INTRODUCTION
Diabetic retinopathy (DR) is the most common microvascular complication of diabetes mellitus and the leading cause of blindness in working age adults, accounting for 8% of the legal blindness in China[1]. Despite intensive studies, the precise mechanism still remains obscure. Longer duration of diabetes[2-3]and poor glycemic control[4]are established risk factors for the development and progression of DR. Early detection and timely treatment of DR could effectively reduce the risk of severe vision loss[5].
Insulin resistance (IR) and pancreatic β-cell dysfunction are two primary determinants of glucose metabolism disorder in type 2 diabetes mellitus (T2DM)[6-7]. The consequent hyperglycemia is closely associated with microvascular complications[8]. Improved understanding about the role of IR and β-cell dysfunction in DR may provide insights for DR prevention at the early stage of T2DM. Several studies have explored the associations between IR/β-cell dysfunction and DR in diabetic patients[9-14]. To date, the relative role of impaired IR and β-cell insulin secretion in the development of DR still remains controversial. In addition, the contributions of IR and β-cell dysfunction to T2DM may be different for obese and non-obese patients as previously reported[15-16]. However,whether the role of IR and β-cell dysfunction in DR differs for obese and non-obese patients is not clear.
The present study aimed to investigate the relationship between IR/β-cell dysfunction and DR in a community-based cohort of Chinese patients with T2DM, and to further explore whether there were differences in the relationship among patients with higher and lower body mass index (BMI).
SUBJECTS AND METHODS
Patient Recruitment and Selection Criteria Patients with T2DM aged 30y or above, as identified from an age-related eye disease screening program in the Desheng Community of urban Beijing, were recruited for the cohort of Beijing Desheng Diabetic Eye Study between November 2009 and June 2012 by using posters, pamphlets, and phone calls. The details of the study have been described elsewhere[17]. Crosssectional baseline data was used for the current study.
Diabetes was defined as either a history of physician diagnosed T2DM being treated with insulin, oral hypoglycemic agents, or diet only, or by a fasting plasma glucose (FPG) concentration of 7.0 mmol/L (126 mg/dL) or more in at least two previous examinations or a random plasma glucose concentration of≥11.1 mmol/L (200 mg/dL). The duration of diabetes was defined as the interval between thefirst definite diagnosis and the time of enrollment into the study. Participants who were treated with insulin, with severe media opacity preventing the classification of retinopathy, with shallow anterior chamber or angle-closure glaucoma preventing mydriasis were excluded for the current study.
The study protocol was approved by the Ethics Committee of the Beijing Tongren Hospital (No.TRECKY2009-07) and adhered to the Helsinki Declaration of medical research. Verbal and written informed consent from the subjects was obtained prior to any investigations.
Questionnaire, Anthropometric and Laboratory Measurements A comprehensive interview using an interviewer-guided questionnaire was conducted by trained staffs collecting data related to potential risk factors for DR,including basic demographic and lifestyle information (such as age, sex, income, educational level, smoking history), and medical history (such as medication, the use of insulin, and history of systemic diseases). Persons currently smoking more than one cigarette/cigar/pipe a day for at least one year were defined as current smokers. Anthropometric parameters included body weight and height, waist circumference and hip circumference. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured in a resting state three times with 5min apart. Hypertension was diagnosed when SBP≥140 mm Hg and/or DBP≥90 mm Hg according to World Health Organization criteria[18], or history of hypertension diagnosed by a doctor, and/or reporting of anti-hypertensive treatment. Height and weight were measured with subjects in light clothing and not wearing shoes by a trained observer. The waist circumference was taken by placing a non-stretchable measuring tape horizontally on the midpoint between the lower part of the 12thrib and the top of the iliac crest, under the mid-axillary line. To the hip circumference, a similar tape was positioned to the maximum circumference around the buttocks, with the subject standing straight, keeping hands by the sides, and facing palms inward. BMI was calculated as weight divided by height squared (kg/m2). Waist-to-hip ratio(WHR) was calculated as waist circumference divided by hip circumference.
Overnight fasting blood samples were collected for measurements of FPG, glycosylated hemoglobin (HbA1c), C-reactive protein (CRP), creatinine, uric acid, total cholesterol (TC),triglycerides (TG), high-density cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) and fasting insulin (FINS). The samples were allowed to stand at room temperature for 30min for coagulation and serum was obtained by centrifugation. The level of FINS was measured through radioimmunoassay using an automated system (Hitachi analyzer 7080, Japan). A first-void, midstream morning spot urine sample was collected, and albuminuria was measured by immunonephelometry (Roche/Cobas C501 analyzer, Ibaraki,Japan), and high albuminuria was defined as ≥20 mg/L[19].
Diabetic Retinopathy Grading One trained ophthalmologist(Yang XF) graded all the images in a masked manner at the University of Wisconsin Fundus Photographic Reading Center, according to the Early Treatment Diabetic Retinopathy Study (ETDRS) standard classification. Retinopathy was considered present if any characteristic lesions as defined by the ETDRS severity grading scale were present, including microaneurysms, hemorrhages, cotton wool spots, intraretinal microvascular abnormalities, hard exudates, venous beading and new vessels[20]. A retinopathy severity score was assigned for each eye according to the ETDRS Diabetes Retinopathy Severity Scale and the score of the worse eye was used for analysis. Eyes were graded according to the following criteria:no DR (NDR, level 10) or any DR (levels 14 and above). Any DR was further divided into mild (levels 14-35), moderate(levels 43-47), and severe DR (level 53 and above). Grading reproducibility was assessed by regrading 5% of the eyes by a senior grader at the University of Wisconsin Fundus Photograph Reading Center. Exact agreement on retinopathy level was 86% and Weighted Kappa was 0.82[21], which are in agreement with published reproducibility from the reading center[22].
