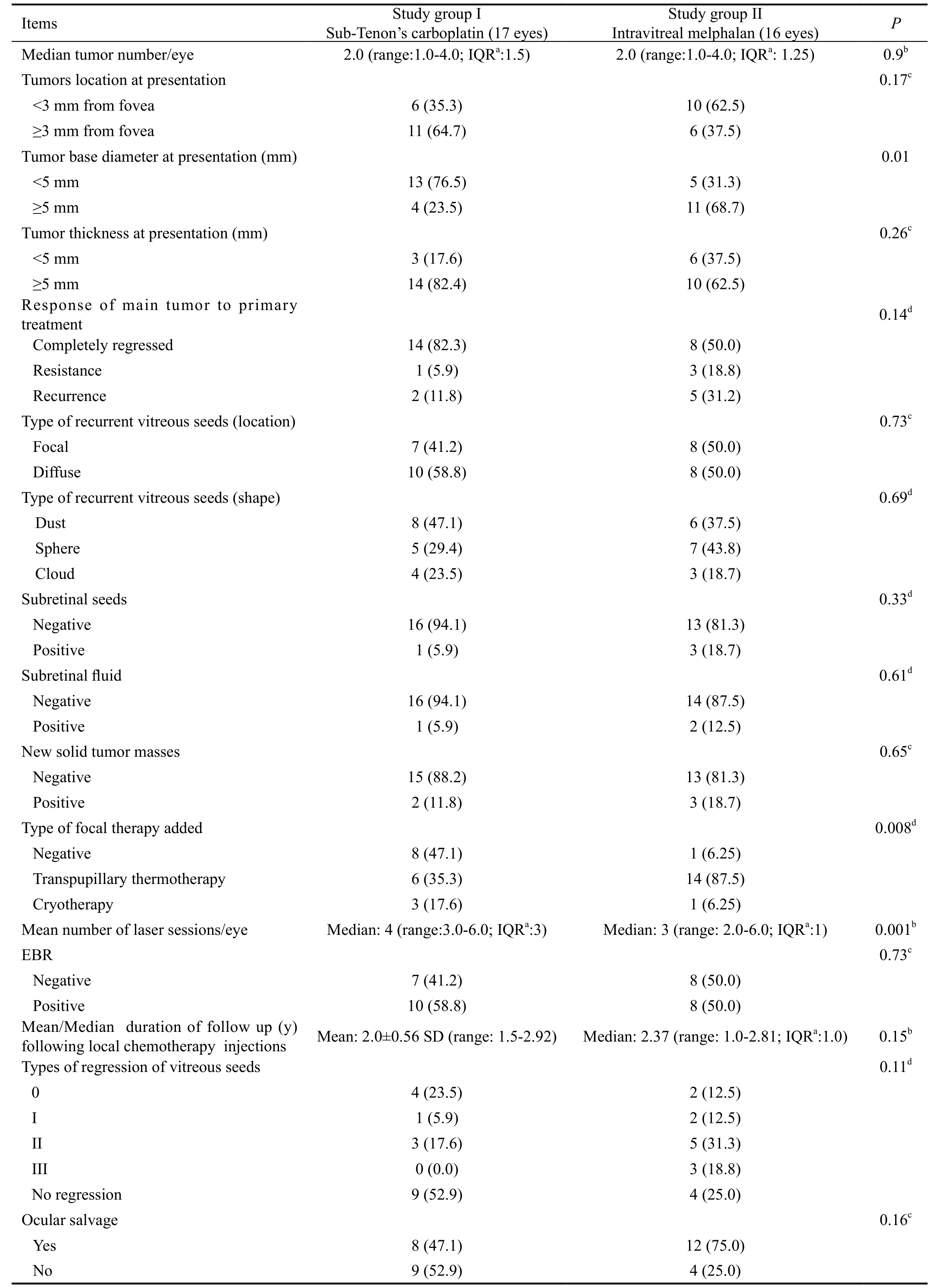
aIQR: Interquartile range;bMann-Whitney test;cFisher’s exact test;dChi-square test. P≤0.05 was statistically significant. EBR: External beam radiotherapy.
