INTRODUCTION
Femtosecond laser-assisted laser in situ keratomileusis (FSLASIK), which involves flap cutting and stromal ablation using femtosecond laser and excimer laser respectively, has been widely applied in myopia correction[1-2]. The wavefrontguided ablation profiles with the latest excimer laser platforms provide a guarantee for the excellent visual, refractive, and aberrometric outcomes of FS-LASIK[3-6]. However, FS-LASIK for correcting high myopia and astigmatism is challenging owing to the associated risk of treatment regression, corneal biomechanics changes, and flap complications[7]. The recently introduced small-incision lenticule extraction (SMILE) is an all-in-one procedure, in which a corneal intrastromal lenticule is fashioned using femtosecond laser and extracted manually through a small peripheral corneal incision[8-9].The size and shape of the extracted lenticule are based on the patient’s refractive error[10]. The new technique, which no longer requires a flap, might reduce some flap-related side effects of FS-LASIK, such as flap dislocation and flap-induced astigmatism[9,11]. Although there is consistent scientific evidence supporting of the efficacy and safety of SMILE for the correction of myopia and astigmatism[8,12], the superiority of SMILE over wavefront-guided FS-LASIK in correcting high myopia and astigmatism have not been demonstrated conclusively. Especially, long-term comparative studies of SMILE versus FS-LASIK in high myopia and myopic astigmatism eyes are far from being established. This may be essential to relieve patients’ concerns about clinical risk following SMILE surgery. The objective of the study was to compare the long-term (3-year) correction of high myopia and myopic astigmatism between SMILE and FS-LASIK in terms of visual acuity, refractive results, aberrations, contrast sensitivity (CS) and dry eye.
SUBJECTS AND METHODS
Participants The study was approved by the Ethics Committee of Shengjing Hospital and comply with the tenets of the Declaration of Helsinki regarding research involving human subjects. This study involved 78 eyes of 40 patients who underwent SMILE and 65 eyes of 33 patients who underwent wavefront-guided FS-LASIK during January 1, 2013 to July 31,2013 at the Refractive Surgery Center of Shengjing Hospital,China Medical University, China. All patients participated in complete follow-up examinations for 3y after surgery.
All patients received a comprehensive preoperative examination for corneal refractive surgery. Patients with normal corneal topography (based on evaluation of Pentacam HR tomagraphs),central corneal thickness ≥500 µm, and calculated residual corneal stromal bed thickness ≥280 µm were informed about the outcome of laser refractive procedures. All patients were informed of full descriptions of the SMILE and wavefrontguided FS-LASIK procedures, including the potential advantages, disadvantages, side effects and complications. The patients decided whichever to choose between the two surgical procedures, and signed the informed consent before treatment.The main inclusion criteria were as below: spherical myopia ranging from -6.0 to -10.0 diopters (D); with or without regular astigmatism up to -3.5 D; corrected distance visual acuity (CDVA) of 20/20 or better; stable refraction in the past 12mo (<0.50 D change of sphere or cylinder); not wearing soft lenses at least 14d before the preoperative evaluation; age between 18 and 40y; with the ability to participate in follow-up examinations for 3y after surgery.
FS-LASIK and SMILE Surgical Procedures A single experienced surgeon performed all surgeries under topical anaesthesia using a standard surgical technique. For FS-LASIK group, superior-hinge flaps were created using a 500 kHz VisuMax femtosecond laser (Carl Zeiss Meditec AG, Germany)with 175 nJ of energy. Femtosecond laser flaps were programmed with 110 µm thickness and 7.9 µm diameter,and 90° side cut angles. Following the flap creation, the spherocylindrical refractive corrections were done with the Carl Zeiss Meditec MEL 80TMexcimer laser system [software version: 3.6; Tissue Saving Ablation (TSA) profiles; standard nomogram]. Wavefront treatment data were measured by WASCA Analyzer (Carl Zeiss, Meditec AG, Germany)according to the Hartmann-Shack technique with 6 mm of pupil size under mesopic conditions and were transferred to MEL 80TMexcimer laser system. The iris registration and pupiltracking system were automatically initiated before ablation.The optical zone was set at 6.0 mm with blend zone at 8 mm.The SMILE surgeries were performed by a Visumax femtosecond laser system (Carl Zeiss, Meditec AG, Germany),with a repetition rate of 500 kHz and pulse energy of 175 nJ following the surgical procedure. A small curved interface cone was used in all cases. The alignment of the refractive lenticule was effectively centered by the fact that the patientfixates coaxially on afixation light when suction was applied,resulting in lenticule formation centered on the corneal vertex of the coaxiallyfixating eye. The femtosecond laser parameters were as follows: cap thickness, 110 μm; cap diameter, 7.6 mm;lenticule diameter, 6.5 mm (with 0.1 mm of transition zone for the correction of astigmatism); side cut angle, 90°; both spot distance and track distance, 4.5 μm for each surface of the lenticule under fast mode; the posterior surface was scanned with the spiral in mode followed by the scanning of the anterior surface with the mode of spiral out. At the end, a 30° incision of approximately 2 mm in length was created at the 120° position for lenticule extraction. Following the cutting procedure, the refractive lenticule was dissected and separated through the side cut and manually removed.
