INTRODUCTION
Fungal keratitis (FK) is a kind of aggressive eye disease which may lead to blindness without in-time treatment[1-3].The majority of FK occurs in tropical or subtropical climates as well as countries with significant agriculture-dependent populations, where ocular trauma is the most common reason.According to the epidemiological studies, FK is mainly caused by plant injury in developing countries and chronic ocular surface disease in developed countries; the other reasons of FK consist mainly of ocular trauma, refractive contact lens wearing as well as long-term addiction to antibiotic or corticosteroid[4-9].Considering of its substantial morbidity and high rate of blindness, seeking for effective solutions is in very urgent.
During the infection process of FK, stimulation of inflammation and clearance of fungi are of most importance. Aspergillus fumigatus (A. fumigatus) is one of the most common reasons that caused FK[10]. Pattern recognition receptors (PRRs)mediated the innate host responses by recognizing pathogenassociated molecular patterns (PAMPs). Well-known PRRs include Toll-like receptors, RIG-I-like receptors, NOD-like receptors, and C-type lectin receptors (CLRs)[11]. Among these PRRs, CLRs play a vital role in recognizing the complex structures, which are composed of carbohydrate residues, of various fungal pathogens[12]. Mincle (also known as Clec4e or Clecsf9) is a newly discovered CLR expressed mainly on innate immune cells such as macrophages, dendritic cells, B cells, and neutrophils[11,13]. Recent studies have shown that Mincle recognizes a variety of ligands from damage-associated molecular patterns (DAMPs), pathogen-associated molecularpatterns (PAMPs) to tumor-associated molecular pattern(TAMPs)[13-22]. Mincle is an activating receptor coupled with the Fc receptor g (FcRg) chain, triggering complex signaling cascades through the CARD9/Bcl10/MALT1 axis towards NF-κB p65 initiation[23]. Based on the above, our study is to explore how Mincle participates in the early stage of A.fumigatus keratitis in mice.
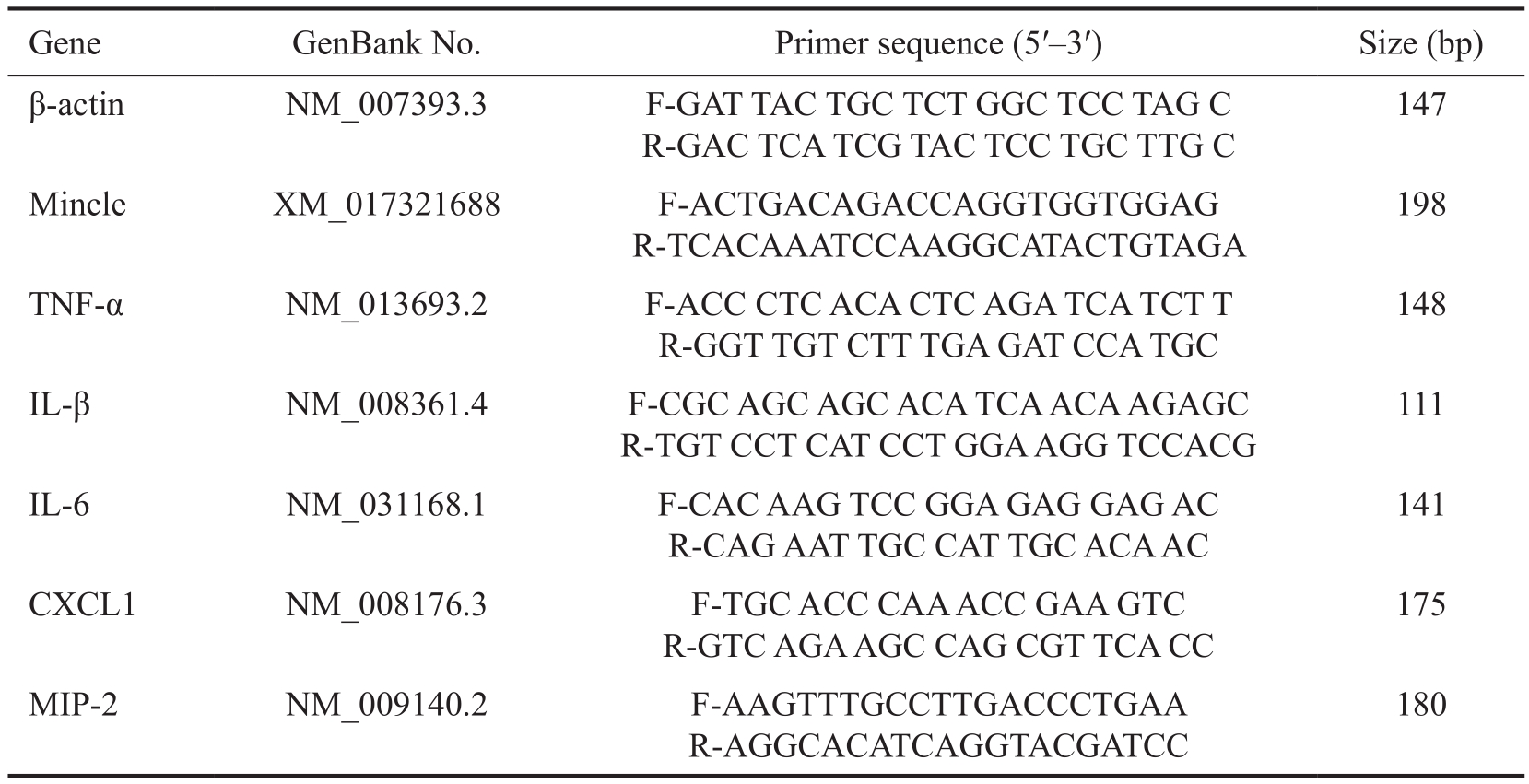
Table 1 Nucleo tide sequences of mouse primers for real-time RT-PCR
MATERIALS AND METHODS
