INTRODUCTION
Endoscopy-assisted ophthalmological surgery is a relatively old technique, although it has not been widely adopted except for managing difficult cases[1-3]. The indications for this technique include goniosynechialysis, retained lens fragments, a posteriorly dislocated intraocular lens, transscleral suture fixation, ciliary body photocoagulation, proliferative vitreoretinopathy (PVR), intraocular foreign bodies, retinal detachment repair (especially for undetectable breaks in the peripheral retina), endophthalmitis, ocular trauma, selecting an intraocular device, and evaluating the surgical strategy[4-5].Vitreoretinal techniques are widely used to treat ophthalmic diseases, and endoscopy-assisted vitrectomy (EV) is attracting increasing amounts of attention. Unlike traditional pars plana vitrectomy (PPV), EV makes it easier to visualize the operativefield in cases with media opacity[6]or a microcornea[4-5], especially when the anterior vitreous (AV) is hidden beneath the iris.
The concept of the AV was first proposed by Lewis and Aaberg[7]in 1988 as being anterior to the posterior insertion of the vitreous base. However, in 1991, Machemer et al[8]suggested that the AV was anterior to the equator of the vitreous base.Although most AV dissections can be recognized, they are difficult to treat because of the complexity and variability of the AV under different conditions[9]. Thus, EV may be useful for overcoming these difficulties.
DEVELOPMENT OF INTRAOCULAR ENDOSCOPES
The first use of an endoscope during ophthalmological surgery was reported in 1934[10], when Thorpe[10]removed an intraocular foreign body using integrated forceps. In 1982, Norris and Cleaseby[11]also performed surgery using a intraocular endoscope with a diameter of 1.7 mm. Furthermore, intraocular lens (IOL) implantations were performed using endoscopes during the 1980s and 1990s[12]. In 1996, Jürgens et al[13]described the use of an endoscope to localize the sulcus during placement of a sulcus-fixated sutured posterior chamber IOL.Moreover, a 20-gauge intraocular endoscope was developed in 1991 for use during endocyclophotocoagulation for glaucoma[14],and various video endoscopes and ophthalmoendoscopes have subsequently been developed for use during vitreoretinal surgery[15]. Since then, several clinical case series have examined the use of EV for different conditions.
CHARACTERISTICS OF INTRAOCULAR ENDOSCOPES
The current intraocular endoscopes project the intraocular images, which are captured using a lens on the distal tip, onto an electronic monitor. In addition, each endoscope has an optical duct illuminating system. Various gradient index lens systems are used, although the E2 or E4 fiber-optic systems(Little Silver, NJ, USA) are widely used. Other endoscope systems are produced by PolyDiagnost (Germany) and Fiber Tech (Tokyo, Japan), which require micro incisions to accommodate the 19-gauge, 20-gauge, or 23-gauge endoscopes. Each endoscope includes a xenon light source(illumination), a charge-coupled device camera (for image capture and monitoring), and an optical laser. The endoscope’s size determines the imaging resolution and thefield of view(FOV), with the 19-gauge endoscopes having 17 000 pixels and a 140° FOV, the 20-gauge endoscopes having 10 000 pixels and a 110° FOV, and the 23-gauge endoscopes having 6000 pixels and a 90° FOV[1,16]. Thus, the specific case determines the sizing of the endoscope that is used[16], and 19-gauge endoscopes have become common in complex cases with a greater need for peripheral imaging[17].
DISTINGUISHING BETWEEN TRADITIONAL MICROSCOPES AND ENDOSCOPES
Traditional microscopes can easily visualize intraocular objects through a clear anterior media, while endoscopes must cross the anterior segment to capture images using their distal tip[1,16,18]. Thus, unlike traditional microscopes,intraocular endoscopes provide a high magnification with panoramic, unobstructed, and undistorted views of the space between the vitreous base and the anterior segments behind the iris[19]. In addition, traditional microscopes provide a topdown perspective from outside the patient’s cornea, while endoscopes provide a unique intraoperative view from inside the vitreous cavity (i.e. the side-on perspective) (Figure 1)[18,20].Nevertheless, surgeons must be comfortable manipulating the endoscope inside the eye, as it is similar to a traditional light guide[20]. Image rotation is possible if the surgeon repositions the probe, and a baseline level of positioning is needed before the probe can be inserted into the eye. Thus, it is important to ascertain the orientation of the endoscopic image on the monitor.