Postoperative Medication and Follow-up As a routine, all patients received the management of an opththalmic solution of Levofloxacin drops 4 times per day for 1wk, a 0.1%fluorometholone drops solution 4 to 1 times per day with a drop decrease per week for 1mo, and an artificial tear drops(Sodium Hyaluronate Eye Drops, Santen, Inc., Japan) 4 times per day for 3mo.
Postoperative time points included 1, 3, 6mo and 1, 2, 3y after surgery. The uncorrected distance visual acuity (UDVA),CDVA, refraction diopters, higher-order aberrations (HOAs,WASCA wavefront analyzer; Carl Zeiss Meditec AG, Jena,Germany), CS (Opetec 6500, Stero Optical) curves, dye eye parameters were gathered and analyzed. The visual acuity was measured using ETDRS chart. The dry eye parameters include tear breakup time (TBUT), and ocular surface disease index(OSDI). The OSDI questionnaire, a 12-question survey, was used to quantify the dry eye symptoms. Each question is given a rank between 0-4. OSDI score=sum of score 625/number of questions answered, ranging from 0-100 score, with lower OSDI scores indicating better results[13].
Table 1 Preoperative demographic data of patients in SMILE and FS-LASIK groups

Data were given as mean±standard deviation with range in parenthesis. SMILE: Small-incision lenticule extraction; FS-LASIK: Femtosecond laser-assisted laser in situ keratomileusis; D: Diopters; SE: Spherical equivalent; CCT: Central corneal thickness; CDVA: Corrected distance visual acuity; logMAR: Logarithm of the minimum angle of resolution.
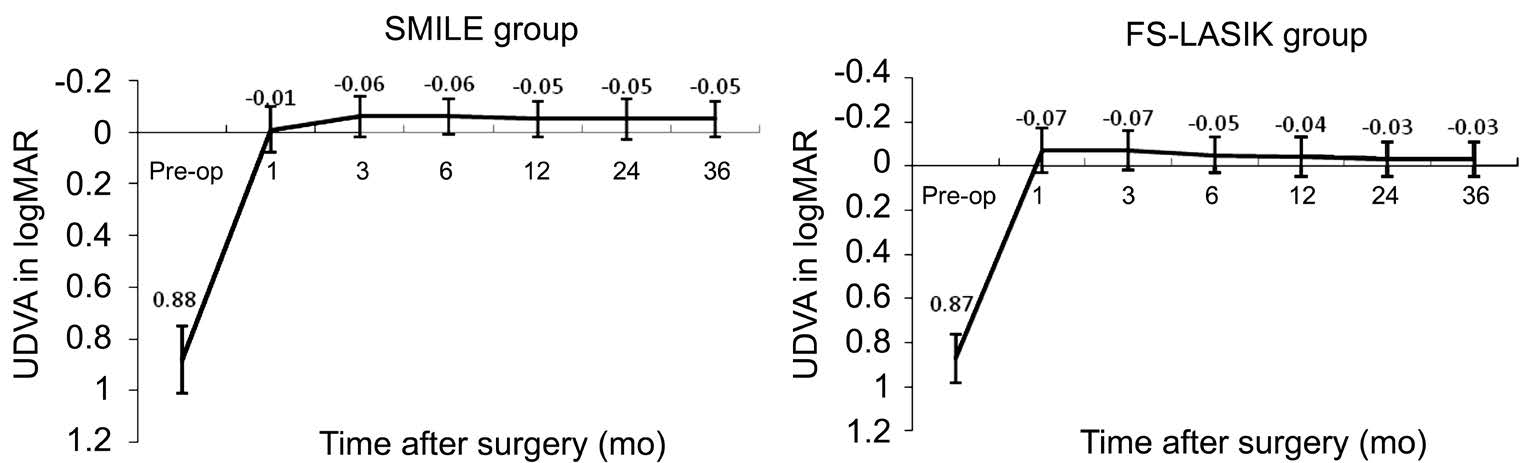
Figure 1 Changes of mean UDVA in logMAR after SMILE and FS-LASIK UDVA: Uncorrected distance visual acuity; SMILE: Smallincision lenticule extraction; FS-LASIK: Femtosecond laser-assisted LASIK.
Statistical Analysis Statistical analysis was performed using SPSS 20.0 statistical analysis software (USA) and reported as mean±standard deviation (SD). Visual acuity outcomes were calculated in logMAR notation. We analyze the multiple difference by using the variance analysis of repeated measurement data. Comparison of continuous variable between the two groups were examined by independent samples t-test, and a Chi-square test was used for statistical analysis of categorical variable at the baseline. Furthermore,we used paired t-test to analyze the difference of spherical equivalent (SE) between 1-month and 3-year postoperatively in both group. Statistical significance level was set at 0.05.
RESULTS
Patient Population The preoperative demographic data of both groups are shown in Table 1. There were no statistically significant differences in terms of the mean age, spherical error,astigmatism, SE, CDVA, central corneal thickness between the two groups. All surgeries were successfully completed without any intraoperative complications. None of the subjects dropped out during the 3-year follow-up period.