A statistically significant difference was found regarding globe salvage in those had new masses (0/3 eyes) and eyes had recurrent seeds only (12/13 eyes) (P=0.007). A statistically significant moderate positive correlation was found between ocular salvage rate and appearance of new tumor mass.Spearman’s correlation coefficient was 0.35 (P=0.045).
All eyes received posterior sub-Tenon’s injections had periorbital edema that resolved with cold fomentation and topical steroid ointment. Motility disturbance due to muscle and orbital fibrosis was developed in 13 eyes (76.5%).Vitreous hemorrhage was developed in two eyes (12.5%)following intravitreal injection that was cleared one month later and localized peripheral salt and pepper retinopathy at site of injection in 5 eyes (31.25%). Two patients (6.25%) had bilateral enucleation with no case of extraocular spread or systemic metastasis during the follow up period.
DISCUSSION
Intravenous chemotherapy involving a 3-drug regimen of vincristine, etoposide and carboplatin is the most widely utilized first-line therapy for intraocular RB. Such therapy aims at chemoreduction, focal therapies can be subsequently used, with the primary goal of eye conservation. Systemic chemotherapy in cases with germline RB gene mutations may decrease the risk of trilateral RB[30]. This modality provides good control of early-stage intraocular RB confined to the retina without subretinal or vitreous involvement[31-32].
More advanced RB characterized by seeding into the subretinal space or the vitreous are classified as Groups C to E. Success rate of systemic chemotherapy is shown to be progressively decrease with increased tumor burden. In Group C eyes where seeding is focal and close to the tumor (<3 mm),good outcomes, with successful tumor control from 67%to 90% of eyes was achieved[33-34]. Previous studies[31-32,34-35]have demonstrated high incidence of intraocular recurrence necessitating EBR and/or enucleation in advanced RB cases,with treatment success achieved in only 9% to 57% of Group D.Several studies[3,7,36-38]reported salvage of about 67% of eyes with vitreous seeds using systemic chemotherapy coupled with EBR however irradiation can induce second non ocular malignant neoplasm especially in patients with the hereditary form of RB in addition to the usual side effects of radiotherapy[38].
By using local chemotherapeutic injections, the intraocular concentration of agents is increased without additional systemic toxicity from increasing intravenous dosages. It was found that intravitreal injections of melphalan in RB with vitreal seeds achieve higher intravitreal levels, which was important in achieving eye preservation[39].
Transgenic mice with RB have also shown significant tumor control with subconjuctival injection of carboplatin,accompanied by little or no local toxicity[40-41]. Periocular injection of carboplatin was used for RB control over two decades, often as an adjunct to systemic chemotherapy. It achieves rapid levels within the vitreous in 30min, with concentration of 6-10 times compared to that achieved by intravenous route, and can last for hours[42-43].
From the present results it was found that posterior sub-Tenon’s injection of carboplatin for treatment of RB with recurrent vitreous seeds has regressing effects in combination with other modalities. Marr et al[16]and Manjandavida et al[3]stated that better efficacy was obtained when adjunctive periocular injection of carboplatin was used along with systemic chemotherapy than when patients have been treated with systemic chemotherapy alone for advanced RB. It is believed that a high intracellular carboplatin level obtained in the tumor tissue is associated with increased tumor control[44]. So further increase in the intraocular concentration of carboplatin by periocular injections is a good adjunct to intravenous systemic chemotherapy for intraocular RB, and is a potential option for decreasing the amounts of intravenous chemotherapy the patients are subjected to[45].