NORMAL STRUCTURES OF THE ANTERIOR VITREOUS
Endoscopy can easily visualize the entire AV, including a circumferential view without scleral indentation or views that are outside of the standard FOV. Furthermore, endoscopy provides a clear and detailed image of the vitreousfibers and gel, the ciliary body, the ora serrata, and the anterior retina, even in cases with myopic, phakic, aphakic, or pseudophakic eyes.Under the subiris, the anterior zonular ligament is attached to the anterior lens capsule in the anterior equatorial region,and the posterior zonular ligament is attached to the posterior lens capsule in the posterior equator[21]. The ciliary body and processes form the pars plicata and consist of the nonpigmentedand pigmented ciliary epithelium, which rest on loose connective tissue or stroma (Figure 2), and the ligament of Wieger can also be clearly visualized[22]. The anterior hyaloid membrane is composed of parallelfibers that adhere to part of the posterior lens capsule, the zonular ligament, the pars plicata, and the ora serrata. In addition, thefibers of the vitreous cortex form a transparent gel without liquefaction or individualization[23-24]. The junction of the AV membrane and the ora serrata is the closest region to the anterior retinal surface[24].In myopic eyes, vitreous fibers and abnormal attachments to the retinal surface may be observed at the border or along the posterior part of the AV base, and may occasionally extend beyond the equator level. In phakic eyes, the zonular and AVfibers are attached over the ciliary process and the pars plicata is rarely visible. In aphakic eyes, the zonular lens is shrunk and the capsule bag is visible because the lens’ haptics are positioned appropriately, whereas the haptics would be in the ciliary sulcus in cases of capsule rupture with or without vitreous gel contraction (Figure 3)[23].

Figure 1 The surgeon’s perspective using a traditional microscope and an endoscope.
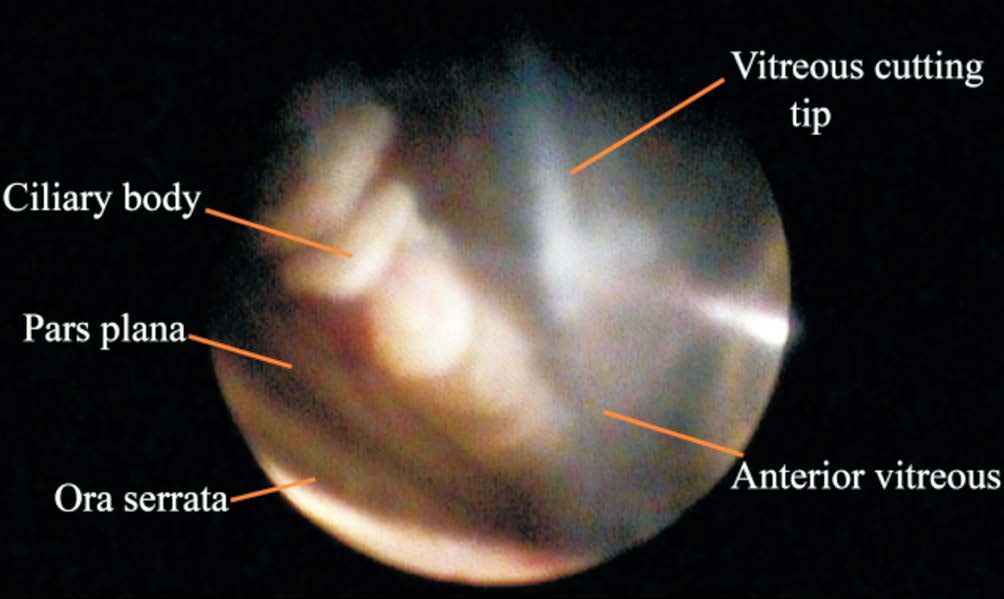
Figure 2 Anterior vitreous in an aphakic eye The vitreous cutting tip, the ciliary body, and the ora serrata are shown.