Visual Acuity Figure 1 shows the changes of UDVA at 3-year follow-up visits for two groups. There were significant differences between the two groups postoperatively (variance analysis of repeated measurement data; F=42.512, P=0.00).In the SMILE group, the best postoperative UDVA (-0.06 in logMAR) was achieved at 3mo after surgery, and remained stable to -0.05 logMAR at 3y postoperatively. In the FS-LASIK group, the best postoperative UDVA (-0.07 in logMAR) was achieved at 1mo after surgery, but decreased gradually to-0.03 logMAR at 3y postoperatively. However, there were no statistically significant difference of the mean UDVA between two groups at 3-year postoperatively (independent samples Student’s t-test; t=-1.59, P=0.13). Figure 2 shows cumulative Snellen postoperative UDVA at 3-year in two groups. Seventytwo (92.3%) of 78 eyes in the SMILE group had an UDVA better than or equal to 20/20, and 78 eyes (100%) had an UDVA better than or equal to 20/25. In the FS-LASIK group,59 (90.8%) of 65 eyes had an UDVA better than or equal to 20/20, 65 eyes (100%) had an UDVA better than or equal to 20/25. The changes of CDVA pre- to post-surgery for two groups were shown in Figure 3. At 3-year postoperatively, of the 78 eyes treated with SMILE, 7 eyes (9.0%) gained one line of CDVA, 1 eye (1.3%) gained two line of CDVA, and 89.7% (70 eyes) were unchanged postoperatively. Of the 65 eyes treated with FS-LASIK, 4 eyes (6.2%) gained one line of CDVA, 2 eyes (3.1%) gained two lines of CDVA, and 59 eyes (90.8%) were unchanged postoperatively. None of them lost ≥1 lines of CDVA in both groups. The results indicate that both the SMILE and the FS-LASIK were effective and safe to correct high myopia and myopic astigmatism.

Figure 2 Cumulative Snellen postoperative uncorrected distance visual acuity at 3-year after SMILE and FS-LASIK SMILE: Smallincision lenticule extraction; FS-LASIK: Femtosecond laser-assisted LASIK.

Figure 3 Distribution of the changes in Snellen lines of corrected distance visual acuity at 3-year after SMILE and FS-LASIK SMILE: Small-incision lenticule extraction; FS-LASIK: Femtosecond laser-assisted LASIK.
Refractive Outcome Mean preoperative SE were -8.11±1.09 and -8.05±1.12 D in SMILE group and FS-LASIK group,respectively. The difference of the mean preoperative SE between two groups were not statistically significant(t=-0.362, P=0.72). Considering the changes of SE from 1 to 36mo after surgery, we found significant differences between the two groups (variance analysis of repeated measurement data; F=82.618, P=0.00). The mean SE in the SMILE group changed from +0.13±0.79 D to -0.01±0.76 D, and the FSLASIK group from +0.46±0.95 D to -0.43±0.82 D. The mean change was 0.14 D in the SMILE treated eyes (paired t-test;t=0.546, P=0.59), and 0.89 D in the FS-LASIK treated eyes(paired t-test; t=5.765, P=0.00). The results indicated that the postoperative refractive outcome in SMILE group was more stable than FS-LASIK group over the 3-year follow-up period(Figure 4).
Higher-order Aberrations Data was analyzed under a 6.0-mm pupil diameter. Preoperative root mean square (RMS) value of HOAs, spherical aberrations, and coma were not significantly different between the SMILE and FS-LASIK groups (P>0.05;Table 2). At 3-year postoperatively, the mean HOAs RMS and spherical aberrations were 0.33±0.10 µm, 0.26±0.31 µm in the SMILE group, and 0.42±0.13 µm, 0.35±0.19 µm in the FS-LASIK group, respectively, for a 6.0 mm pupil. Both thepostoperative HOAs and spherical aberrations in the SMILE treated eyes were apparently less than those in the FS-LASIK treated eyes (P=0.00). However, the postoperative mean coma RMS was higher in the SMILE treated eyes (0.59±0.26 μm)than in the FS-LASIK treated eyes (0.29±0.14 μm, P=0.00).
Table 2 The comparisons of the RMS values of different higherorder aberrations for a 6 mm pupil between the SMILE and FSLASIK group
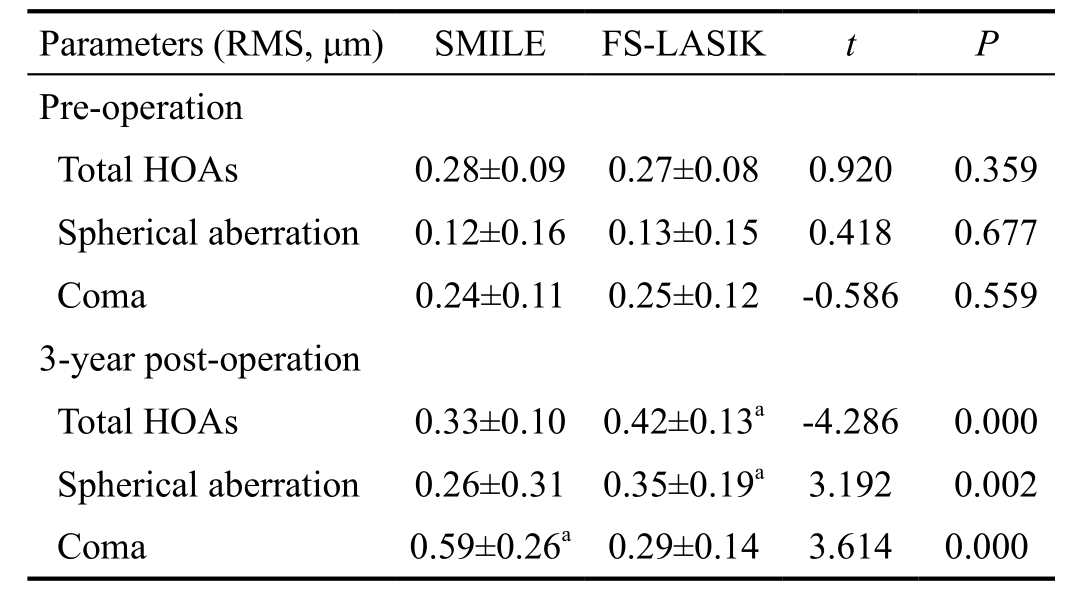
SMILE: Small-incision lenticule extraction; FS-LASIK: Femtosecond laser-assisted LASIK; RMS: Root mean square; HOAs: Higher-order aberrations.aSignificant differences between the SMILE and FSLASIK group.