In the present study regarding the response of secondary vitreous seeds to posterior sub-Tenon’s injection of carboplatin;23.5% of eyes were salvaged in conjunction with systemic chemotherapy without EBR especially when the primary tumors were controlled. Shields et al[27]in a retrospective study reported their experience with the efficacy of periocular carboplatin injections over a 12y period. Their eyes were in advanced stages than the present study. Totally 2/33 (6%) were in Group E, 30/33 (90%) in Group D and 1/33 (3%) in Group C eyes. Viable vitreous seeding was found in 28/33 (85%),recurrent retinal tumor in 2/33 (6%) and advanced subretinal seeding in 3/33 (9%) of eyes. The median number of injections and mean were three, maximum of 11, and a minimum of one injection per eye. Of 13 eyes (39%) had avoided enucleation.All salvaged eyes in their study were in Group D with the exception of one Group C eye. No Group E eyes were salvaged.Ocular survival at 36mo was 36%. Eleven of the 13 salvaged eyes received concurrent treatment with chemotherapy in 30%,EBR in 46%, or brachytherapy in 8%. Two of the salvaged eyes (16%) were treated with periocular injections alone. These data do not support its use as monotherapy. The use of multiple treatment modalities either simultaneously, before and/or after injections including EBR or plaque radiotherapy in their study and also an overall number of injections of 102 reaching 11 times in some treated eyes might explain the difference in the results with the present study regarding ocular salvage.
Table 3 Ocular salvage rates among the study groups based on international classification of RB and the use of different treatment modalities n (%)
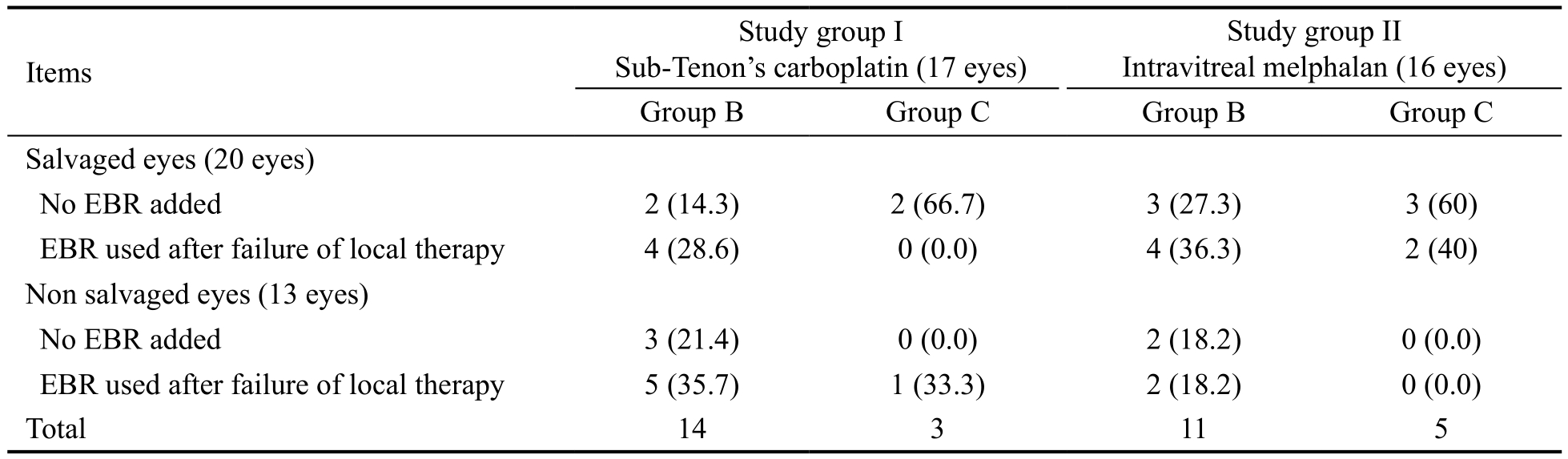
EBR: External beam radiotherapy.
A case report was published in 2010 of a 5mo old baby with bilateral RB (Reese Ellsworth Group Vb of left eye and Group Ia of right eye at that time) received one initial cycle of intravenous carboplatin and was treated focally with laser in both eyes but had persistent vitreal seeds in the more advanced left eye. That eye received 11 injections of periocular carboplatin with additional laser treatments which led to resolution of the vitreous seeds with no recurrence for 9y.This is because periocular injection can control small vitreal tumors[17].