Corneal Infection Female C57BL/6 mice (Cavens Laboratory Animal Ltd., Changzhou, China), 8 weeks old were anesthetized with chloral hydrate (400 mg/kg) by intraperitoneal injection and operated using a stereoscopic microscope at 40× magnification.Every single eye was examined before operation to eliminate the interference of other ocular diseases. Cornea epithelia in the center of right eyes were removed a 3-4 mm diameter size of circle, 5 μL aliquot containing 1×108CFU/mL of A. fumigatus were applied to the cornea surface (strain 3.0772, General Microbiological Culture Collection Center, Beijing, China).After that, corneas were covered with a self-made contact lens to prevent fungi loss. Left eyes were taken as control groups. Mice were examined at 1 and 3d post infection (dpi)to monitor the disease. Clinical score was recorded for each eye after infection according to an established scale[24]to grade the disease severity statistically and slit lamp examination was implemented daily using digital camera. Animals were treated humanely and in accordance with the regulations and guidelines of Qingdao University Institutional Animal Care as well as ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Mincle Neutralization and Agitation Goat anti-mouse Mincle neutralizing antibody (5 μg/5 μL, R & D Systems,Minneapolis, MN, USA) or control goat IgG (5 μg/5 μL, R & D Systems, Minneapolis, MN, USA) was subconjunctiv ally injected to the right eyes of C57BL/6 mice 1d before infection.After that, corneas were treated with A. fumigatus for1 to 3d.Mincle agonist TDB (2 μg/5 μL, Invivogen, San Diego, CA,USA) or control 0.5% DMSO (5 μL, Solarbio, Beijing, China)was subconjunctivally injected to the right eyes of C57BL/6 mice 1d before infection, followed with A. fumigatus infection for 1 to 3d.
Total RNA isolation and RT-PCR Corneas were collected and total RNA was separated using RNAiso plus reagent(TaKaRa, Dalian, China) according to the product manual, and then quantified with a NanoDrop 2000C Spectrophotometers(Thermo Scientific, West Palm Beach, FL, USA). Total RNA(1 μg) was converted to cDNA using the reverse transcription kit (TaKaRa, Dalian, China) and then amplified using SYBR Premix Ex Taq (TaKaRa, Dalian, China) as described in the previous article[25]. Relative mRNA levels were determined by threshold cycle (CT) method compared with normalization to β-actin. Each experiment was repeated three times. Primer sequences are listed in the Table 1.