Contrast Sensitivity Function At night environment (under a 5 mm pupil size condition), the CS values between two groups at 1.5, 3, 6, 12, and 18 cycles per degree were not statistically significant (P>0.05; Figure 5). At 3y postoperatively, there also were no statistically significant differences found in SMILE and FS-LASIK surgeries at all spatial frequencies (P>0.05;Figure 5).
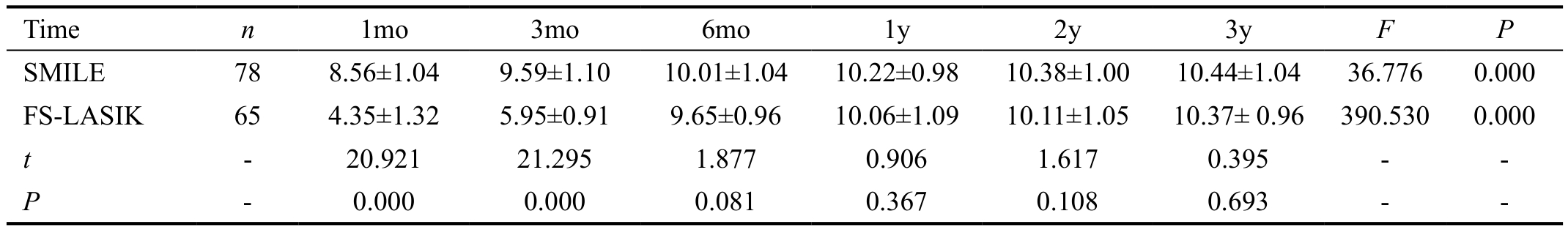
Ocular Surface Disease Index In terms of OSDI, we found significant difference at different time points between the two groups postoperatively (variance analysis of repeated measurement data; F=120.318, P=0.00). Table 3 showed postoperative OSDI scores at each time point for two groups.Figure 6 summarizes mean preoperative and postoperative OSDI scores at each time point for two groups. Mean preoperative OSDI scores were 6.73±3.25 in the SMILE group and 6.68±3.11 in the FS-LASIK group, and there was no significant difference between the two groups (t=0.432,P=0.61). The mean OSDI scores in the FS-LASIK group were increased significantly at 1mo (12.63±4.51) and 3mo(9.32±4.18) postoperatively relative to preoperative scores(t=3.081, P=0.00). However, the postoperative significant increases of OSDI scores in the SMILE group were only found at 1mo postoperatively (9.08±3.88, P=0.00). In addition,mean OSDI scores were significantly worse in the FS-LASIK treated eyes than in the SMILE treated eyes at 1mo and 3mo postoperatively (independent samples t-test; t=-11.831, -8.389,P=0.00, 0.00). There were no significant difference in the mean OSDI scores between the two groups at other follow-up time (P>0.05).
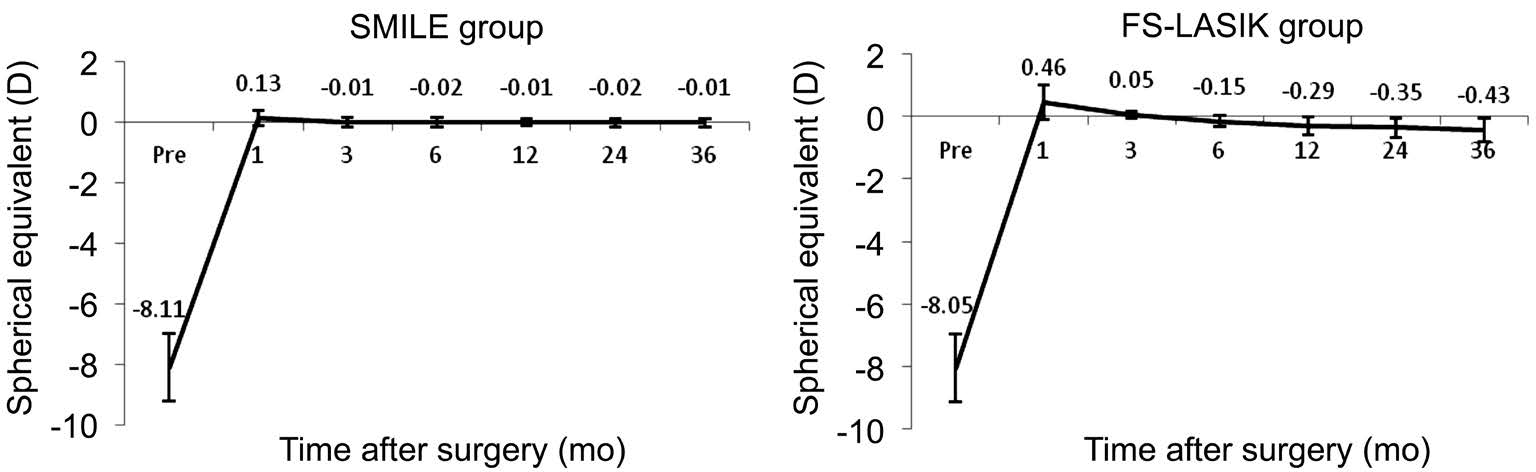
Figure 4 Changes of spherical equivalent from 1mo to 3y after SMILE and FS-LASIK SMILE: Small-incision lenticule extraction; FSLASIK: Femtosecond laser-assisted LASIK.