There was an earlier trial[15]of use of subconjuctival carboplatin as a treatment of intraocular RB to avoid the risks of use of systemic chemotherapy like anemia, neutropenia, infections and organ toxicities. The study included 13 eyes in 11 patients with bilateral RB. Those eyes were classified as Reese-Ellsworth Group Vb at the time of enrollment into the study,whereas 6 had only retinal or subretinal disease. Seven(64%) of the 11 patients had been treated previously with an intravenous carboplatin-containing chemotherapy regimen,and 4 of those had also received EBR previously. A median of 3 injections per eye. The median dose administered per injection was 20 mg. Three offive eyes with vitreous seeding had impressive responses. These three patients who had major responses of their vitreous disease had all previously received both carboplatin-containing systemic chemotherapy and EBR before the disease progression. At the end of their study, they recommended evaluation of various devices used to noninvasively deliver various drugs to the vitreous and the possibility of development of a multimodality protocol that use a combination of both systemic and locally administered chemotherapy in intraocular RB.
Manjandavida et al[3]stated that intensive management with primary high dose of systemic chemotherapy and concurrent periocular carboplatin, and EBR selectively in chemotherapy failure provides gratifying outcome in RB with vitreous seeds.They saved by their different protocol of treatment, 95%, 85%and 57.5% of eyes in Groups C, D and E respectively.
The use of intravitreal melphalan for recurrent or resistant vitreous seeds had promising results in the previous studies[12,25,46-49]. In the present study, it saved 37.5% of eyes receiving this modality.In a study of 23 eyes with active vitreous seeds, Munier et al[12]reported globe salvage in 87% of eyes with complete remission of vitreous seeds over a mean follow-up period of 2y. Intravitreal melphalan 20-30 μg was injected every 7-10d via the pars plana approach. The median number of injections was 6.5 (range: 2-8).
Seregard and Singh et al[49]studied 11 eyes treated with intravitreal melphalan and reported the globe salvage in 100%eyes at a mean follow-up of 9mo. Intravitreal melphalan 20-30 μg was injected every month via the pars plana approach with concomitant triple freeze-thaw cryotherapy to the injection site. The median number of injections was 6.0 (range: 2-6).This was not comparable to the present study because of the difference in dose of melphalan injected which was up to 30 μg and the median number of injection which was 6 times/eye in their study.
Lee et al[50]in a retrospective study on 8 eyes used combined intravitreal injection of melphalan combined with intraarterial chemotherapy (3-5 sessions/eye) in all eyes that were previously treated with intravenous chemotherapy. They used a median number of melphalan injections of 3.5 (range: 2-4)with a globe salvage rate of 87.5%. The high rate of ocular salvage compared to the present study was due to combination of intra-arterial injections in all cases.
Ji et al[51]conducted a study to evaluate the efficacy of intravitreal melphalan for vitreous seeds from RB in 19 Chinese patients. The patients received multiple intravitreal injections of 20 μg melphalan. Successful control of vitreous seeds was achieved in 16 of 19 eyes (84.21%) and globe retention was achieved in 73.68% (14/19) eyes.
They found a significant difference in response to intravitreal melphalan for cloud, spheres, and dust seeds. Cloud was the most resistant to control and dust was the easiest. The median number of injections to control was 9, 6, and 3 respectively.This was reported in the present study and previous studies[13,25].Factors predict vitreous seeds regression stated by Manjandavida et al[3]were bilateral RB, absence of subretinal fluid, absence of subretinal seeds and tumor base diameter of less than 16 mm.The present study found that there was no correlation between these factors and regression of vitreous seeds and eye salvage except type of vitreous seeds and new solid tumor growth. This difference may be due to large number of cases (101 eyes)studied on a retrospective way over 10y in their study.
No case of extraocular extension or systemic metastasis reported in the present study owing to the precautions used to minimize this risk during intravitreal injection as described by Munier et al[12]in the form of repetitive freeze and thaw cycles at the injection site when pulling out needle and irrigation with sterile distilled water submersion on the surface of the eye for at least 3min after injection. Smith et al[52]reported that local and systemic tumor spread following intravitreal melphalan injection is rare especially by the use of safety enhancing injection techniques.