Mincle and Polymorphonuclear Immunostaining Mice eye balls were cleaned by 0.01 mol/L PBS and then embedded in OCT compound (Tissue-Tek; Miles, Elkhart, IN, USA). After frozen with liquid nitrogen, 10 micrometer sections were cut in the horizontal plane by Frozen Slicer (Leica, Germany).Slides were fixed in acetone for 10min, washed with 0.01 M PBS and then blocked with blocking solution [0.01 mol/L PBS, 2.5% BSA and Donkey IgG (1:100)] for 30min at room temperature. The primary antibody for Mincle is rabbit antimouse Mincle antibody (1:200, Bioss, Boston, USA), primary antibody for neutrophil is rabbit anti-polymorphonuclear(anti-PMN) antibody NIMP-R14 (1:100, Santa Cruz, USA).Secondary antibody for both Mincle and PMN is donkey anti-rabbit antibody-FITC. The protocol was described previously[25]. After immunostaining, sections were observed with fluorescence microscopy (Leica, Germany).
Enzyme-linked Immunosorbent Assay All individual corneas taken for enzyme-linked immunosorbent assay(ELISA) analysis were homogenized in 500 μL PBS with 0.1% Tween 20 with a protease inhibitor cocktail (Cwbiotech,Beijing, China) and centrifuged at 5000 g for 10min. Normal and infected corneas were removed at 1 dpi from C57BL/6 mice (n=6/group/time) and 100 μL of each sample was assayed in duplicate for IL-1β, TNF-α, and IL-6 protein according to the manufacturer’s instructions (Dakewe, Shenzhen, China).
Griess Assay Corneas were pretreated with agonist TDB or neutralizing antibody along with their negative control DMSO or IgG. Each cornea was split with cell and tissue lysis buffer for nitric oxide (NO) assay (Beyotime, Shanghai, China), and supernatant of each sample was collected. NO levels were determined using the Griess reagent (Beyotime, Shanghai,China) by measuring levels of the stable end product nitrite.The results were expressed as the mean micromoles of nitrite per sample 6 SEM.
Statistical Analysis Differences were considered significant for P<0.05 in comparing two measurements. Comparison among groups was evaluated by unpaired, two-tailed Student’s t-test. All experiments were done in triplicate. The data from a representative experiment were expressed as mean±SD.
RESULTS
Mincle Expression in Corneas of C57BL/6 Mice During A.fumigatus Infection C57BL/6 mice corneas were infected with A. fumigatus and the Mincle mRNA levels were detected in normal, 12h, 1, 3d after infection respectively. There were little amount of Mincle mRNA expressed in the normal cornea tissue. When stimulated with A. fumigatus, Mincle mRNA levels were significantly elevated and peaked at 1d (Figure 1A; P<0.01). Then we choose 1d as infection time to perform the following experiments. C57BL/6 mice were divided into groups randomly, and were pretreated with Mincle agonist TDB or Mincle neutralizing antibody (MincleAb), taking DMSO or IgG as control reagent before infection. Mincle mRNA levels were detected by RT-PCR. As shown in the pictures, Mincle mRNA levels increased significantly when pretreated with TDB and decreased when pretreated with MincleAb correspondingly (Figure 1B, 1C; P<0.01, P<0.01)The expression of Mincle protein levels also showed the same trend when determined with immunostaining (Figure 1D, 1E).
Influence of Mincle on the Prognosis of Mice Fungal Keratitis Photographs taken by slit-lamp microscope show that Mincle agonist TDB treatment (Figure 2C) reduced the severity of FK compared with the DMSO control group(Figure 2B) on 1d after A. fumigatus infection. There were more chance for mice in the TDB group to survive compared with DMSO control group. A significant difference was also observed in clinical scores of the TDB treated corneas versus the DMSO control group after infection (Figure 2D; P<0.01).On the contrary, corneas pretreated with MincleAb suffered from severe FK characterized with larger ulcer proportion,dense opacity or even corneal perforation (Figure 2G). Also,there are significant difference in clinical scores between the MincleAb treated corneas and the IgG control group on 1dpi(Figure 2H; P<0.01).
In order to investigate the deeper mechanism between Mincle expression and the prognosis of mice FK, we conducted a series of experiments subsequently, focused on how Mincle affected the expression of different cytokines and chemokines.