Figure 3 Anterior vitreous in an pseudophakic eye The intraocular lens, anterior vitreous cavity, ciliary body, and ora serrata are shown.
CLINICAL APPLICATION AND OUTCOMES
Table 1 summarizes the outcomes from a number of cases series that used EV to treat AV pathologies[20,25-41]. Single-case reports were excluded.
PATHOLOGIES OF THE ANTERIOR VITREOUS
Anterior Vitreous Retraction, Prolapse, or Incarceration Vitreous retraction with/without retinal breaks can usually bedetected during anterior PVR[20,30], where the anterior smooth invisible gel is usually replaced by high-density agglomeration,whereas the contractive gel or fibers connect to the anterior and posterior parts of the vitreous base and the circumferential retina[20]. Therefore, persistent tractive forces exist around the vitreous base and the circumferential retina, which may lead to hemorrhage in the retinal surface. Vitreous prolapse usually occurs because of a capsular defect, and contraction of the vitreous gel orfibers could lead to IOL dislocation or a powerful tractive force being exerted on the retina, leading to retinal tearing[13,42]. Vitreous incarceration often occurs in cases involving surgical incision[43].
Table 1 Summary of studies that evaluated endoscopy-assisted vitrectomy for anterior vitreous pathologies
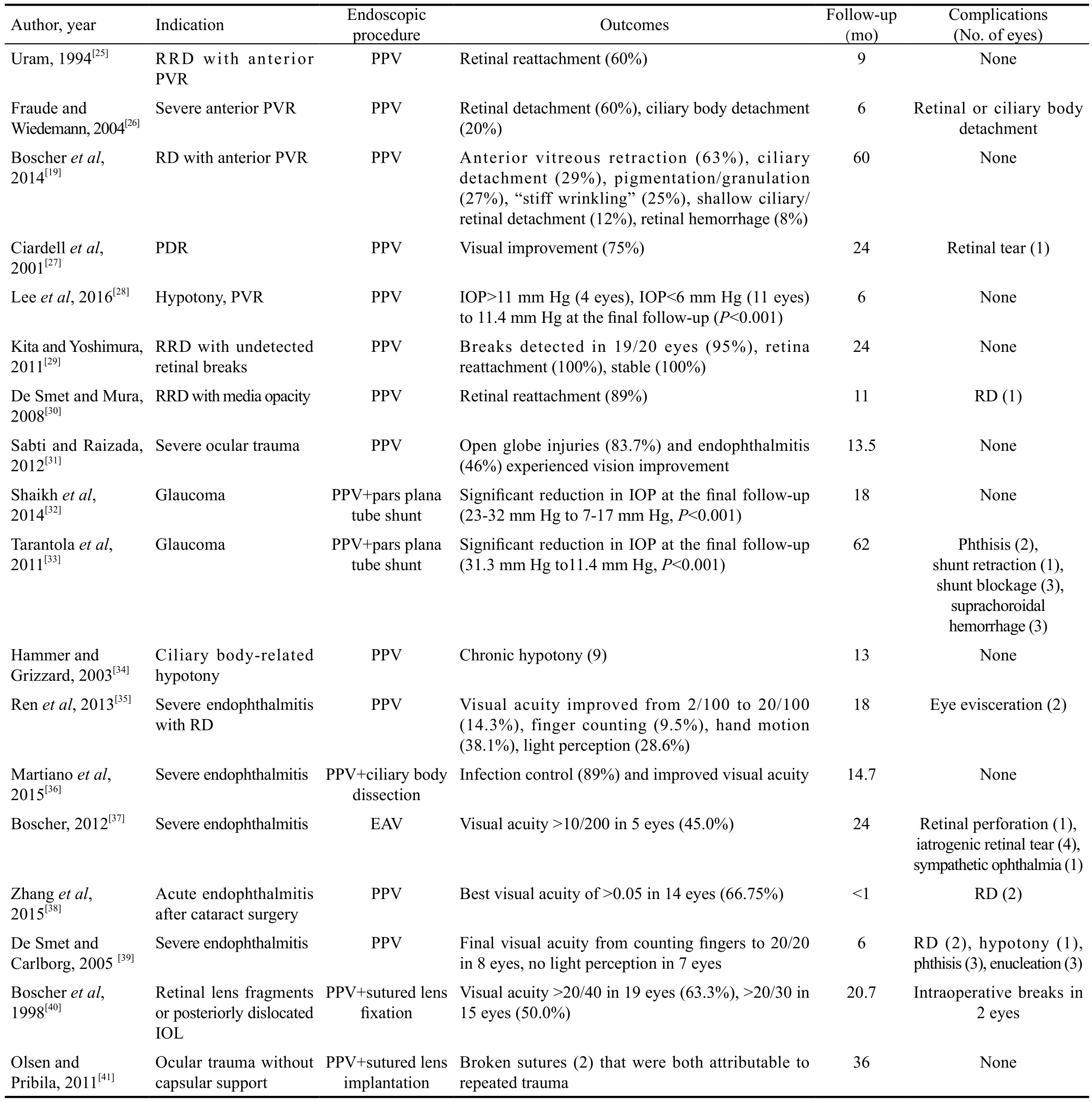
PPV: Pars plana vitrectomy; EAV: Endoscopy assisted vitrectomy; PVR: Proliferative vitreoretinopathy; PDR: Proliferative diabetic retinopathy; IOP: Intraocular pressure; RD: Retinal detachment; RRD: Re-retinal detachment.
ANTERIOR VITREOUS ADHESION
In aphakic eyes, vitreous adhesion to the iris is often observed in the AV regions of the vitreoretinal junction. This is usually associated with various pathological conditions involving the pupil, such as inflammation, foreign matter, or a neoplasm behind the iris. Vitreous adhesion to the anterior retina can also lead to peripheral retinal tearing or fibrovascular proliferation[44].
