INTRODUCTION
Limbal dermoids are congenital benign tumors consisting of tissues of ectodermal and mesodermal origin, most commonly located in the inferotemporal quadrant[1]. They may occur as single or multiple lesions and have a raised, whitish,finely-vascularized bulbous appearance[2]. These slow growing lesions are occasionally associated with a decreased visual acuity due to astigmatism, obscuration of the visual axis, and the presence of coexisting amblyopia[3]. Indications for surgical excision include astigmatism, involvement of the visual axis,poor cosmesis, and discomfort due to the cilia or disturbance to the ocular surface tearfilm[3-5]. Nevertheless, it is not clear what the most appropriate surgical procedure is and when it should be carried out[3,6]. This varies depending on the lesion’s size and area of involvement, as well as the patient’s request for removal due to psychosocial and cosmetic concerns[6]. Possible surgical approaches include simple excision or shaving of the lesion, superficial keratectomy, and lamellar or penetrating keratoplasty. The literature is limited in the number of studies reporting the surgical results of limbal dermoids. The purpose of this study is to describe a technique of lamellar keratoplasty for the removal of congenital limbal dermoids and to evaluate postoperative outcomes.
METHODS
The charts of all patients who underwent limbal dermoid excision with lamellar corneoscleral graft transplantation at the Bascom Palmer Eye Institute of the University of Miami Miller School of Medicine, between January 2003 and July 2013, were retrospectively reviewed. Patient demographic data were collected. For all patients, preoperative and postoperative photographs were obtained to assess cosmetic results. Dermoid size and the meridian on which the lesion was situated were recorded. Comparisons of best-corrected visual acuity (BCVA),astigmatic power and spherical equivalent, before and after surgery, were performed. Due to the small patients’ number non-parametric statistical tests were used. Vector analysis was used to evaluate the change in astigmatism after surgery.
The study was approved by the Internal Review Board of the University of Miami Miller School of Medicine and complied with the principles outlined in the Declaration of Helsinki.
Surgical Technique Surgeries were performed under general anesthesia or with peribulbar nerve block and intravenous sedation. The limbal dermoid was measured and its corneal and scleral borders were marked. The conjunctiva was dissected and undermined to reveal bare sclera around the lesion. Partial thickness trephination of the lesion using a trephine was done.This was followed by lamellar delineation starting from the corneal site and using a crescent knife. Lamellar delineationwas repeated as needed to dissect until most of the opacity was excised and the remaining deep cornea was relatively clear.Cautery was applied as needed. The tumor bed was measured separately for the corneal and scleral parts. A similar thickness corneoscleral graft was trephined from a whole globe donor.Grafts were the same size as the lesions size of oversized by 0.25-0.5 mm. The corneoscleral graft was then dissected in a lamellar fashion using a crescent knife. The graft was secured to the host cornea and sclera using interrupted 10-0 nylon sutures. Finally, the conjunctiva was sutured with 8-0 vicryl sutures. At the end of the procedure, subconjunctival antibiotics and corticosteroids were injected. Starting the following day, patients were given trimethoprim/polymyxin B drops 4 times a day and prednisolone acetate drops 4 times a day. Trimethoprim/polymyxin B drops were given until cornea was fully epithelized and prednisolone acetate drops were slowly tapered down, typically during a 6mo period. No additional immunosuppressant medications were given. All surgeries and follow up appointments were performed by one surgeon (Forster RK). Histopathologic confirmation of limbal dermoids was verified in all lesions.
Table 1 Demographic data for patients who underwent a corneoscleral graft for limbal dermoids

IT: Inferotemporal; I: Inferior; T: Temporal; NV: Neovascularization.
Table 2 Preoperative and postoperative visual acuity and refractive data for patients who underwent corneoscleral graft for limbal dermoids
BCVA: Best-corrected visual acuity (Snellen).
RESULTS AND DISCUSSION
Eight patients were included in the study-all were operated on as primary surgical treatment. Follow-up time was 31.1±19.6mo. Patient No.4 missed follow-up. Indications for surgery included unfavorable cosmetic appearance, high astigmatism or ocular irritation. The mean age of patients on the day of surgery was 13.0±9.0y (range 6.0-33.0y, 62.5%female). Six patients had a single limbal dermoid. One patient had 3 adjacent limbal dermoids and 1 patient had an additional lipodermoid which was not excised. Mean dermoid size was 7.75±1.83 mm (range 6.0-12.0 mm) and mean corneoscleral graft was 7.88±1.79 mm (range 6.0-12.0 mm).The most common location of the dermoid was inferotemporal(Table 1).