Figure 5 Comparison of contrast sensitivity between SMILE and FS-LASIK SMILE: Small-incision lenticule extraction; FS-LASIK:Femtosecond laser-assisted LASIK.
Table 3 The comparisons of OSDI between the SMILE and FS-LASIK group postoperatively
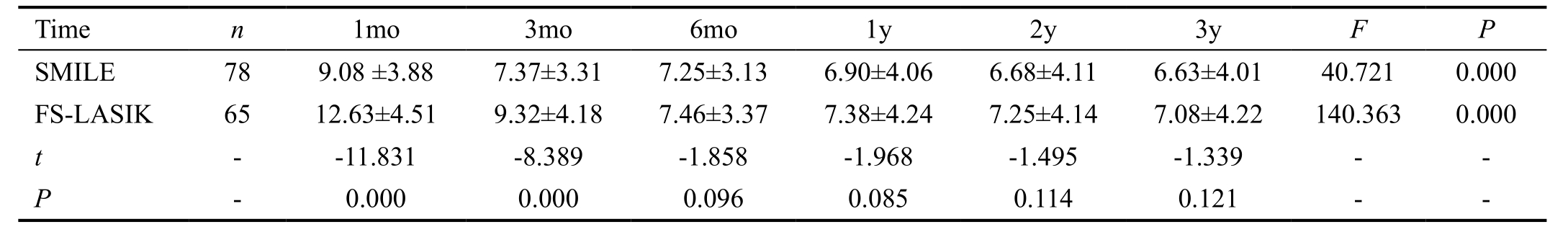
OSDI: Ocular surface disease index; SMILE: Small-incision lenticule extraction; FS-LASIK: Femtosecond laser-assisted LASIK.
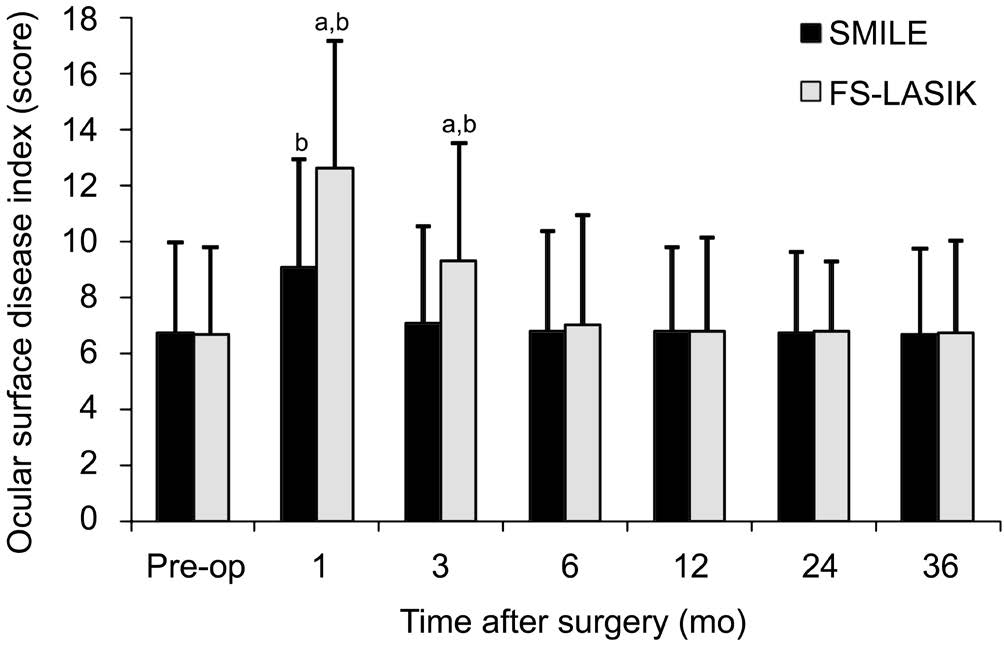
Figure 6 Comparison of ocular surface disease index between the SMILE group and FS-LASIK group pre- and post-operatively SMILE: Small-incision lenticule extraction; FS-LASIK: Femtosecond laser-assisted LASIK.aSignificant differences between the SMILE group and FS-LASIK group;bSignificant differences compared with preoperative level.