Assessment of Insulin Resistance and β-cell Function The Homeostatis Model Assessments (HOMA) were employed for evaluating IR (marked as HOMA IR) and β-cell function(marked as HOMA β-cell)[23]. The detailed calculation was done by the following formula: HOMA IR=FPG (mmol/L)×FINS (mU/L)/22.5, HOMA β-cell=20×FINS (mU/L)/[FPG (mmol/L)-3.5][23]. Since the HOMA model is not suitable for T2DM cases treated with insulin[12], these were excluded from the present study.
Statistical Analysis Statistical analysis was performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). The data were expressed as numbers (%) for categorical variables,mean±standard deviation (SD) for normally distributed variables. Variables with a skewed distribution were expressed as medians, the lower and upper quartiles. Differences in clinical characteristics of participants with or without DR were compared using independent sample t-tests or Mann-Whitney U test for continuous variables as appropriate, and Chi-square test for categorical variables. Selected indexes of the population stratified by HOMA IR or HOMA β-cell quartiles were analyzed with ANOVA or Kruskal-Wallis H test for continuous variables as appropriate and Chi-squared tests for categorical variables. Multiple logistic regression model was adopted to investigate whether HOMA IR and HOMA β-cell were independently associated with the presence of DR after adjusting for the potential confounding factors. Results were expressed as odds ratio (OR), 95% confidence intervals(CI) and P value. P value less than 0.05 was considered as statistically significant.
RESULTS
A total of 1466 subjects with T2DM were recruited from a local Desheng Community of urban Beijing for the cohort of Beijing Desheng Diabetic Eye Study. After excluding those participants who were treated with insulin (n=352) or had missing data of FINS (n=96), and further excluding those with poor quality of retinal photographs (n=10), a total of 1008 subjects were included for the final analysis. Of the 1008 subjects, 406 (40.3%) were men and 602 (59.7%) were women, mean age was 64.87±8.28y, ranging from 34 to 86y. Any DR (levels 14 and above) was diagnosed in 27.6%(n=278) subjects, of which 253 (25.1%) had mild (levels 14-35),12 (1.2%) had moderate (levels 43-47), and 13 (1.3%) had severe (levels 53 and above) DR.
Baseline characteristics of the study population were shown in Table 1. Compared with the group of NDR, persons diagnosed with any DR were more likely to be male (P=0.004), had a lower level of monthly income (P=0.03), longer duration of diabetes (P<0.001), younger age when DM onset (P<0.001),more likely to be current smoker (P=0.01) and have high albuminuria (P=0.004). Moreover, DR persons were found to have higher levels of SBP (P=0.002), DBP (P=0.036),creatinine (P=0.01), FPG (P<0.001), HbA1c (P<0.001) and HOMA IR (P=0.028), but lower levels of CRP (P=0.048), TG(P=0.045) and HOMA β-cell (P=0.005).
Table 2 showed the selected parameters of the study participants stratified by quartiles of HOMA IR value. In unadjusted analyses of quartiles, increased HOMA IR was associated with higher levels of BMI (P<0.001), WHR (P<0.001), SBP(P=0.002), DBP (P<0.001), CRP (P<0.001), FPG (P<0.001),TG (P<0.001), HbA1c (P<0.001), FINS (P<0.001), high albuminuria (P=0.032), and lower HDL-C (P<0.001).
Table 3 showed the selected parameters of the study participants stratified by quartiles of HOMA β-cell value. In unadjusted analyses of quartiles, decreased HOMA β-cell was associated with younger age when DM onset (P<0.001), longer T2DM duration (P<0.001), current smoking (P=0.001), lower levels of BMI (P<0.001), WHR (P=0.011), FINS (P<0.001),creatinine (P<0.001), uric acid (P<0.001), and higher levels of SBP (P=0.01), DBP (P=0.002), CRP (P=0.034), FPG(P<0.001), TC (P<0.001), LDL-C (P=0.021), and HbA1c(P<0.001).
Table 4 showed the independent associations between baseline HOMA IR/HOMA β-cell and the presence of any DR by multiple logistic regression. After adjusting for confounding factors including established risk factors and variables with P≤0.2 as shown in Table 1 (sex, monthly income, education,DM duration, age when DM onset, current smoking, BMI,SBP, DBP, CRP, creatinine, TG, HbA1c, high aluminuria),when all participants were pooled together, the presence of any DR was not correlated with HOMA IR (OR 1.51, 95%CI:0.87-2.61, P=0.14) or HOMA β-cell (OR 0.71, 95%CI 0.40-1.26, P=0.25).