Mean BCVA before and after surgery was 0.18±0.17 (20/31,range 20/20-20/50) and 0.17±0.20 (20/31, range 20/15-20/50),respectively (P=0.29, Wilcoxon Signed rank test). Spherical equivalent was 1.3±1.6 D before surgery and 0.7±1.5 D after surgery (P=0.40, Wilcoxon Signed rank test). The mean astigmatic power before and after surgery was 2.4±2.0 D and 1.5±1.3 D, respectively (P=0.17, Wilcoxon Signed rank test).Vector analysis revealed a mild change in the astigmatism with mean “d” of 3.6±2.2 (0.56-6.89) (Table 2).
No intra- or postoperative complications were documented. No rejection events occurred and none of the grafts failed during the follow-up period. In general, patients had good cosmetic outcome with good anatomic integrity (Figures 1, 2).
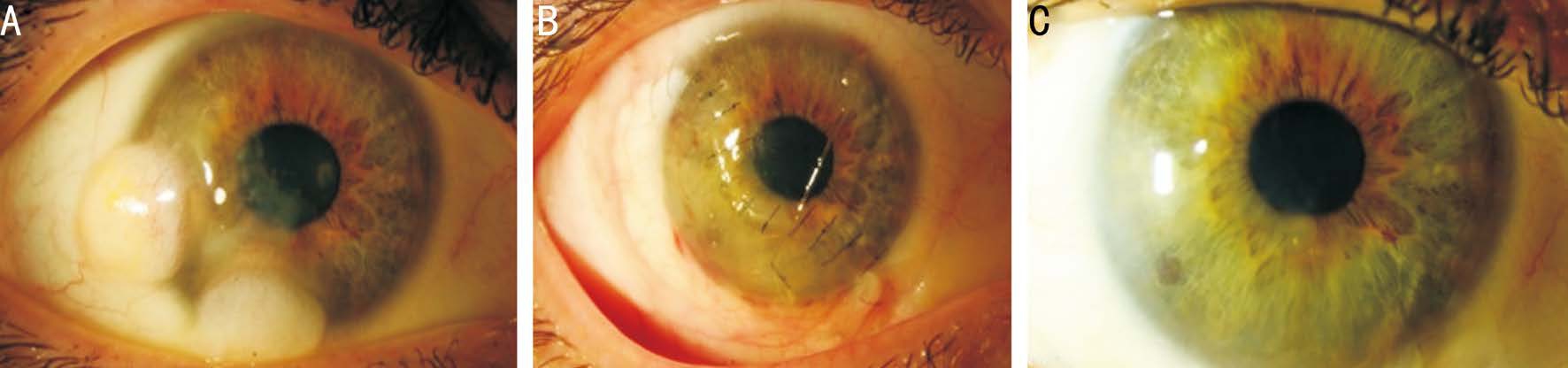
Figure 1 Patient No.7 A: Limbal dermoids before surgery; B: One month after removal of the limbal dermoid with corneoscleral graft; C: Four years after surgery. There is a very mild opacity at the cornea graft-host junction.
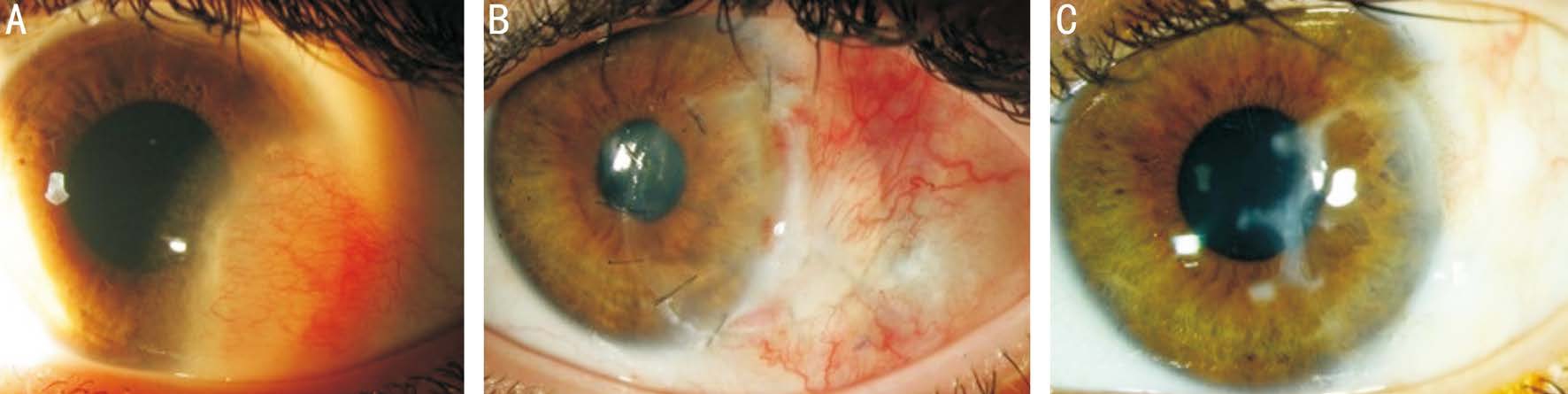
Figure 2 Patient No.5 A: Limbal dermoid before surgery; B: One month after removal of the limbal dermoid with corneoscleral graft; C: One year after surgery. There is moderate opacity/scarring at the cornea graft-host junction, possibly because of graft-host thickness difference or due to wound gape.