Ciliary Body Detachment and Cyclodialysis Cleft The common causes of ciliary detachment and cyclodialysis cleft include eye trauma and iatrogenic interventions[43].Ciliary body detachment is always accompanied by retinal or choroidal detachment[45]. In most cases, retinal detachment occurs within a limited area and with a clear boundary.Furthermore, anterior retinal detachment rarely occurs in combination with ciliary detachment, unless there is extremely severe vitreous contraction, cyclitic membrane contraction,or hypotony. Cyclodialysis clefts are more rare than ciliary detachment. All of these pathological changes can be clearly and directly visualized using an endoscope.
Anterior Neovascularization Neovascularization in the anterior retina or in the iris is always observed in cases of proliferative diabetic retinopathy or retinal vein occlusion,especially when ischemia is observed. This is mainly the result of ischemia in areas with long-standing anterior loop traction and loss of blood supply into the anterior retinal vessels.Furthermore, neovascularization is related to angiogenesis that is controlled by various cytokines, mainly including vascular endothelial growth factor,fibroblast growth factor and insulinlike growth factor. This process can create a vicious circle that aggravates the ischemia and hypoxia[46]and potentially can lead to anterior hemorrhage.
Anterior Retinal or Subretinal Proliferation Anterior retinal or subretinal proliferation can be observed in cases involving proliferative diabetic retinopathy, proliferative vitreoretinopathy, or prolonged complex retinal detachment,which causes severe retinal contraction with the stifffibers[20].Thesefibers adhere tightly to the retina, which can create a sort of “retinal anterior loop” or “subretinal anterior loop” that can also lead to hemorrhage or tearing.