According to the cut-off value of obesity for Asians(BMI≥25 kg/m2)[24], the subjects were divided into groups of BMI≥25 kg/m2(n=528) and BMI<25 kg/m2(n=480) (Table 4),and any DR was diagnosed in 151 (28.6%) and 127 (26.5%)subjects respectively. The level of HOMA IR were significantly different between the group of patients with BMI≥25 kg/m2and BMI<25 kg/m2(median: 4.82 vs 2.98, P<0.001), and HOMA β-cell were also significantly different between the two groups (median: 73.70 vs 56.64, P<0.001). In the group of BMI≥25 kg/m2, the presence of any DR was associated positively with HOMA IR (OR 2.46, 95%CI: 1.18-5.12,P=0.016) and negatively with HOMA β-cell (OR 0.40,95%CI: 0.19-0.87, P=0.021) after adjustment for covariates.In the group of BMI<25 kg/m2, the presence of any DR was not associated with HOMA IR (OR 1.00, 95%CI: 0.43-2.33, P=1.00) or HOMA β-cell (OR 1.41, 95%CI: 0.60-3.32,P=0.43) after adjustment.
Table 1 Characteristics of the study participants with type 2 diabetes mellitus
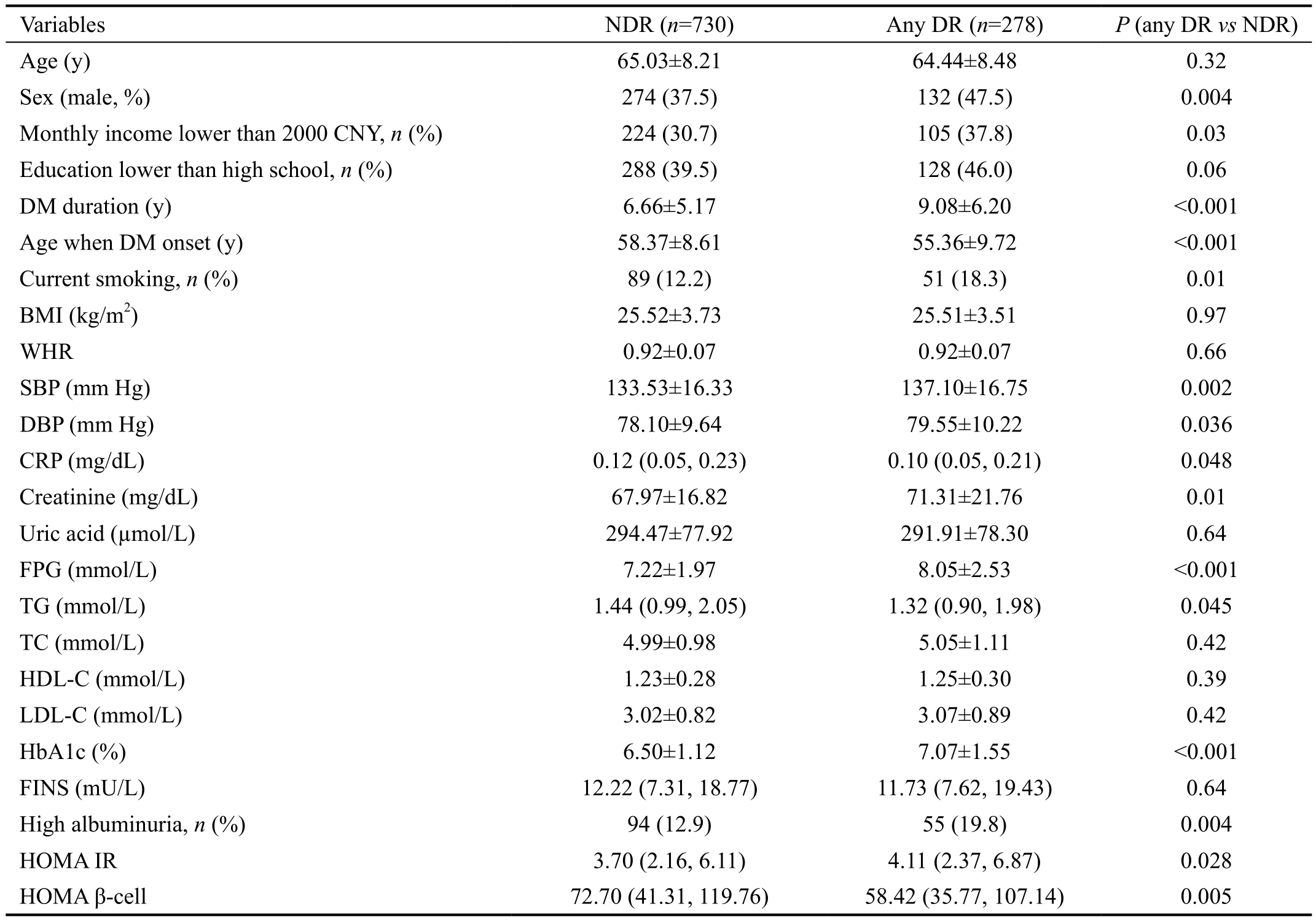
CNY: China Yuan; BMI: Body mass index; WHR: Waist and hip ratio; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; CRP:C-reactive protein; FPG: Fasting plasma glucose; TG: Triglycerides; TC: Total cholesterol; HDL-C: High-density lipoprotein cholesterol;LDL-C: Low-density lipoprotein cholesterol; HbA1c: Glycosylated hemoglobin A1c; FINS: Fasting insulin; HOMA IR: Homeostasis model assessment of insulin resistance; HOMA β-cell: Homeostasis model assessment of β-cell function. The data were expressed as numbers (%) for categorical variables, mean±standard deviation (SD) for normally distributed variables. While variables with a skewed distribution, including Triglycerides, FINS, HOMA IR and HOMA β-cell were expressed as medians, and the lower and upper quartiles. Differences between any DR (diabetic retinopathy) and NDR (no diabetic retinopathy) groups were compared using independent t-test or the Mann-Whitney U test as appropriate. Differences between proportions were analyzed using Chi-squared test. P<0.05 was considered statistically significant.