Ji et al[51]reported vitreous hemorrhage developed in 2 cases,cataract in 3 cases, a localized peripheral salt-and pepper retinopathy in 8 eyes and pupil posterior synechia was noted in 1 case which was comparable to the present study regarding the occurrence of vitreous hemorrhage owing to the use of a dose of 20 μg/0.1 mL which is generally accepted to be safer for the retina and the eye[13,20,48,53].
Transient periocular swelling and redness occur in the majority of patients following periocular carboplatin injection[15-16,54].More serious side effects (endophthalmitis, motility restriction and conjunctival scarring) usually developed after three injections per eye, each performed at 3-4wk interval. This is due to direct toxicity of carboplatin[51]. Ocular motility disturbance in the present study was (76.5%) which was lower comparable to a previous study of Mulvihill et al[54]. They detected limitation of ocular motility in all 12 eyes (100%)of 10 RB patients injected with periocular carboplatin and significantfibrosis was proved by histopathology of enucleated eyes. Kim et al[55]had a case of 5mo old infant had bilateral RB and received periocular carboplatin for 3 times in the more advanced eye and developed orbital fibrosis and fat necrosis following the third injection. Marr et al[16]lost patients during follow up and the data available on forced duction test was not available to them in their retrospective study, so they did not report this side effect. Fibrin sealant sustained release carboplatin may be used to limit the number of injections and prevent diffusion of the drug into the orbit[56]. According to that, the decision to use posterior sub-Tenon’s carboplatin injection must weigh the risks and benefits and should be discussed thoroughly with the parents.
Intravitreal melphalan injection could save 6 eyes (37.5%)versus 4 eyes (23.5%) following posterior sub-Tenon’s injection and these rates were doubled following EBR use.There was no statistically significant difference between the two groups. This result was different from previous results regarding the efficacy of intravitreal melphalan. It might be due to some study limitations; lack of randomization, small number of cases in each group, different study population characteristics, cases of RB with secondary vitreous seeding were included only in the study, location of the tumor (more tumors near the fovea in the present study), dosage and numbers of injection.
In conclusion, this was thefirst prospective study comparing the safety and efficacy of local injection of carboplatin in the posterior sub-Tenon’s space and melphalan into the vitreous cavity for treatment of RB with secondary vitreous seeds. It was found that both techniques can salvage 30.3% of eyes without the need for EBR. Additional 30.3% of eyes were salvaged by EBR following failure of local therapies. There was a trend toward superiority of intravitreal melphalan in ocular salvage however, no statistically significant difference was found between the two groups.
ACKNOWLEDGEMENTS
The authors thank all members of Ocular Oncology Clinic in Ophthalmology Department, Ain Shams University, Cairo, Egypt.
Conflicts of Interest:Said AMA, None; Aly MG, None;Rashed HO, None; Rady AM, None.
REFERENCES
1 Brink A, Correa ZM, Geller J, Abruzzo T, Augsburger JJ. Managing the consequences of aggressive conservative treatment for refractory retinoblastoma with vitreous seeding. Arq Bras Olfalmol 2014;77(4):256-258.
2 Gombos DS, Cauchi PA, Hungerford JL, Addison P, Coen PG, Kingston JE. Vitreous relapse following primary chemotherapy for retinoblastoma:is adjuvant diode laser a risk factor? Br J Ophthalmol 2006;90(9):1168-1172.3 Manjandavida FP, Honavar SG, Reddy VA, Khanna R. Management and outcome of retinoblastoma with vitreous seeds. Ophthalmology 2014;121(2):517-524.
4 Shields CL, Bianciotto CG, Jabbour P, Ramasubramanian A, Lally SE,Griffin GC, Rosenwasser R, Shields JA. Intraarterial chemotherapy for retinoblastoma: report No 1, control of retinal tumors, subretinal seeds,and vitreous seeds. Arch Ophthalmol 2011;129(11):1399-1406.
5 Munier FL. Classification and management of seeds in retinoblastoma.Ellsworth Lecture Ghent August 24th 2013. Ophthalmic Genet 2014;35(4):193-207.