Effect of Mincle on Cytokine Expression In order to investigate how cytokines changed along with the upregulation and down-regulation of Mincle, mice corneas were divided into different groups randomly.
Firstly, we explored the expression of cytokines when corneas were pretreated with Mincle agonist TDB. C57BL/6 mice were divided into 4 groups randomly: blank control group,AF group, DMSO+AF group and TDB+AF group. Among them, TDB and DMSO were injected subconjunctive 1d before the surgery. The mRNA and protein levels of IL-1β,TNF-α, IL-6 were detected by RT-PCR and ELISA. Our data showed that mRNA levels of IL-1β,TNF-α and IL-6 were significantly increased in the TDB+AF group compared with DMSO+AF group, as well as in AF group compared with blank control group, both P<0.01 (Figure 3A-3C). There was no statistically significant between AF group and DMSO+AF group. As for the protein levels of cytokines above, data from ELISA experiments indicated that IL-1β, TNF-α, IL-6 protein levels were significantly promoted in the TDB+AF group compared with DMSO+AF group (Figure 3D-3F; P<0.01). It is consistent with the trend of gene change.
After that we suppressed Mincle expression with neutralizing antibody to investigate the corresponding change of cytokines in mice cornea. Similarly, the mice were divided into 4 groups randomly: blank control group, AF group, MincleAb+AF group and IgG+AF group. The results were presented as below.The mRNA levels of IL-1β, TNF-α and IL-6 got reduced on different degrees after infection pretreated with MincleAb compared with the group pretreated with IgG (Figure 4A-4C;P<0.01). The changes of protein expressions were consistent with gene (Figure 4D-4F; P<0.01).