DISCUSSION
The baseline cross-sectional data from the cohort of Beijing Desheng Diabetic Eye Study suggest that the presence of any DR is associated with higher IR and lower β-cell function in the group of patients with higher BMI (≥25 kg/m2), but this association was not found in the group of patients with lower BMI (<25 kg/m2).
T2DM is characterized by peripheral and hepatic IR and/or pancreatic β-cell dysfunction[25]. IR is defined as an inadequate physiological response of peripheral tissues to insulin action,resulting in metabolic and hemodynamic disturbances[26]. IR was also associated with endothelial dysfunction, increased inflammation and cardiovascular disease[27-29]. Meanwhile,pancreatic β-cells can increase the production of insulin to compensate for IR appropriately, and its dysfunction was central to the development and progression of T2DM[30]and microalbuminuria[29]. In our univariate analysis, higher IR and lower β-cell function were associated with a cluster of metabolic abnormalities including obesity, hypertension,dyslipidemia, hyperglycemia and inflammation, which were conventional risk factors for DR.
IR and/or β-cell dysfunction had also been suggested to be related to DR for T2DM patients[9,12,14]. For example, a hospital-based study showed that the presence of DR was associated with a greater tissue resistance to insulin action instead of inadequate insulin secretion[9]. Moreover, a cohort study of newly diagnosed T2DM patients showed that DR was associated with β-cell failure rather than IR[14], and a community-based study suggested that DR was both associated with IR and β-cell dysfunction in T2DM[12]. In this current study, however, we failed tofind the association between IR/β-cell dysfunction and the presence of DR after adjusting forpossible covariates when all participants were polled together,whereas the onset of moderate NPDR or above (≥level 43) was associated with HOMA β-cell (OR 0.46, 95%CI: 0.23-0.90,P=0.025), but not with HOMA IR (OR 1.43, 95%CI: 0.77-2.67, P=0.26). The discrepancies between previous studies and the current study may be attributed to different methods for the assessment of IR/β-cell function and DR severity or racial differences.
Table 2 Characteristics of the participants with type 2 diabetes mellitus divided by quartiles of HOMA IR
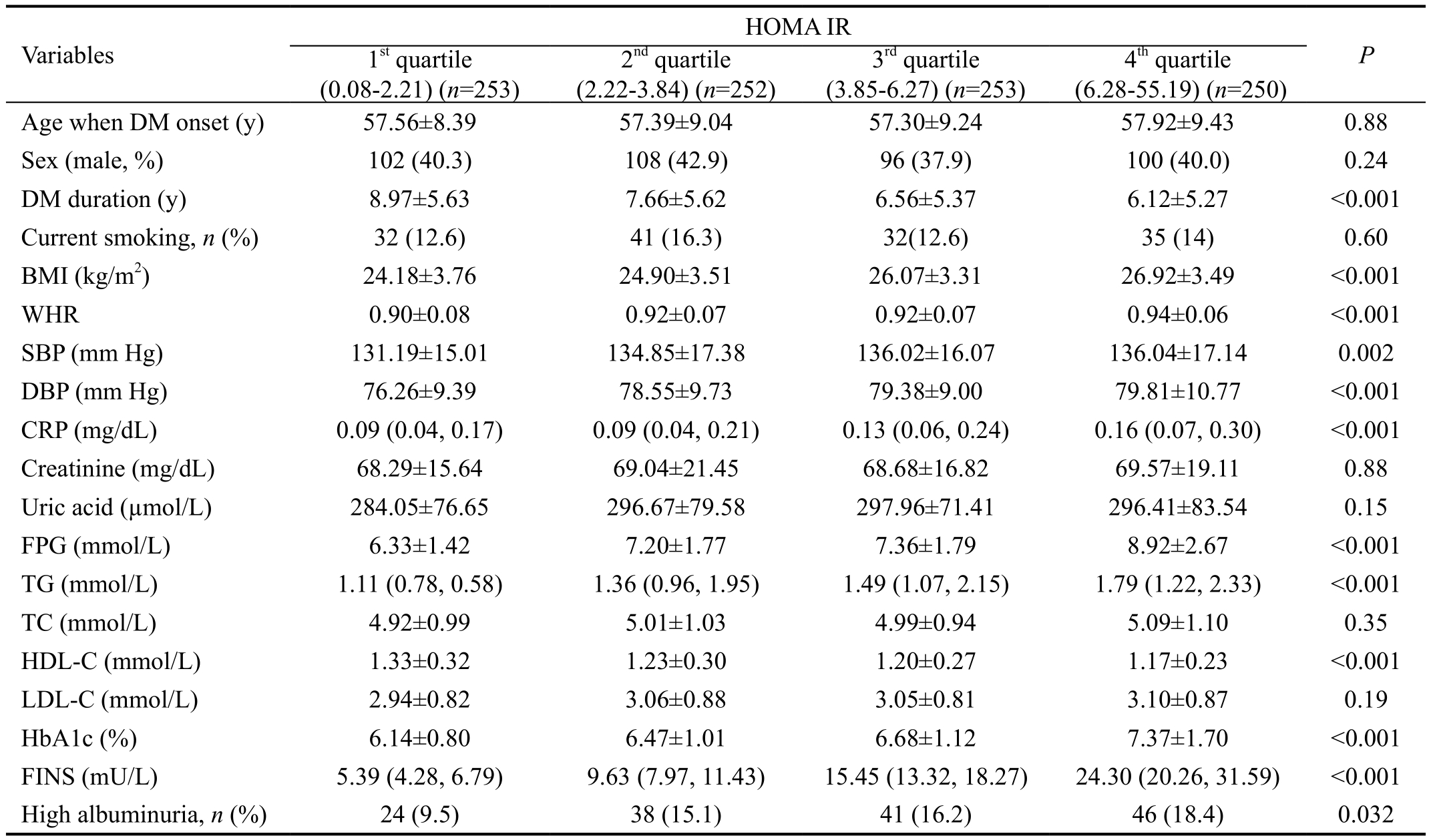
HOMA IR: Homeostasis model assessment of insulin resistance; BMI: Body mass index; WHR: Waist and hip ratio; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; CRP: C-reactive protein; FPG: Fasting plasma glucose; TG: Triglycerides; TC: Total cholesterol;HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; HbA1c: Glycosylated hemoglobin A1c; FINS:Fasting insulin. While variables with a skewed distribution, including CRP, TG and FINS, were expressed as medians, the lower and upper quartiles. Groups were compared using ANOVA or Kruskal-Wallis H test as appropriate. P<0.05 was considered statistically significant.