Tear Break-up Time The mean TBUT in both groups were shorter significantly after surgery relative to their preoperative level, and we found significant difference at different time points between the two groups postoperatively (variance analysis of repeated measurement data; F=256.530, P=0.000).However, the mean TBUT returned to the preoperative TBUT values at the third month postoperatively in the SMILE treated eyes, whereas at the sixth month postoperatively in the FSLASIK treated eyes. The result was shown in Table 4 below.We found significant differences between the two groups postoperatively. That is to say, higher TBUT scores were found in the SMILE group than in the FS-LASIK group at postoperative 1mo and 3mo (P=0.00; Figure 7).
DISCUSSION
Femtosecond laser technology to create corneal flaps enables a large increase in the flap safety of LASIK[1-2]. Wavefrontguided FS-LASIK becomes the most common corneal refractive surgery because of its excellent refractive outcomes[6]. SMILE has gained widespread acceptance in corneal refractive surgery because it is flapless. In the current research, we not only compared the visual acuity, refractive results, but also analyzed HOAs, CS, and the dry eye parameters at 3-year after SMILE and FS-LASIK for the correction of high myopia and myopic astigmatism.
Table 4 The comparisons of TBUT between the SMILE and FS-LASIK group postoperatively
SMILE: Small-incision lenticule extraction; FS-LASIK: Femtosecond laser-assisted LASIK.

Figure 7 Comparison of tear break-up time between the SMILE and FS-LASIK group pre- and post-operatively SMILE: Smallincision lenticule extraction; FS-LASIK: Femtosecond laser-assisted LASIK.aSignificant difference between the SMILE group and FS-LASIK group;bSignificant differences compared with preoperative level.
In this study, the best postoperative UDVA were achieved at 1mo after FS-LASIK, and at 3mo after SMILE. The reason for the delayed visual recovery after SMILE was probably due to the corneal edema and healing response after extracted the lenticule, which is different from the FS-LASIK procedure[8,14-15]. However, the values of UDVA at 3-year post-surgery between two groups were not statistically significant. At 3-year postoperatively, UDVA of 20/20 or better was achieved in 92.3% after SMILE and 90.8% after FSLASIK, which is lower than thefindings by Liu et al[15]. Liu et al[15]reported that 96% of treated eyes in SMILE group and 99% treated eyes in FS-LASIK group, respectively, achieved 20/20 or better UDVA at 6-month postoperatively. Possible reasons for the difference of UDVA between our report and that of Liu et al[15]could be a relatively lower preoperative mean SE (-5.22±1.70 D in SMILE group and -5.18±1.93 D in FS-LASIK group) in their patients as compared to our study population. Another reason may be their relatively short-term follow-up. When Han et al[16]investigated the predictability and stability of SMILE, they found that UDVA was better than or equal to 20/20 in 92% of eyes at 4y after SMILE,which is similar to the findings of our study (92.3% of eyes at 3y after SMILE). With regard to safety, none of them lost any line of CDVA in both groups at 3y after surgery, which is consistent with the studies by others[15,17]. The results showed that both SMILE and FS-LASIK demonstrating the similar effectiveness and security in correcting high myopia and myopic astigmatism.
A genera concern of refractive surgery is always the longterm stability, especially in high myopic eyes. In LASIK, a mean regression of 0.63-0.97 D was reported after 6-7y[18-19]. The myopic regression may be due to multiple factors, such as high corrections, small optical area, weak corneal biomechanics,low preoperative pachymetry and low residual stromal bed,and young patients[20]. Considering refractive regression after LASIK, we usually add additional magnitude to the SE for high myopic eyes. In this study, we added -0.75 D and -0.25 D to preoperative SE for FS-LASIK group and SMILE group,respectively, and observed the postoperative changes of SE from 1mo to 3y after FS-LASIK and SMILE techniques. We found that mean SE at 1mo was +0.46 D and at 3y decreased to -0.43 D after FS-LASIK. This corresponds to 0.89 D of regression within the 3-year period in FS-LASIK group.In contrast, no significant changes of SE occurred between postoperative follow-ups in SMILE group, though mean SE was decreased from +0.13 D at 1mo to -0.01 D at 3y after SMILE. No significant changes of SE after SMILE were also reported by Han et al[16]. Their results demonstrate 0.17 D decrease of mean SE after SMILE with 4y of follow up. However,Pedersen et al[21]assessed 3-year refractive and visual outcomes after SMILE in patients with high myopia and found a significant regression of 0.36 D in total corneal refractive power but not in subjective refraction. Blum et al[12]found the regression was -0.48 D over a 5-year follow-up after SMILE surgery, and they suggest this might be because the increase of the axial length leads to the growth of the eyeball rather than the true regression of cornea levels. Our results indicate the favorable stability of SMILE over FS-LASIK for correction of high myopia and astigmatism with 3y of follow-up. Hence,further investigations on regression following SMILE surgery are required[12].
It is well known that HOAs and especial spherical aberrations after LASIK are increased[22-23], with some increases in aberrations being produced by flap creation alone[24-25]. The increase of aberrations is the main factor affecting the visual quality after surgery[26]. In the current study, we used the Hartmann-Shack WASCA aberrometer to measures the wholeeye wavefront aberrations and compared HOAs after SMILE and FS-LASIK at the analysis diameter of 6 mm. We found that the postoperative HOAs and spherical aberrations in the SMILE treated eyes were markedly less than those in the FSLASIK treated eyes, but the postoperative mean coma RMS was higher in the SMILE treated eyes than in the FS-LASIK treated eyes at 3-year postoperatively. Several comparative studies of SMILE versus FS-LASIK by others[11,15,17,27]also found that SMILE eyes had more coma postoperatively and FS-LASIK eyes had more spherical aberration postoperatively.The higher level of coma after SMILE was thought to be associated with the presence of mild levels of treatment decentration[11,15-16]. The lower induction of spherical aberration after SMILE was thought to be related to the larger ablation zone and less changes in the corneal shape of the SMILE procedure[14,28-29].