In the current study we found that lamellar excision of large limbal dermoids with matched partial thickness corneoscleral grafts is associated with favorable long-term cosmetic outcomes. The procedure is safe and demonstrates a very low risk for postoperative complications. Corneal astigmatism may decrease after the surgery, but the change is expected to be mild and insignificant.
Possible indications for surgical intervention include corneal astigmatism, growth and encroachment over the pupillary area (by the tumor itself or the lipid ring), poor cosmesis,uncertain diagnosis, irritation or foreign body sensation,dellen, and inadequate lid closure[1-2,7]. It could be assumed that lamellar dissection of the lesion will act like a relaxing incision and that deeper dissection would flatten the cornea in the same meridian, however it cannot be confirmed from our data. According to our results, visual acuity and the degree of astigmatism did not change significantly following surgery.Our results are in agreement with most previous studies[3-5,8].Robb[5]reported that the preoperative astigmatism persisted postoperatively with little change in its orientation or value,regardless of age at the time of surgery. On the other hand,anecdotal reports suggest that early surgery may reduce astigmatism[9]. To contrast with these reports, Watts et al[3]found that a significant number of patients had an increase in astigmatism postoperatively. Shen et al[10]reported that patients with preoperative astigmatism of 6 diopters or more were found to have a marked decrease in astigmatism after surgery,and that patients with less astigmatism were found to have an insignificant increase in astigmatism after surgery.
At this time, the surgical approach to limbal dermoids depends mainly on surgeon preference and experience. Size and depth of the lesion are other important factors. Surgical approaches include simple excision, superficial keratectomy,lamellar keratoplasty and penetrating keratoplasty[6]. Simple keratectomy with corneal tattooing showed a good cosmetic outcome, and could be an alternative option for surgery,especially when a donor cornea is not available[11]. However,persistent keratoepithelial defect, postoperative corneal vascularization, pseudopterygium and scar formation are the major complications reported after simple shaving[8,12]. A recent study suggested adding sutureless limboconjunctival autograft in order to overcome the remaining corneal opacity and conjunctivalization which are a result of limbal cell deficiency[13].
Complications relating to penetrating keratoplasty include wound leak, glaucoma, graft rejection and failure. All of these complications are rare in anterior lamellar keratoplasty[14].Our preferred surgical technique for limbal dermoids is partial thickness trephination followed by lamellar delineation with partial-thickness corneoscleral graft implantation. If the dermoid is very superficial then simple keratectomy with or without tattooing might be sufficient, however in all our cases the dermoid was embedded in the cornea and simple keratectomy was inadequate. For our surgical approach,the presence of a translucent cornea deep to the lesion is mandatory. If the dermoid involves the anterior chamber, then penetrating keratoplasty is indicated. In some instances, it is hard to know whether the dermoid has penetrated the cornea into the anterior chamber. Deep pigmentation in the lesion may suggest dermoid-iris touch. Anterior segment ocular coherence tomography can aid with the preoperative planning, although this test may be hard to accomplish in young children. A thorough slit lamp examination in all of our cases revealed a deep translucent corneal tissue. Therefore, there was no need for anterior segment ocular coherence tomography.
The advantages of lamellar delineation dermoid excision with corneoscleral graft transplantation include good host-graft apposition and anatomically matched cornea-sclera to the missing cornea and sclera, respectively. This technique might offer an additional advantage in young children in whom the immunologic system is very active. Graft failure attributable to graft rejection seen in penetrating keratoplasty is rare in anterior lamellar keratoplasty[15]. Nevertheless, this technique is undoubtedly more challenging than simple excision or full keratoplasty. The surgeon should be experienced in shelving the limbal dermoid and avoiding Descemet membrane perforation, which is the main challenge in this technique, as well as dissecting a uniform thickness lamellar corneoscleral graft. Variation in the surgical technique is possible, as whether to start the lamellar dissection from the corneal or the scleral site and whether to use a crescent knife or a blunt instrument,which may provide smoother dissection with decreased risk of perforation. We use a whole globe donor which offers the surgeon the option to prepare a lamellar corneoscleral graft which will best-fit the corneoscleral host. Nevertheless, if Descemet membrane perforation occurs during the dermoid dissection, the surgeon can easily convert to penetrating keratoplasty.