6 Parness-Yossifon R, Bryar PJ, Weinstein JL, Strikumaran D, Mets MB.Sudden dispersion of retinoblastoma shortly after initial chemotherapy treatment. Am J Ophthalmol 2009;147(5):903-906.
7 Shields CL, Honavar SG, Shields JA, Demirci H, Meadows AT,Naduvilath TJ. Factors predictive of recurrence of retinal tumors, vitreous seeds, and subretinal seeds following chemoreduction for retinoblastoma.Arch Ophthalmol 2002;120(4):460-464.
8 Abramson DH, Marr BP, Dunkel IJ, Brodie S, Zabor EC, Driscoll SJ, Gobin YP. Intra-arterial chemotherapy for retinoblastoma in eyes with vitreous and/or subretinal seeding: 2-year results. Br J Ophthalmol 2012;96(4):499-502.
9 Shields CL, Kaliki S, Shah SU, Bianciotto CG, Liu D, Jabbour P, Griffin GC, Shields JA. Minimal exposure (one or two cycles) of intra-arterial chemotherapy in the management of retinoblastoma. Ophthalmology 2012;119(1):188-192.
10 Shields CL, Kaliki S, Al-Dahmash S, Rojanaporn D, Leahey A, Griffin G, Jabbour P, Shields JA. Management of advanced retinoblastoma with intravenous chemotherapy then intra-arterial chemotherapy as alternative to enucleation. Retina 2013;33(10):2103-2109.
11 Shields CL, Manjandavida FP, Lally SE, Pieretti G, Arepalli SA,Caywood EH, Jabbour P, Shields JA. Intra-arterial chemotherapy for retinoblastoma in 70 eyes outcomes based on the international classification of retinoblastoma. Ophthalmology 2014;121(7):1453-1460.
12 Munier FL, Gaillard MC, Balmer A, Soliman S, Podilsky G, Moulin AP, Beck-Popovic M. Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibition to conditional indications. Br J Ophthalmol 2012;96(8):1078-1083.
13 Ghassemi F, Shields CL, Ghadimi H, Khodabandeh A, Roohipoor R. Combined intravitreal melphalan and topotecan for refractory or recurrent vitreous seeding from retinoblastoma. JAMA Ophthalmol 2014;132(8):936-941.
14 Francis JH, Marr BP, Brodie SE, Gobin P, Dunkel IJ, Abramson DH. Intravitreal melphalan as salvage therapy for refractory retinal and subretinal retinoblastoma. Retin Cases Breif Rep 2016;10(4):357-360.
15 Abramson DH, Frank CM, Dunkel IJ. A phase I/II study of subconjunctival carboplatin for intraocular retinoblastoma. Ophthalmology 1999;106(10):1947-1950.
16 Marr BP, Dunkel IJ, Linker A, Abramson DH. Periocular carboplatin for retinoblastoma: long-term report (12 years) on efficacy and toxicity.Br J Ophthalmol 2012;96(6):881-883.
17 Leng T, Cebulla CM, Schefler AC, Murray TG. Focal periocular carboplatin chemotherapy avoids systemic chemotherapy for unilateral,progressive retinoblastoma. Retina 2010;30(4 Suppl):S66-S68.
18 Friedrich MJ. Retinoblastoma therapy delivers power of chemotherapy with surgical precision. JAMA 2011;305(22):2276-2278.
19 Linn Murphree A. Intraocular retinoblastoma: the case for a new group classification. Ophthalmol Clin North Am 2005;18(1):41-53.
20 Francis JH, Schaiquevich P, Buitrago E, Del Sole MJ, Zapata G,Croxatto JO, Marr BP, Brodie SE, Berra A, Chantada GL, Abramson DH. Local and systemic toxicity of intravitreal melphalan for vitreous seeding in retinoblastoma: a preclinical and clinical study. Ophthalmology 2014;121(9):1810-1817.