Table 3 Characteristics of the participants with type 2 diabetes mellitus divided by quartiles of HOMA β-cell
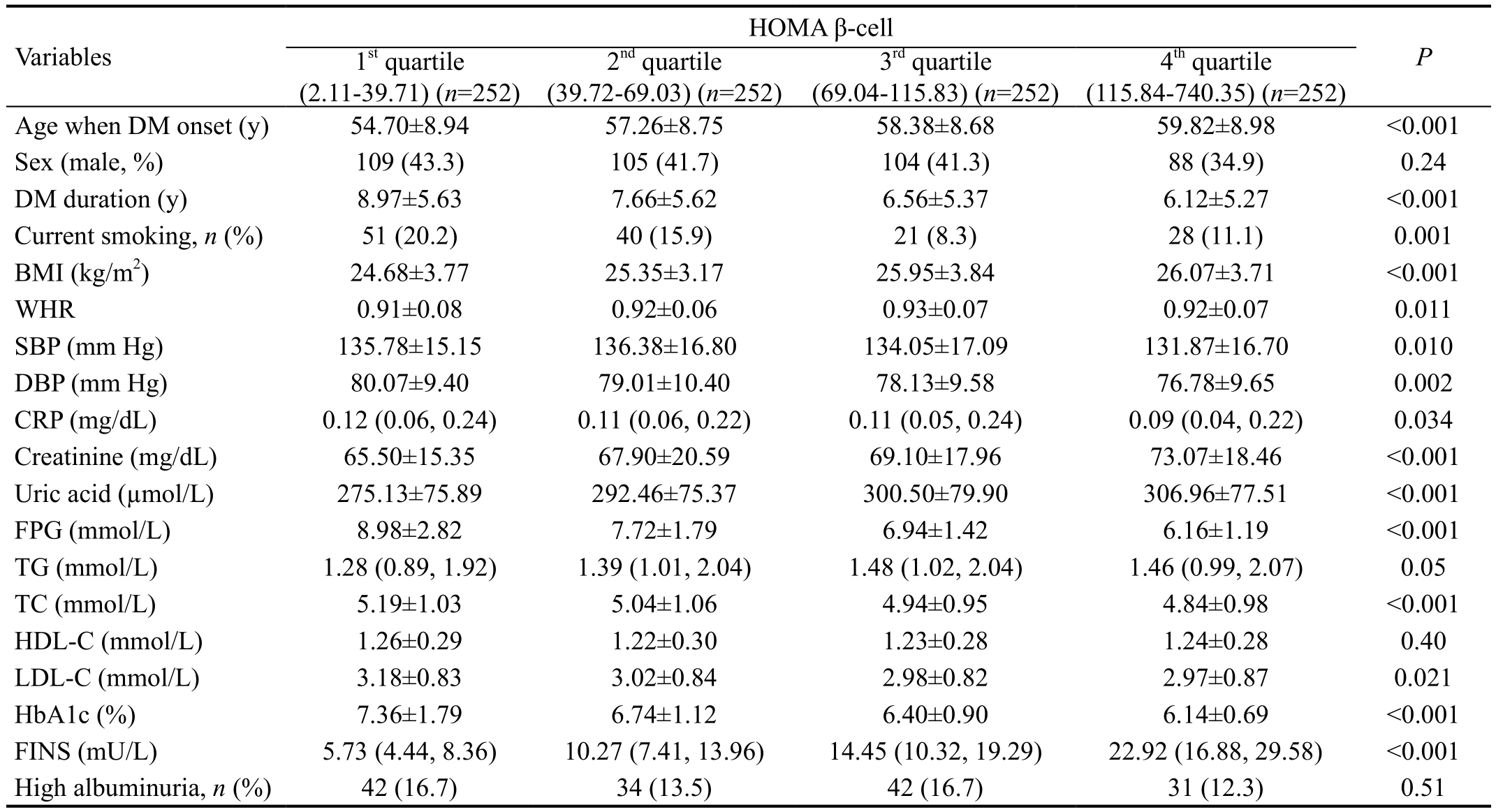
HOMA β-cell: Homeostasis model assessment of β-cell function; BMI: Body mass index; WHR: Waist and hip ratio; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; CRP: C-reactive protein; FPG: Fasting plasma glucose; TG: Triglycerides; TC: Total cholesterol;HDL-C: High-density lipoprotein cholesterol; LDL-C: Low-density lipoprotein cholesterol; HbA1c: Glycosylated hemoglobin A1c; FINS:Fasting insulin. While variables with a skewed distribution, including CRP, TG and FINS, were expressed as medians, the lower and upper quartiles. Groups were compared using ANOVA or Kruskal-Wallis H test as appropriate. P<0.05 was considered statistically significant.