The retrospective nature and small sample size of our study are its main limitations. Indeed, limbal dermoid surgery is not a common procedure as this is a rare tumor with a prevalence of 1 in 10 000[1]. None of the patients had graft rejection. As mean follow-up time was 31.1mo and graft rejection can occur many after keratoplasty, it is possible that longer follow up will show graft rejection episodes in some patients. If graft rejection occurs then intensive topical corticosteroids treatment is needed with possibly short course of oral corticosteroids.In these cases, failure of the graft is possible, resulting in poor cosmetic outcome. When dermoid is small, asymptomatic and cosmetically acceptable, surgery is not indicated. When surgery is needed, we believe that lamellar corneoscleral keratoplasty offers an excellent option. This technique is safe and offers good appearance and tectonic stability. No deterioration in the visual acuity is expected postoperatively.Significant improvement in the amount of astigmatism is not likely to occur following surgery.
ACKNOWLEDGEMENTS
Authors’ contributions:Conception and design (Spierer O,Gologorsky D, Adler E, Forster RK); Data collection (Spierer O, Gologorsky D); Analysis and interpretation of the data(Spierer O, Gologorsky D, Adler E, Forster RK); Provision of patients, materials, or resources (Forster RK); Administrative,technical, or logistical support (Gologorsky D, Adler E, Forster RK); Writing the article (Spierer O); Critical revision of article(Gologorsky D, Adler E, Forster RK); and Final approval of article (Spierer O, Gologorsky D, Adler E, Forster RK).
Foundations:Supported by the Research to Prevent Blindness Unrestricted Grant (New York, USA); the National Eye Institute core grant (No.P30-EY14801) (Bethesda, Maryland, USA).
conflicts of Interest:Spierer O, None; Gologorsky D, None;Adler E, None; Forster RK, None.
REFERENCES
1 Mansour AM, Barber JC, Reinecke RD, Wang FM. Ocular choristomas.Surv Ophthalmol 1989;33(5):339-358.
2 Pirouzian A. Management of pediatric corneal limbal dermoids. Clin Ophthalmol 2013;7:607-614.
3 Watts P, Michaeli-Cohen A, Abdolell M, Rootman D. Outcome of lamellar keratoplasty for limbal dermoids in children. J AAPOS 2002;6(4):209-215.
4 Scott JA, Tan DT. Therapeutic lamellar keratoplasty for limbal dermoids. Ophthalmology 2001;108(10):1858-1867.
5 Robb RM. Astigmatic refractive errors associated with limbal dermoids.J Pediatr Ophthalmol Strabismus 1996;33(4):241-243.
6 Pirouzian A, Ly H, Holz H, Sudesh RS, Chuck RS. Fibrin-glue assisted multilayered amniotic membrane transplantation in surgical management of pediatric corneal limbal dermoid: a novel approach. Graefes Arch Clin Exp Ophthalmol 2011;249(2):261-265.
7 Trubnik V, Conley R, Ritterband DC, Udell I, Thompson G, Shih C,McCormick SA, Milman T. Progressive growth in epibulbar complex choristomas: report of 2 cases and review of literature. Cornea 2011;30(11):1267-1269.
8 Panton RW, Sugar J. Excision of limbal dermoids. Ophthalmic Surg 1991;22(2):85-89.
9 Burillon C, Durand L. Solid dermoids of the limbus and the cornea.Ophthalmologica 1997;211(6):367-372.
10 Shen YD, Chen WL, Wang IJ, Hou YC, Hu FR. Full-thickness central corneal grafts in lamellar keratoscleroplasty to treat limbal dermoids.Ophthalmology 2005;112(11):1955.
11 Cha DM, Shin KH, Kim KH, Kwon JW. Simple keratectomy and corneal tattooing for limbal dermoids: results of a 3-year study. Int J Ophthalmol 2013;6(4):463-466.
12 Panda A, Ghose S, Khokhar S, Das H. Surgical outcomes of epibulbar dermoids. J Pediatr Ophthalmol Strabismus 2002;39(1):20-25.
13 Jeong J, Song YJ, Jung SI, Kwon JW. New surgical approach for limbal dermoids in children: simple excision, corneal tattooing, and sutureless limboconjunctival autograft. Cornea 2015,34(6):720-723.
14 Keane M, Coster D, Ziaei M, Williams K. Deep anterior lamellar keratoplasty versus penetrating keratoplasty for treating keratoconus.Cochrane Database Syst Rev 2014;(7):CD009700.
15 Ashar JN, Pahuja S, Ramappa M, Vaddavalli PK, Chaurasia S, Garg P. Deep anterior lamellar keratoplasty in children. Am J Ophthalmol 2013;155(3):570-574.e1.