21 Ericson LA, Rosengren BH. Present therapeutic resources in retinoblastoma. Acta Ophthalmol (Copenh) 1961;39:569-576.
22 Karcioglu ZA. Fine needle aspiration biopsy (FNAB) for retinoblastoma. Retina 2002;22(6):707-710.
23 Tuncer S, Balci Ö, Tanyildiz B, Kebudi R, Shields CL. Intravitreal lower-dose (20 μg) melphalan for persistent or recurrent retinoblastoma vitreous seeds. Ophthalmic Surg Lasers Imaging Retina 2015;46(9):942-948.
24 Munier FL, Gaillard MC, Balmer A, Beck-Popovic M. Intravitreal chemotherapy for vitreous seeding in retinoblastoma: Recent advances and perspectives. Saudi J Ophthalmol 2013;27(3):147-150.
25 Francis JH, Abramson DH, Gaillard MC, Marr BP, Beck-Popovic M,Munier FL. The classification of vitreous seeds in retinoblastoma and response to intravitreal melphalan. Ophthalmology 2015;122(6):1173-1179.
26 Shields CL, Shields JA. Basic understanding of current classification and management of retinoblastoma. Curr Opin Ophthalmol 2006;17(3): 228-234.27 Shields CL, Santos MC, Diniz W, Gündüz K, Mercado G, Cater JR,Shields JA. Thermotherapy for retinoblastoma. Arch Ophthalmol 1999;117(7):885-893.
28 Shields JA, Shields CL. Intraocular tumors- text and Atlas. Philadelphia,PA, USA: WB Saunders Company; 1992.
29 Harnett AN, Hungerford J, Lambert G, Hirst A, Darlinson R, Hart B,Trodd TC, Plowman PN. Modern lateral external beam (lens sparing)radiotherapy for retinoblastoma. Ophthalmic Paediatr Genet 1987;8(1):53-61.
30 Chung CY, Medina CA, Aziz HA, Singh AD. Retinoblastoma:evidence for stage-based chemotherapy. Int Ophthalmol Clin 2015;55(1):63-75.
31 Shin JY, Kim JH, Yu YS, Khwarg SI, Choung HK, Shin HY, Ahn HS. Eye-preserving therapy in retinoblastoma: prolonged primary chemotherapy alone or combined with local therapy. Korean J Ophthalmol 2010;24(4):219-224.
32 Bartuma K, Pal N, Kosek S, Holm S, All-Ericsson C. A 10 - year experience of outcome in chemotherapy treated hereditary retinoblastoma.Acta Ophthalmol 2014;92(5):404-411.
33 Shields CL, Mashayekhi A, Au AK, Czyz C, Leahey A, Meadows AT,Shields JA. The international classification of retinoblastoma predicts chemoreduction success. Ophthalmology 2006;113(12):2276-2280.
34 Künkele A, Jurklies C, Wieland R, Lohmann D, Bornfeld N, Eggert A, Schulte JH. Chemoreduction improves eye retention in patients with retinoblastoma: a report from the German retinoblastoma reference centre.Br J Ophthalmol 2013;97(10):1277-1283.
35 Cohen VM, Kingston J, Hungerford JL. The success of primary chemotherapy for group D heritable retinoblastoma. Br J Ophthalmol 2009;93(7):887-890.
36 Kingston JE, Hungerford JL, Madreperla SA, Plowman PN. Results of combined chemotherapy and radiotherapy for advanced intraocular retinoblastoma. Arch Ophthalmol 1996;114(11):1339-1343.
37 Shields CL, Shields JA, Needle M, de Potter P, Kheterpral S, Hamada A, Meadows AT. Combined chemoreduction and adjuvant treatment for intraocular retinoblastoma. Ophthalmology 1997;104(12):2101-2111.
38 Abramson DH , Frank CM. Second nonocular tumors in survivors of bilateral retinoblastoma: a possible age effect on radiation-related risk.Ophthalmology 1998;105(4):573-579.
39 Kaneko A, Suzuki S. Eye-preservation treatment of retinoblastoma with vitreous seeding. Jpn J Clin Oncol 2003;33(12):601-607.
40 Murray TG, Cicciarelli N, O’Brien JM, Hernández E, Mueller RL,Smith BJ, Feuer W. Subconjunctival carboplatin therapy and cryotherapy in the treatment of transgenic murine retinoblastoma. Arch Ophthalmol 1997;115(10):1286-1290.