Table 4 Multiple logistic regression of association between HOMA IR/β-cell and diabetic retinopathy
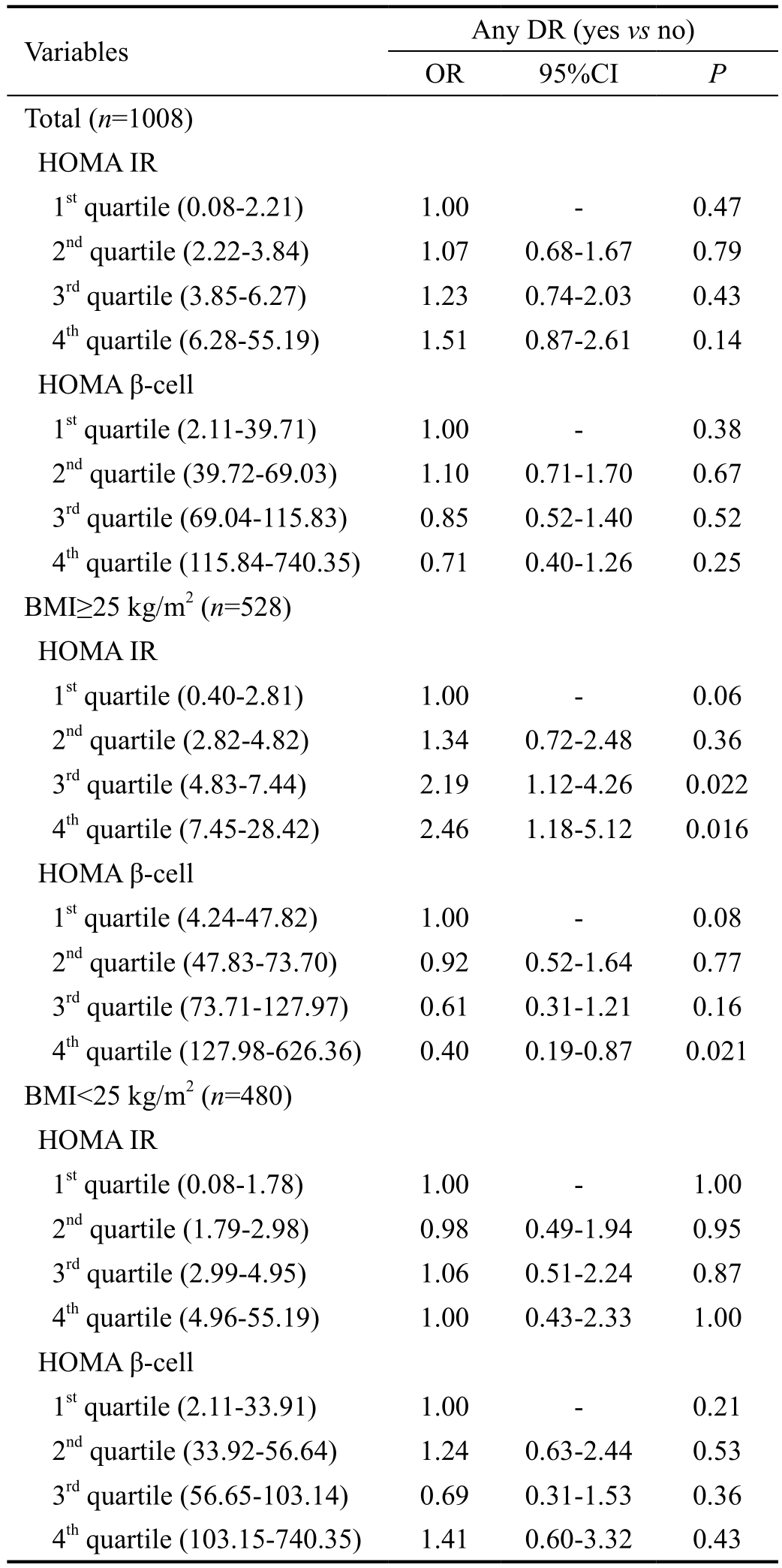
HOMA IR: Homeostasis model assessment of insulin resistance;HOMA β-cell: Homeostasis model assessment of β-cell function;DR: Diabetic retinopathy; DM: Diabetes mellitus; BMI: Body mass index; SBP: Systolic blood pressure; DBP: Diastolic blood pressure;CRP: C-reactive protein; TG: Triglycerides; HbA1c: Glycosylated hemoglobin A1c. OR: Odds ratio; CI: Confidence interval. Other confounding factors controlled included: sex, monthly income,education, DM duration, age when DM onset, current smoking, BMI,SBP, DBP, CRP, creatinine, TG, HbA1c, high albuminuria. P<0.05 was considered statistically significant.