Indications for Endoscopy-assisted Vitrectomy in the Anterior Vitreous Intraocular endoscopy can facilitate the visualization and manipulation of the anterior structures,which can lead to better efficacy and anatomical outcomes in cases that are treated using EV (vs other surgical options)[20].This is because the endoscope can easily reach the areas of the posterior iris to manage retained lens matter, anterior PVR, and complex retinal detachments.
In the ciliary sulcus, endoscopy can facilitate complete capsulectomy during vitreolensectomy in cases with uveitis and in cases with retained pseudophake lens matter causing chronic uveitis[47]. In addition, endoscopy can be used to manage ciliary body and posterioriris pathologies, such as cyclitic membranes causing cyclodialysis cleft or ciliary detachment and hypotony[31], which can be addressed by removing the epiciliary membranes. Furthermore, endoscopy is not distorted by scleral indentation and provides a clear view of AV contraction[25-26], as well as the relationship between the AV gel and the pars plana-based glaucoma shunt tube[32-33].Therefore, endoscopy can facilitate more precise and complete vitreous clearance during vitrectomy, and reduce the risk of anterior proliferation resulting in severe anterior PVR,hypotony, and re-retinal detachment.
There are several benefits to using EV during the repair of retinal detachment. For example, it provides better visualization for retinal repair or re-attachment in cases with anterior media opacity[30-31]. In addition, it is a useful tool for identifying undetectable retinal breaks, especially in pseudophakic or aphakic eyes and cases with complex retinal detachment[29]. Furthermore, EV is an effective approach for subretinal surgery, removal of the subretinal membranes, or managing PVR, as it provides tracking along the subretinal surface from a relatively small retinotomy at a remote site and can also facilitate subretinal fluid drainage. Sonoda et al[48]have also used an ophthalmological endoscope to remove subretinal fluid without performing endodrainage retinotomy and successfully performed retinal re-attachment.
Some case series have also reported that EV is useful for open globe injuries with or without intraocular foreign bodies(IOFBs) and severe endophthalmitis[35-37]. The anterior and posterior segment pathologies deteriorated soon after these ocular injuries, and endoscopy helped facilitate better IOFB removal than that under a conventional microscopic view.Moreover, endoscopy reduced the probability of retinal tearing or vitreous hemorrhage, especially in cases with IOFBs located in the anterior retina or pre-existing endophthalmitis. Other reports have indicated that ophthalmological endoscopy can preserve visual acuity in cases that would otherwise require delay of surgery because of hazy media or the non-availability of a donor cornea for simultaneous penetrating keratoplasty[31].
Pediatric Vitreoretinal Surgery Although few studies have examined EV for children, endoscopy provides clear advantages during complex pediatric vitreoretinal surgery. In this context,the common pediatric vitreoretinal pathologies include retinopathy of prematurity, tractional retinal detachment, and familial exudative vitreoretinopathy. Furthermore, pediatric eyes have a unique anatomy and physiology[16-17], as well as a high risk of aggressive and widespread PVR, especially in cases with traumatic retinal detachment and open globe injuries[20]. Endoscopy reduces the risk in these situations, as it can directly guide the surgery with minimal manipulationand the greatest surgical effect. For example, pediatric tractional retinal detachmentcan extend anteriorly very close to the lens and pars plicata, which is associated with a high risk of iatrogenic retinal break or lens trauma during sclerotomy.Endoscopy can help prevent these iatrogenic events.
Manipulation During Endoscopy-assisted Vitrectomy and Its Learning Curve The use of EV is challenging, and this technique has a steep learning curve[1,18,23]. Thefirst issue is the indirect viewing of the surgical field on a monitor,which requires training to adapt to the pseudo stereopsis,rather than traditional true stereopsis. In addition, the side-on intraoperative perspective requires the ability to discern the endoscope’s orientation and manually control the probe tip,which requires precise hand-eye coordination. Thus, novice surgeons may experience difficulty with EV.
The second issue is that bimanual surgery is impossible, as one hand must control the endoscope. Furthermore, the endoscope provides a highly magnified image, which can provide a false sense of security regarding distance from anatomical structures.Thus, care is needed to avoid harming the retina or uvea, which can lead to iatrogenic ocular and choroidal hemorrhage.