Effect of Mincle on Expression of Chemokines and Neutrophil Infiltration The mRNA levels of CXC chemokine ligand 1(CXCL1) and macrophage inflammatory (MIP)-2 in mice FK were detected when Mincle expression was up-regulated with TDB or down-regulated with MincleAb. Grouping method is the same with the previous section. Both of the two chemokines were significantly increased in TDB+AF group compared with DMSO+AF control group (Figure 5A, 5B;P<0.01) and decreased in MincleAb+AF group compared with IgG+Ab group (Figure 5D, 5E; P<0.01).
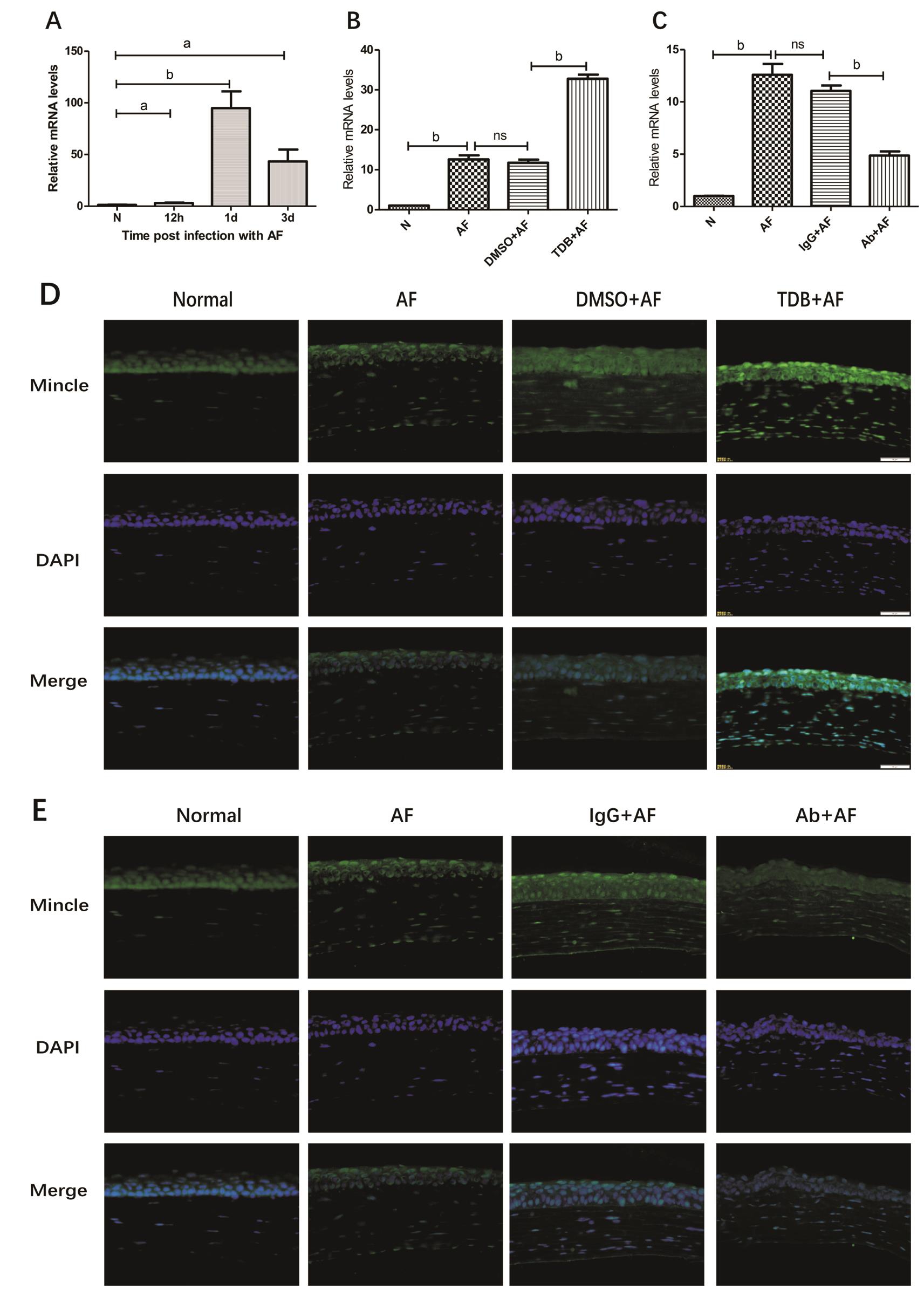
Figure 1 Mincle expression in corneas of C57BL/6 mice during A. fumigatus infection A: Relative mRNA levels of Mincle in mice corneas at different time points after infection; B, C: Relative mRNA levels of Mincle in mice corneas when pretreated with Mincle agonist TDB(TDB+AF) and Mincle neutralizing antibody (Ab+AF) along with their control agent DMSO (DMSO+AF) and IgG (IgG+AF) in 1dpi; D, E:Immuno fluorescence images (×400) showing expression of Mincle in the corneas of normal, AF, DMSO+AF, TDB+AF, IgG+AF and Ab+AF on 1dpi. Blue: Nuclear staining (DAPI); Green: Mincle staining. Magnification: 40×.aP<0.05,bP<0.01.
Followed with the expression of chemokines, we investigated the degree of neutrophil in filtration using immunostaining. As shown in the pictures, neutrophils can be hardly discovered in normal cornea tissues. After A. fumigatus infection, the number of neutrophils was obviously increased. What’s more, in the TDB pretreated group we observed a great deal of neutrophils gathered around the lesion site (Figure 6A), and in the MincleAb group,aggregation of neutrophils was inhibited (Figure 6B).