The contribution of IR and β-cell dysfunction to T2DM may be different for obese and non-obese patients[15-16]. Elevated BMI has been shown to be an established risk factor for T2DM[31]and diabetic complications[32]. In the subgroup analysis after stratification by BMI, our data suggest that increased IR and decreased β-cell function were statistically significantly associated with the presence of any DR for the group of patients with BMI≥25 kg/m2, but this association was not found for those with BMI<25 kg/m2. For obese subjects, pro-inflammatory cytokines are highly expressed in adipose tissue, which contributes to a chronic low-grade in flammation and IR[33-34]. Coincidently, the level of CRP, the most extensively studied biomarker of systemic in flammation,is higher in obese subjects than non-obese subjects in the present study (median: 0.14 mg/dL vs 0.09 mg/dL, P<0.001).Moreover, adipose tissue exhibits a higher lipolysis rate, which leads to elevated levels of free fatty acids[34]. Higher levels of free fatty acids are known to be involved in the activation of inflammatory pathway, which may further cause IR and β-cell dysfunction[35-36]. We speculate that higher IR together with lower β-cell function may accelerate the development of DR under the low-grade inflammatory environment in diabetic patients with higher BMI. Although β-cell dysfunction was taken as the main etiological factor of T2DM in nonobese patients[16], we failed to show the association between lower β-cell function and DR for participants with lower BMI, suggesting that β-cell dysfunction may not have direct relationship with DR for diabetic patients with lower BMI.
Limitations of the study should be noted. Being a crosssectional survey, the study explored only the association between IR/β-cell dysfunction and DR for T2DM patients,but a causal relationship cannot be established and needs to be verified in future longitudinal cohort studies. Moreover, the application condition of HOMA model requires exclusion of those participants treated with insulin, therefore only a small number of subjects with severe DR could be included into the study, making it nearly impossible to further analyze the relationship between IR/β-cell dysfunction and DR severity.
In conclusion, our data showed that IR and β-cell dysfunction may be related to the presence of DR for subjects with higher BMI in the studied Chinese patients with T2DM, whereas we failed to find the association in the subgroup of patients with lower BMI, implying that the clinical value of IR and β-cell dysfunction for the risk of DR may need to be evaluated differently for obese and non-obese patients, and hopefully provide personalized early interventions for diabetes with different individual characteristics. Further studies to confirm this observation would be warranted.
ACKNOWLEDGEMENTS
The authors thank Ronald R Danis, James L. Reimers and Ashwini Narkar, staff at the Fundus Photograph Reading Center, Department of Ophthalmology and Visual Sciences,University of Wisconsin, for their important contributions on the diabetic retinopathy grading and quality control.
Authors’ contributions:Li YY: data acquisition, analysis and interpretation, drafting of manuscript; Yang XF, Gu H:data acquisition, analysis and interpretation, critical revision of manuscript. Snellingen T, Liu XP, Liu NP: conception,design, data acquisition, analysis and interpretation, critical revision of manuscript. All authors read and approved thefinal manuscript.
Foundations:Supported by the Beijing Natural Science Foundation (No.7131007); National Basic Research Program of China (973 Program; No.2007CB512201).
conflicts of Interest:Li YY, None; Yang XF, None; Gu H,None; Snellingen T, None; Liu XP, None; Liu NP, None.
REFERENCES
1 Xie X, Xu L, Yang H, Wang S, Jonas JB. Frequency of diabetic retinopathy in the adult population in China: the Beijing Eye Study 2001.Int Ophthalmol 2009;29(6):485-493.
2 Dowse GK, Humphrey AR, Collins VR, Plehwe W, Gareeboo H,Fareed D, Hemraj F, Taylor HR, Tuomilehto J, Alberti KG, Zimmet PZ.Prevalence and risk factors for diabetic retinopathy in the multiethnic population of Mauritius. Am J Epidemiol 1998;147(5):448-457.
3 el Haddad OA, Saad MK. Prevalence and risk factors for diabetic retinopathy among Omani diabetics. Br J Ophthalmol 1998;82(8):901-906.
4 Kohner EM, Aldington SJ, Stratton IM, Manley SE, Holman RR,Matthews DR, Turner RC. United Kingdom Prospective Diabetes Study,30: diabetic retinopathy at diagnosis of non-insulin-dependent diabetes mellitus and associated risk factors. Arch Ophthalmol 1998;116(3):297-303.
5 Hill L, Makaroff LE. Early detection and timely treatment can prevent or delay diabetic retinopathy. Diabetes Res Clin Pract 2016;120:241-243.
6 LeRoith D. Beta-cell dysfunction and insulin resistance in type 2 diabetes: role of metabolic and genetic abnormalities. Am J Med 2002;113 Suppl 6A:3S-11S.
7 Li CL, Tsai ST, Chou P. Relative role of insulin resistance and beta-cell dysfunction in the progression to type 2 diabetes--The Kinmen Study.Diabetes Res Clin Pract 2003;59(3):225-232.
8 Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376(9739):419-430.
9 Maneschi F, Mashiter K, Kohner EM. Insulin resistance and insulin deficiency in diabetic retinopathy of non-insulin-dependent diabetes.Diabetes 1983;32(1):82-87.
10 Suzuki K, Watanabe K, Motegi T, Kajinuma H. High prevalence of proliferative retinopathy in diabetic patients with low pancreatic B-cell capacity. Diabetes Res Clin Pract 1989;6(1):45-52.