Third, it is important to center the target area in the FOV during endoscopy surgery, as the imaging is controlled by the endoscope’s tip. Thus, surgical maneuvers at the edge of the FOV are associated with an increased risk of iatrogenic trauma. In addition, lighting adjustment may be necessary,as the available light is a product of the distance between the endoscope tip and the target area. Thus, when working at high magnifications and short distances, the illumination intensity should be reduced. Moreover, it is important to keep the endoscope’s tip clean in order to avoid obstruction or blurring of the endoscopic image. Finally, crossing beyond the pole of the lens should be avoided.
EV is also affected by the surgeon’s technique, the vitreous cutting equipment, and the vitreous gel viscosity[1,18,23]. For example, if the vitreous cutting machine is a Venturi-type pump, the pump only controls aspiration, and the vacuum’s power is controlled by a foot pedal, which is affected by the surgeon’s experience[23]. Furthermore, it is difficult to select a proper aspiration flow and cutting speed, which is based on both the specific anatomy and gel viscosity. Thus, the effective aspiration flow depends on the specific gel viscosity,the infusion gradient, the cutting mode, the vitrectomy probe,and other factors. Moreover, each surgeon tends to select parameters that fit their technique and habits after they have evaluated gel viscosity. In general, high cutting speeds are required for highly viscous gel and near the target pathology,while decreased cutting speed is warranted when the probe reaches the root of the gel. Therefore, extensive EV training is essential to the successful use of this technique, and it is preferable to practice using an artificial eye if possible.
CONCLUSION
Intraocular endoscopy plays a crucial role in vitreoretinal surgery for cases with media opacity, a small pupil, or iris adhesion, as it can facilitate clear and accurate AV dissection.Thus, it is likely that EV will become an increasingly valuable technique in thisfield.
ACKNOWLEDGEMENTS
The authors thank Editage (www.editage.cn) for English language editing.
Authors’ contributions:Zou YP and Zou XL conceived of the review. Yu YZ drafted the manuscript. Zou YP supplied the images.
Foundations:Supported by the Guangzhou Science and technology Foundation of Guangdong Province (No.2014J4100035;No.2014KP000071).
conflicts of Interest:Yu YZ, None; Zou YP, None; Zou XL,None.
REFERENCES
1 Wong SC, Lee TC, Heier JS. 23-Gauge endoscopic vitrectomy. Dev Ophthalmol 2014;54:108-119.
2 Yoshitake S, Oh H, Kita M. Endoscope-assisted vitrectomy for retinal detachment in an eye with microcornea. Jpn J Ophthalmol 2012;56(6):613-616.
3 Farias CC, Ozturk HE, Albini TA, Berrocal AM, Amescua G, Betancurt C, Parel JM, Oliveros MC, Gibbons A, Vargas JM, Perez VL. Use of intraocular video endoscopic examination in the preoperative evaluation of keratoprosthesis surgery to assess visual potential. Am J Ophthalmol 2014;158(1):80-86.e2.
4 Goldberg RA, Hariprasad SM. Endoscopy remains a valuable tool for vitreoretinal surgery. Ophthalmic Surg Lasers Imaging Retina 2015;46(6):606-608.
5 Francis BA, Kwon J, Fellman R, Noecker R, Samuelson T, Uram M,Jampel H. Endoscopic ophthalmic surgery of the anterior segment. Surv Ophthalmol 2014;59(2):217-231.
6 Chun DW, Colyer MH, Wroblewski KJ. Visual and anatomic outcomes of vitrectomy with temporary keratoprosthesis or endoscopy in ocular trauma with opaque cornea. Ophthalmic Surg Lasers Imaging 2012;43(4):302-310.