41 Hayden BH, Murray TG, Scott IU, Cicciarelli N, Hernandez E, Feuer W, Fulton L, O’Brien JM. Subconjunctival carboplatin in retinoblastoma:impact of tumor burden and dose schedule. Arch Ophthalmol 2000;118(11):1549-1554.
42 Mendelsohn ME, Abramson DH, Madden T, Tong W, Tran HT, Dunkel IJ. Intraocular concentrations of chemotherapy following systemic or local administration. Arch Ophthalmol 1998;116(9):1209-1212.
43 Hayden BC, Jockovich ME, Murray TG, Voigt M, Milne P, Kralinger M, Feuer WJ, Hernandez E, Parel JM. Pharmacokinetics of systemic versus focal carboplatin chemotherapy in the rabbit eye: possible implication in the treatment of retinoblastoma. Invest Ophthalmol Vis Sci 2004;45(10):3644-3649.
44 Duffull SB, Robinson BA. Clinical pharmacokinetics and dose optimisation of carboplatin. Clin Pharmacokinet 1997;33(3):161-183.
45 Kalita D, Shome D, Jain VG, Chadha K, Bellare JR. In vivo intraocular distribution and safety of periocular nanoparticle carboplatin for treatment of advanced retinoblastoma in humans. Am J Ophthalmol 2014;157(5):1109-1115.
46 Brodie SE, Munier FL, Francis JH, Marr B, Gobin YP, Abramson DH.Persistence of retinal function after intravitreal melphalan injection for retinoblastoma. Doc Ophthalmol 2013;126(1):79-84.
47 Ghassemi F, Shields CL. Intravitreal melphalan for refractory or recurrent vitreous seeding from retinoblastoma. Arch Ophthalmol 2012;130(10):1268-1271.
48 Shields CL, Manjandavida FP, Arepalli S, Kaliki S, Lally SE, Shields JA. Intravitreal melphalan for persistent or recurrent retinoblastoma vitreous seeds: Preliminary results. JAMA Ophthalmol 2014;132(3):319-325.
49 Seregard S, Singh AD. Retinoblastoma: direct chemotherapeutic drug delivery into the vitreous cavity. Br J Ophthalmol 2012;96(4):473-474.
50 Lee JH, Han JW, Hahn SM, Lyu CJ, Kim DJ, Lee SC. Combined intravitreal melphalan and intravenous/intra-arterial chemotherapy for retinoblastoma with vitreous seeds. Graefes Arch Clin Exp Ophthalmol 2016;254(2):391-394.
51 Ji X, Hua P, Li J, Li J, Zhao J, Zhao P. Intravitreal melphalan for vitreous seeds: initial experience in China. J Ophthalmol 2016;2016:4387286.
52 Smith SJ, Smith BD. Evaluating the risk of extraocular tumour spread following intravitreal injection therapy for retinoblastoma: a systematic review. Br J Ophthalmol 2013;97(10):1231-1236.
53 Smith SJ, Smith BD, Mohney BG. Ocular side effects following intravitreal injection therapy for retinoblastoma: a systematic review. Br J Ophthalmol 2014;98(3):292-297.
54 Mulvihill A, Budning A, Jay V, Vandenhoven C, Heon E, Gallie BL, Chan HS. Ocular motility changes after subtenon carboplatin chemotherapy for retinoblastoma. Arch Ophthalmol 2003;121(8):1120-1124.
55 Kim JW, Yau JW, Moshfeghi D, Fishman M. Orbital fibrosis and intraocular recurrence of retinoblastoma following periocular carboplatin.J Pediatr Ophthalmol Strabismus 2010;47:e1-e4.
56 Van Quill KR, Dioguardi PK, Tong CT, Gilbert JA, Aaberg TM Jr, Grossniklaus HE, Edelhauser HF, O'Brien JM. Subconjunctival carboplatin in fibrin sealant in the treatment of transgenic murine retinoblastoma. Ophthalmology 2005;112(6):1151-1158.