11 Katsumori K, Wasada T, Kuroki H, Arii H, Saeki A, Aoki K, Saito S,Omori Y. Prevalence of macro- and microvascular diseases in non-insulindependent diabetic and borderline glucose-intolerant subjects with insulin resistance syndrome. Diabetes Res Clin Pract 1995;29(3):195-201.
12 Tung TH, Shih HC, Tsai ST, Chou P, Chen SJ, Lee FL, Chuang SY,Liu JH. A community-based study of the relationship between insulin resistance/beta-cell dysfunction and diabetic retinopathy among type II diabetics in Kinmen, Taiwan. Ophthalmic Epidemiol 2007;14(3):148-154.
13 Stolk RP, Vingerling JR, de Jong PT, Diels I, Hofman A, Lamberts SW,Pols HA, Grobbee DE. Retinopathy, glucose, and insulin in an elderly population. The Rotterdam Study. Diabetes 1995;44(1):11-15.
14 Roy Chowdhury S, Thomas RL, Dunseath GJ, Peter R, Rees DA,North RV, Luzio SD, Owens DR. Diabetic retinopathy in newly diagnosed subjects with type 2 diabetes mellitus: contribution of β-cell function. J Clin Endocrinol Metab 2016;101(2):572-580.
15 Liu J, Wang Y, Hu Y, Leng S, Wang G. Comparison of β-cell dysfunction and insulin resistance correlating obesity with type 2 diabetes:a cross-sectional study. Journal of Diabetes and Its Complications 2016;30(5):898-902.
16 Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of beta-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 2006;29(5):1130-1139.
17 Yang X, Deng Y, Gu H, et al. Relationship of retinal vascular calibre and diabetic retinopathy in Chinese patients with type 2 diabetes mellitus:the Desheng Diabetic Eye Study. British J Ophthalmol 2016;100(10):1359-1365.
18 1999 World Health Organization-International Society of Hypertension Guidelines for the Management of Hypertension. Guidelines Subcommittee.J Hypertens 1999;17(2):151-183.
19 Lepore G, Maglio ML, Nosari I, Dodesini AR, Trevisan R. Costeffectiveness of two screening programs for microalbuminuria in type 2 diabetes. Diabetes Care 2002;25(11):2103-2104; author reply 2104.
20 Wong TY, Klein R, Islam FM, Cotch MF, Folsom AR, Klein BE,Sharrett AR, Shea S. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol 2006;141(3):446-455.
21 Yang XF, Deng Y, Gu H, et al. C-reactive protein and diabetic retinopathy in Chinese patients with type 2 diabetes mellitus. Int J Ophthalmol 2016;9(1):111-118.
22 Li HK, Hubbard LD, Danis RP, Esquivel A, Florez-Arango JF, Ferrier NJ, Krupinski EA. Digital versusfilm fundus photography for research grading of diabetic retinopathy severity. Invest Ophthalmol Vis Sci 2010;51(11):5846-5852.
23 Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF,Turner RC. Homeostasis model assessment: insulin resistance and betacell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28(7):412-419.
24 WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies.Lancet 2004;363(9403):157-163.
25 DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle,liver. A collusion responsible for NIDDM. Diabetes 1988;37(6):667-687.
26 Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R.Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care 2003;26(12):3320-3325.
27 Syed Ikmal SI, Zaman Huri H, Vethakkan SR, Wan Ahmad WA.Potential biomarkers of insulin resistance and atherosclerosis in type 2 diabetes mellitus patients with coronary artery disease. Int J Endocrinol 2013;2013:698567.
28 Xun P, Wu Y, He Q, He K. Fasting insulin concentrations and incidence of hypertension, stroke, and coronary heart disease: a meta-analysis of prospective cohort studies. Am J Clin Nutr 2013;98(6):1543-1554.
29 Mulvey CK, McNeill AM, Girman CJ, et al. Differential associations of oral glucose tolerance test-derived measures of insulin sensitivity and pancreatic beta-cell function with coronary artery calcification and microalbuminuria in type 2 diabetes. Diabetes Care 2014;37(1):124-133.
30 Halban PA, Polonsky KS, Bowden DW, et al. Beta-cell failure in type 2 diabetes: postulated mechanisms and prospects for prevention and treatment. Diabetes Care 2014;37(6):1751-1758.
31 Sanada H, Yokokawa H, Yoneda M, Yatabe J, Sasaki Yatabe M,Williams SM, Felder RA, Jose PA. High body mass index is an important risk factor for the development of type 2 diabetes. Intern Med 2012;51(14):1821-1826.
32 Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol 1984;102(4):527-532.
33 Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, in flammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci 2014;15(4):6184-6223.
34 Smith JD, Borel AL, Nazare JA, Haffner SM, Balkau B, Ross R,Massien C, Alméras N, Després JP. Visceral adipose tissue indicates the severity of cardiometabolic risk in patients with and without type 2 diabetes: results from the INSPIRE ME IAA study. J Clin Endocrinol Metab 2012;97(5):1517-1525.
35 Haghighatdoost F, Hosseinzadeh-Attar MJ, Kabiri A, Eshraghian M,Esmaillzadeh A. Effect of substituting saturated with monounsaturated fatty acids on serum visfatin levels and insulin resistance in overweight women: a randomized cross-over clinical trial. Int J Food Sci Nutr 2012;63(7):772-781.
36 Tan C, Voss U, Svensson S, Erlinge D, Olde B. High glucose and free fatty acids induce beta cell apoptosis via autocrine effects of ADP acting on the P2Y(13) receptor. Purinergic Signal 2013;9(1):67-79.