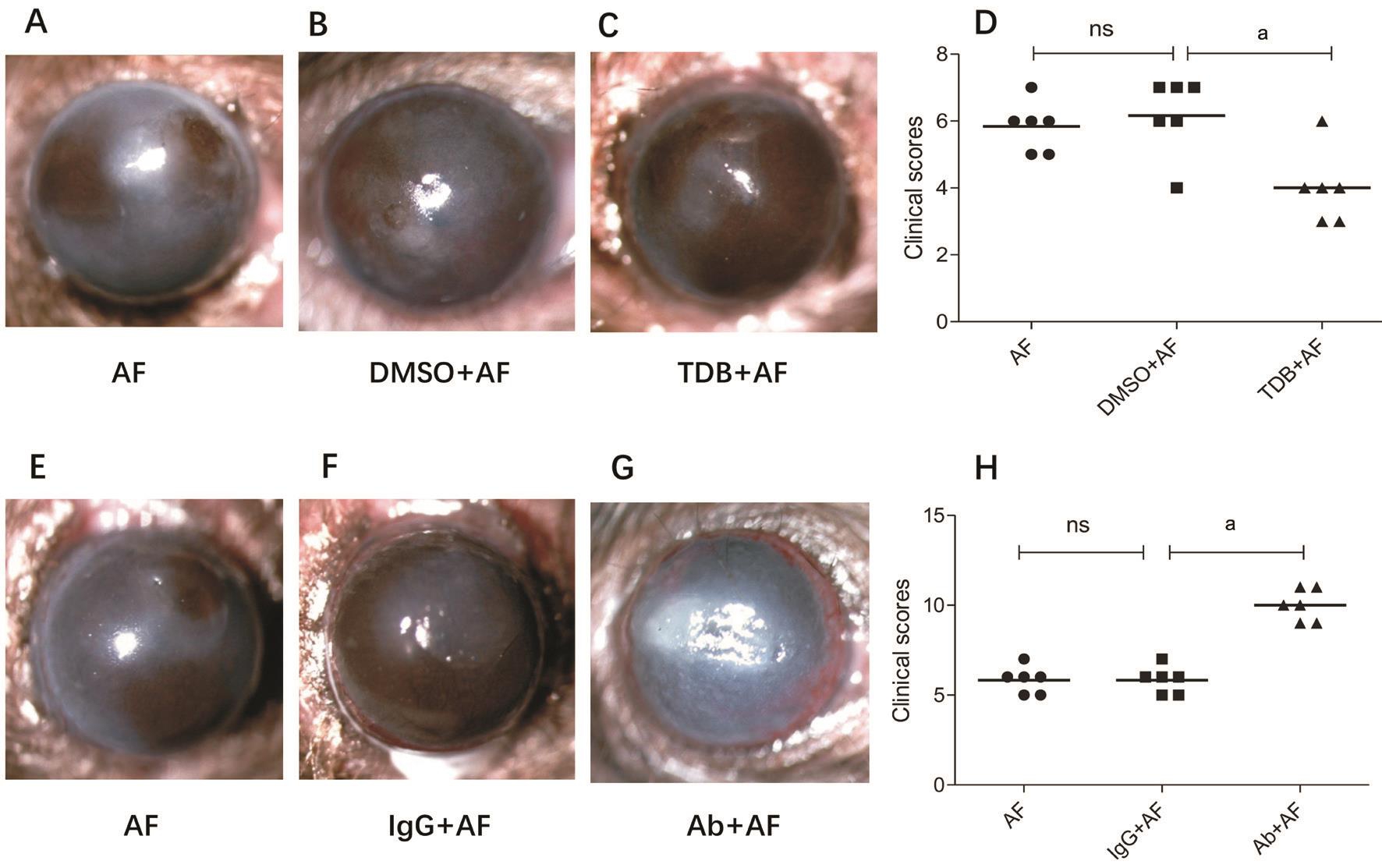
Figure 2 Mincle influences the prognosis of mice FK A-C, E-G: Photographs taken by slit-lamp microscope of mice corneas 1d post A.fumigatus infection when pretreated with DMSO, TDB, IgG or MincleAb; D, H: Clinical scores of the different treatment groups.aP<0.01.