In our opinion, although HOAs reflect the objective quality of vision, CS re flects the subjective quality of vision from the patient’s perspective, which is a crucial parameter for patients’satisfaction. In this study, although there were statistically significant differences in the characteristics of HOA induction between the SMILE and FS-LASIK groups, no significant differences in mesopic CS values for all spatial frequencies were found between two groups at 3y postoperatively. In another comparative analysis of CS after SMILE and FSLASIK, both procedures yielded no statistically significant differences from baseline to 6mo after surgery[30].
As we know, one of the most feared and common complications of traditional refractive surgeries is dry eye[31-32]. Dry eye is not only a simple disease causing patients feel uncomfortable about the deterioration of quality of life, but also impairs visual function, CS and ocular HOAs[33-34]. SMILE uses the femtosecond laser system as an all-in-one device for lenticule processing and replacement of small incised corneal flap.Therefore, the ocular surface of eyes treated with SMILE were healthier than those with FS-LASIK surgery. Li et al[13]found that patients in the SMILE group had less corneal staining and greater central corneal sensitivity scores than patients in the FS-LASIK group. Consistent with their studies, our study demonstrated significantly lower OSDI scores and longer TBUT values in the SMILE group compared with the FSLASIK group at 1mo and 3mo postoperatively. Our data also showed a faster recovery of ocular surface injury in SMILE group than in FS-LASIK group, which should be mainly attributed to the slight of corneal nerve damage and a more regular corneal surface during the new flapless technique of SMILE[13,35].
In conclusion, both SMILE and wavefront-guided FSLASIK provide good visual outcomes. Both SMILE and FSLASIK demonstrated the similar effectiveness and security in correcting high myopia and myopic astigmatism. SMILE surgery achieved more stable refractive outcome and better control of early postoperative dry eye as compared to wavefrontguided FS-LASIK.
ACKNOWLEDGEMENTS
conflicts of Interest:Xia LK, None; Ma J, None; Liu HN,None; Shi C, None; Huang Q, None.
REFERENCES
1 Farjo AA, Sugar A, Schallhorn SC, Majmudar PA, Tanzer DJ,Trattler WB, Cason JB, Donaldson KE, Kymionis GD. Femtosecond lasers for LASIK flap creation: a report by the American Academy of Ophthalmology. Ophthalmology 2013;120(3):e5-e20.
2 Kymionis GD, Kankariya VP, Plaka AD, Reinstein DZ. Femtosecond laser technology in corneal refractive surgery: a review. J Refract Surg 2012;28(12):912-920.
3 Smadja D, Santhiago MR, Tellouck J, De Castro T, Lecomte F, Mello GR, Touboul D. Safety and efficacy of wavefront-guided myopic laser in situ keratomileusis using a new wavefront sensor technology: first 100 cases. J Cataract Refract Surg 2015;41(8):1588-1593.
4 Schallhorn SC, Venter JA, Hannan SJ, Hettinger KA. Clinical outcomes of wavefront-guided laser in situ keratomileusis to treat moderate-to-high astigmatism. Clin Ophthalmol 2015;9:1291-1298.
5 Shaheen MS, Massoud TH, Ezzeldin H, Khalifa MA. Four-year visual,refractive, and contrast sensitivity outcomes after wavefront-guided myopic LASIK using an advanced excimer laser platform. J Refract Surg 2013;29(12):816-822.
6 Prakash G, Srivastava D, Suhail M. Femtosecond laser-assisted wavefront-guided lasik using a newer generation aberrometer: 1-year results. J Refract Surg 2015;31(9):600-606.
7 dos Santos AM, Torricelli AA, Marino GK, Garcia R, Netto MV,Bechara SJ, Wilson SE. Femtosecond laser-assisted LASIK flap complications. J Refract Surg 2016;32(1):52-59.
8 Sekundo W, Kunert KS, Blum M. Small incision corneal refractive surgery using the small incision lenticule extraction (SMILE) procedure for the correction of myopia and myopic astigmatism: results of a 6 month prospective study. Br J Ophthalmol 2011;95(3):335-339.
9 Ivarsen A, Asp S, Hjortdal J. Safety and complications of more than 1500 small-incision lenticule extraction procedures. Ophthalmology 2014;121(4):822-828.
10 Vestergaard A, Ivarsen AR, Asp S, Hjortdal JØ. Small-incision lenticule extraction for moderate to high myopia: predictability,safety, and patient satisfaction. J Cataract Refract Surg 2012;38(11):2003-2010.
11 Piñero DP, Teus MA. Clinical outcomes of small-incision lenticule extraction and femtosecond laser-assisted wavefront-guided laser in situ keratomileusis. J Cataract Refract Surg 2016;42(7):1078-1093.
12 Blum M, Täubig K, Gruhn C, Sekundo W, Kunert KS. Five-year results of small incision lenticule extraction (ReLEx SMILE). Br J Ophthalmol 2016;100(9):1192-1195.