7 Lewis H, Aaberg TM. Anterior proliferative vitreoretinopathy. Am J Ophthalmol 1988;105(3):277-284.
8 Machemer R, Aaberg TM, Freeman HM, Irvine AR, Lean JS, Michels RM. An updated classification of retinal detachment with proliferative vitreoretinopathy. Am J Ophthalmol 1991;112(2):159-165.
9 Charteris DG, Downie J, Aylward GW, Sethi C, Luthert P. Intraretinal and periretinal pathology in anterior proliferative vitreoretinopathy.Graefes Arch Clin Exp Ophthalmol 2007;245(1):93-100.
10 Thorpe HE. Ocular endoscope: instrument for removal of intravitreous non magnetic foreign bodies. Trans Am Acad Ophthalmol Otolaryngol 1934;39:422-424.
11 Norris JL, Cleasby GW. Intraocular foreign body removal by endoscopy. Ann Ophthalmol 1982;14(4):371-372.
12 Uram M. Ophthalmic laser microendoscope endophotocoagulation.Ophthalmology 1992;99(12):1829-1832.
13 Jürgens I, Lillo J, Buil JA, Castilla M. Endoscope-assisted transscleral suturefixation of intraocular lenses. J Cataract Refract Surg 1996;22(7):879-881.
14 Kaplowitz K, Kuei A, Klenofsky B, Abazari A, Honkanen R. The use of endoscopic cyclophotocoagulation for moderate to advanced glaucoma.Acta Ophthalmol 2015;93(5):395-401.
15 Eguchi S, Araie M. A new ophthalmic electronic videoendoscope system for intraocular surgery. Arch Ophthalmol 1990;108(12):1778-1781.
16 Wong SC, Lee TC, Heier JS, Ho AC. Endoscopic vitrectomy. Curr Opin Ophthalmol 2014;25(3):195-206.
17 Hubbard GI, Engelbrecht N, Wong S, Lee T. Endoscopic vitrectomy in children. Retina Times 2012;30:18-20.
18 Kawashima S, Kawashima M, Tsubota K. Endoscopy-guided vitreoretinal surgery. Expert Rev Med Devices 2014;11(2):163-168.
19 Boscher C, Kuhn F. An endoscopic overview of the anterior vitreous base in retinal detachment and anterior proliferative vitreoretinopathy.Acta Ophthalmol 2014;92(4):e298-e304.
20 Marra KV, Yonekawa Y, Papakostas TD, Arroyo JG. Indications and techniques of endoscope assisted vitrectomy. J Ophthalmic Vis Res 2013;8(3):282-290.
21 Liu JQ, Li FM. Practical ophthalmology, second edition. People’s Medical Publishing House 2002:30-33.
22 Murthy GJ, Murthy PR, Murthy KR, Kulkarni VV, Murthy KR.A study of the efficacy of endoscopic cyclophotocoagulation for the treatment of refractory glaucomas. Indian J Ophthalmol 2009;57(2):127-132.
23 Boscher C, Kuhn F. Endoscopic evaluation and dissection of the anterior vitreous base. Ophthalmic Res 2015;53(2):90-99.
24 Stephen J. Ryan, David R. Hinton. Retina, Fifth Edition, Volume I, part two: 482-516.
25 Uram M. Laser endoscope in the management of proliferative vitreoretinopathy. Ophthalmology 1994;101(8):1404-1408.
26 Faude F, Wiedemann P. Vitreoretinal endoscope for the assessment of the peripheral retina and the ciliary body after large retinectomies in severe anterior PVR. Int Ophthalmol 2004;25(1):53-63.
27 Ciardella AP, Fisher YL, Carvalho C, Slakter JS, Bryan RG, Sorenson JA, Spaide RF, Freund KB, Guyer DR, Yannuzzi LA. Endoscopic vitreoretinal surgery for complicated proliferative diabetic retinopathy.Retina 2001;21(1):20-27.
28 Lee GD, Goldberg RA, Heier JS. Endoscopy-assisted vitrectomy and membrane dissection of anterior proliferative vitreoretinopathy for chronic hypotony after previous retinal detachment repair. Retina 2016;36(6):1058-1063.