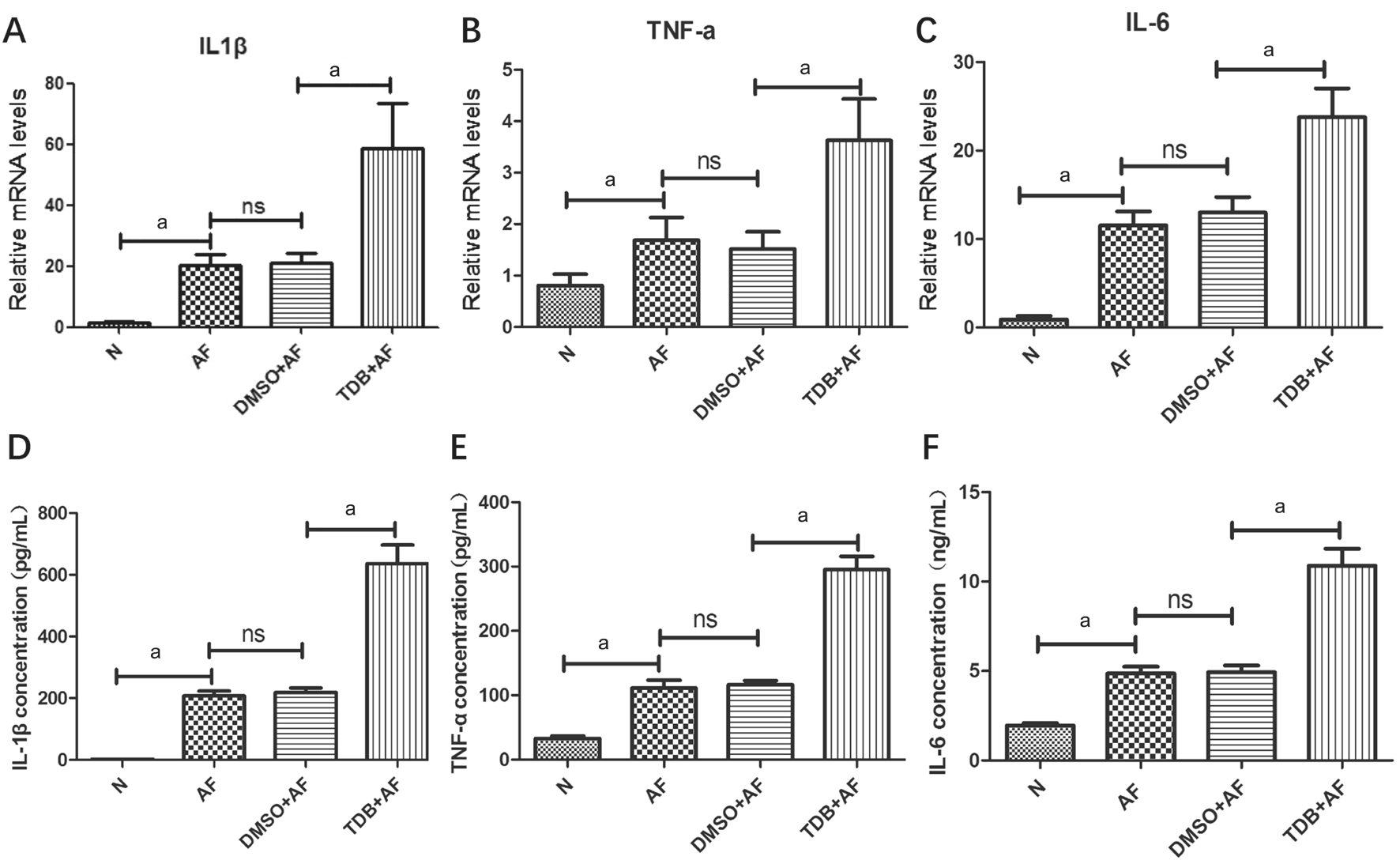
Figure 3 The expression of cytokines when Mincle is agonist by TDB Relative mRNA levels of IL-1β (A), TNF-α (B) and IL-6 (C) in corneas of different mice groups 1dpi treated with DMSO or TDB. Protein concentration of IL-1β (D; pg/mL), TNF-α (E; pg/mL) and IL-6 (F; ng/mL)in corneas of different mice groups 1dpi treated with DMSO or TDB.aP<0.01.
Regulation of Mincle on the Release of NO in Mice Corneas Next, to investigate how Mincle regulates the release of NO in mice corneas, the concentrations of NO were measured by Griess reaction before and after infection. Mice corneas were divided into 6 groups randomly: normal, AF infected without other treatment, DMSO+AF, TDB+AF, IgG+AF and MincleAb+AF. In our study, NO concentration was statistically increased when pretreated the cornea with Mincle agonist TDB compared with the DMSO control group, and approximately two times higher compared with the stable normal group (Figure 5C; P<0.01). Simultaneously, the degree of NO concentration was decreased when pretreated with MincleAb compared with IgG control group (Figure 5F;P<0.01). Summing up the above, we can perceive that there was a significantly positive correlation between Mincle expression and NO concentration.