13 Li M, Zhao J, Shen Y, Li T, He L, Xu H, Yu Y, Zhou X. Comparison of dry eye and corneal sensitivity between small incision lenticule extractionand femtosecond LASIK for myopia. PLoS One 2013;29;8(10):e77797.
14 Shah R, Shah S, Sengupta S. Results of small incision lenticule extraction: all-in-one femtosecond laser refractive surgery. J Cataract Refract Surg 2011;37(1):127-137.
15 Liu M, Chen Y, Wang D, Zhou Y, Zhang X, He J, Zhang T, Sun Y,Liu Q. Clinical outcomes after SMILE and femtosecond laser-assisted lasik for myopia and myopic astigmatism: a prospective randomized comparative study. Cornea 2016;35(2):210-216.
16 Han T, Zheng K, Chen Y, Gao Y, He L, Zhou X. Four-year observation of predictability and stability of small incision lenticule extraction. BMC Ophthalmol 2016;16(1):149.
17 Ganesh S, Gupta R. Comparison of visual and refractive outcomes following femtosecond laser-assisted LASIK with SMILE in patients with myopia or myopic astigmatism. J Refract Surg 2014;30(9):590-596.
18 Zalentein WN, Tervo TM, Holopainen JM. Seven-year follow-up of LASIK for myopia. J Refract Surg 2009;25(3):312-318.
19 Sekundo W, Bönicke K, Mattausch P, Wiegand W. Six-year follow-up of laser in situ keratomileusis for moderate and extreme myopia using afirst-generation excimer laser and microkeratome. J Cataract Refract Surg 2003;29(6):1152-1158.
20 Alió JL, Soria F, Abbouda A, Peña-García P. Laser in situ keratomileusis for -6.00 to -18.00 diopters of myopia and up to -5.00 diopters of astigmatism: 15-year follow-up. J Cataract Refract Surg 2015;41(1):33-40.
21 Pedersen IB, Ivarsen A, Hjortdal J. Three-year results of small incision lenticule extraction for high myopia: refractive outcomes and aberrations.J Refract Surg 2015;31(11):719-724.
22 Oshika T, Klyce SD, Applegate RA, Howland HC, El Danasoury MA. Comparison of corneal wavefront aberrations after photorefractive keratectomy and laser in situ keratomileusis. Am J Ophthalmol 1999;127(1):1-7.
23 Chen S, Feng Y, Stojanovic A, Jankov MR 2nd, Wang Q. IntraLase femtosecond laser vs mechanical microkeratomes in LASIK for myopia:a systematic review and meta-analysis. J Refract Surg 2012;28(1):15-24.
24 Porter J, MacRae S, Yoon G, Roberts C, Cox IG, Williams DR.Separate effects of the microkeratome incision and laser ablation on the eye’s wave aberration. Am J Ophthalmol 2003;136(2):327-337.
25 Pallikaris IG, Kymionis GD, Panagopoulou SI, Siganos CS, Theodorakis MA, Pallikaris AI. Induced optical aberrations following formation of a laser in situ keratomileusis flap. J Cataract Refract Surg 2002;28(10):1737-1741.
26 Oshika T. Quantitative assessment of quality of vision. Nippon Ganka Gakkai Zasshi 2004;108(12):770-807;discussion 808.
27 Agca A, Ozgurhan EB, Demirok A, Bozkurt E, Celik U, Ozkaya A,Cankaya I, Yilmaz OF. Comparison of corneal hysteresis and corneal resistance factor after small incision lenticule extraction and femtosecond laser-assisted LASIK: a prospective fellow eye study. Cont Lens Anterior Eye 2014;37(2):77-80.
28 Reinstein DZ, Archer TJ, Randleman JB. Mathematical model to compare the relative tensile strength of the cornea after PRK, LASIK, and small incision lenticule extraction. J Refract Surg 2013;29(7):454-460.
29 Zhu X, Zou L, Yu M, Qiu C, Chen M, Dai J. Comparison of postoperative visual quality after SMILE and LASEK for high myopia: a 1-year outcome. PLoS One 2017;12(8):e0182251.
30 Vestergaard AH, Grauslund J, Ivarsen AR, Hjortdal JØ. Efficacy, safety,predictability, contrast sensitivity, and aberrations after femtosecond laser lenticule extraction. J Cataract Refract Surg 2014;40(3):403-411.
31 Shtein RM. Post-LASIK dry eye. Expert Rev Ophthalmol 2011;6(5):575-582.
32 Chao C, Golebiowski B, Stapleton F. The role of corneal innervation in LASIK-induced neuropathic dry eye. Ocul Surf 2014;12(1):32-45.
33 Wang Y, Xu J, Sun X, Chu R, Zhuang H, He JC. Dynamic wavefront aberrations and visual acuity in normal and dry eyes. Clin Exp Optom 2009;92(3):267-273.
34 Montés-Micó R, Cáliz A, Alió JL. Wavefront analysis of higher order aberrations in dry eye patients. J Refract Surg 2004;20(3):243-247.
35 Li M, Niu L, Qin B, Zhou Z, Ni K, Le Q, Xiang J, Wei A, Ma W, Zhou X. Confocal comparison of corneal reinnervation after small incision lenticule extraction (SMILE) and femtosecond laser in situ keratomileusis(FS-LASIK). PLoS One 2013;8(12):e81435.