29 Kita M, Yoshimura N. Endoscope-assisted vitrectomy in the management of pseudophakic and aphakic retinal detachments with undetected retinal breaks. Retina 2011;31(7):1347-1351.
30 de Smet MD, Mura M. Minimally invasive surgery-endoscopic retinal detachment repair in patients with media opacities. Eye (Lond)2008;22(5):662-665.
31 Sabti KA, Raizada S. Endoscope-assisted pars plana vitrectomy in severe ocular trauma. Br J Ophthalmol 2012;96(11):1399-1403.
32 Shaikh AH, Khatana AK, Zink JM, Miller DM, Petersen MR, Correa ZM, Riemann CD. Combined endoscopic vitrectomy with pars plana tube shunt procedure. Br J Ophthalmol 2014;98(11):1547-1550.
33 Tarantola RM, Agarwal A, Lu P, Joos KM. Long-term results of combined endoscope-assisted pars plana vitrectomy and glaucoma tube shunt surgery. Retina 2011;31(2):275-283.
34 Hammer ME, Grizzard WS. Endoscopy for evaluation and treatment of the ciliary body in hypotony. Retina 2003;23(1):30-36.
35 Ren H, Jiang R, Xu G, Chang Q, Lv J, Chen Q, Wang W. Endoscopyassisted vitrectomy for treatment of severe endophthalmitis with retinal detachment. Graefes Arch Clin Exp Ophthalmol 2013;251(7):1797-1800.
36 Martiano D, L’helgoualc’h G, Cochener B. Endoscopy-guided 20-G vitrectomy in severe endophthalmitis: report of 18 cases and literature review. J Fr Ophtalmol 2015;38(10):941-949.
37 Boscher C. Endoscopy-assisted vitrectomy for severe endophthalmitis.Retin Physician 2012;9:38-58.
38 Zhang J, Han F, Zhai X. Clinical analysis of 23-gauge vitrectomy for the treatment of acute endophthalmitis after cataract surgery. Eur J Ophthalmol 2015;25(6):503-506.
39 De Smet MD, Carlborg EA. Managing severe endophthalmitis with the use of an endoscope. Retina 2005;25(8):976-980.
40 Boscher C, Lebuisson DA, Lean JS, Nguyen-Khoa JL. Vitrectomy with endoscopy for management of retained lens fragments and/or posteriorly dislocated intraocular lens. Graefes Arch Clin Exp Ophthalmol 1998;236(2):115-121.
41 Olsen TW, Pribila JT. Pars plana vitrectomy with endoscope-guided sutured posterior chamber intraocular lens implantation in children and adults. Am J Ophthalmol 2011;151(2):287-296.
42 Shah VA, Gupta SK, Chalam KV. Management of vitreous loss during cataract surgery under topical anesthesia with transconjunctival vitrectomy system. Eur J Ophthalmol 2003;13(8):693-696.
43 Hikichi T, Kitamei H, Kosaka S, Shioya S, Takami K. Intraoperative endoscopic observation of sclerotomy site after cannula removal for 23-gauge vitrectomy. Clin Ophthalmol 2014;8:477-481.
44 Kishi S. Vitreous anatomy and the vitreomacular correlation. Jpn J Ophthalmol 2016;60(4):239-273.
45 Ioannidis AS, Barton K. Cyclodialysis cleft: causes and repair. Curr Opin Ophthalmol 2010;21(2):150-154.
46 Campochiaro PA. Ocular neovascularization. J Mol Med 2013;91(3):311-321.
47 Manabu Sasahara, Junichi Kiryu, Nagahisa Yoshimura. Endoscopeassisted transscleral suturefixation to reduce the incidence of intraocular lens dislocation. J Cataract Refract Surg 2005;31:1777-1780.
48 Sonoda Y, Yamakiri K, Sonoda S, Uchino E, Doi N, Sakamoto T.Endoscopy-guided subretinal fluid drainage in vitrectomy for retinal detachment. Ophthalmologica 2006;220(2):83-86.