DISCUSSION
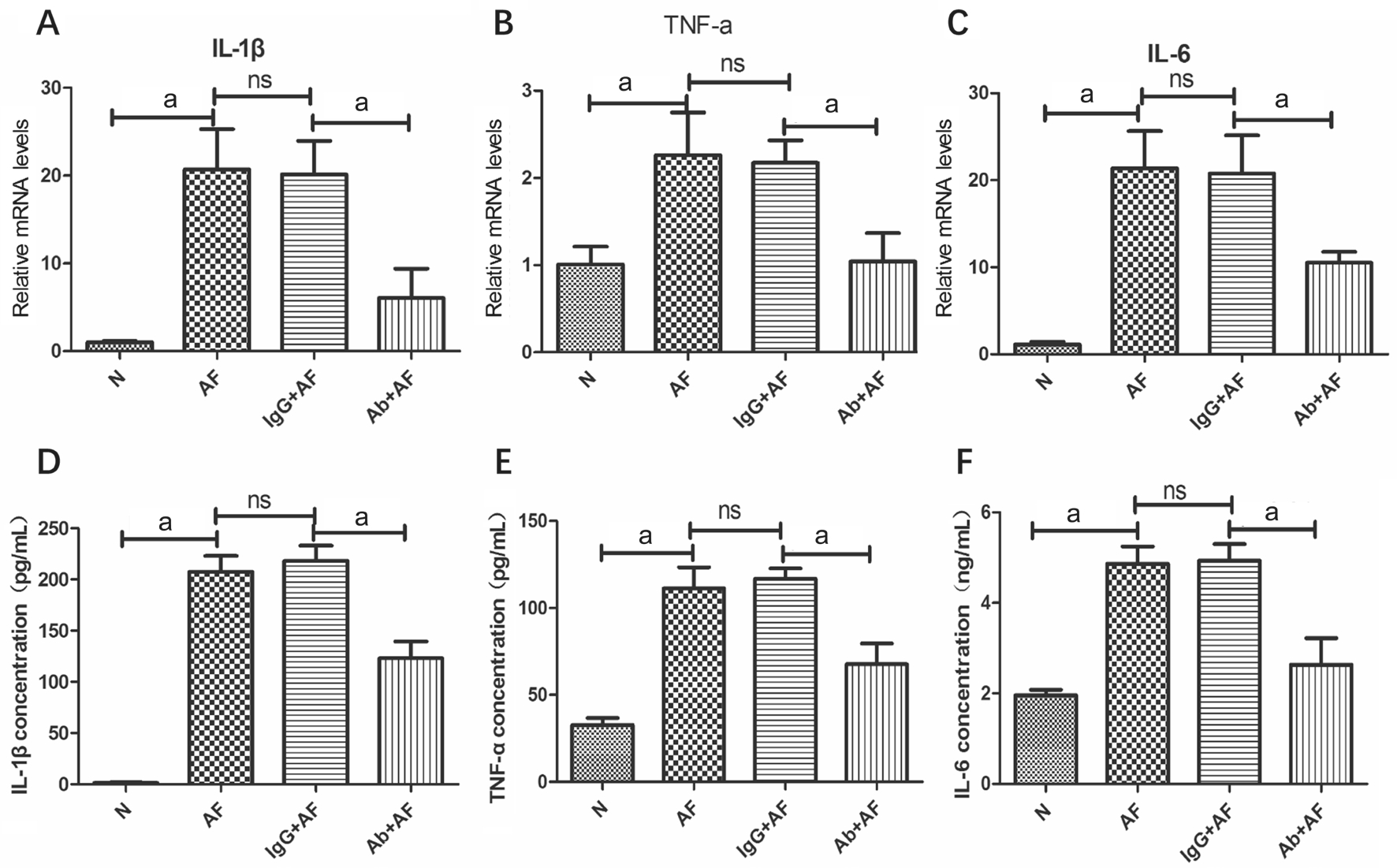
Figure 4 The expression of cytokines when Mincle is inhibited by Mincle neutralizing antibody Relative mRNA levels of IL-1β (A), TNF-α(B) and IL-6 (C) in corneas of different mice groups 1dpi treated with IgG or MincleAb. Protein concentration of IL-1β (D; pg/mL),TNF-α (E; pg/mL) and IL-6 (F; ng/mL) in corneas of different mice groups 1dpi. treated with IgG or MincleAb.aP<0.01.
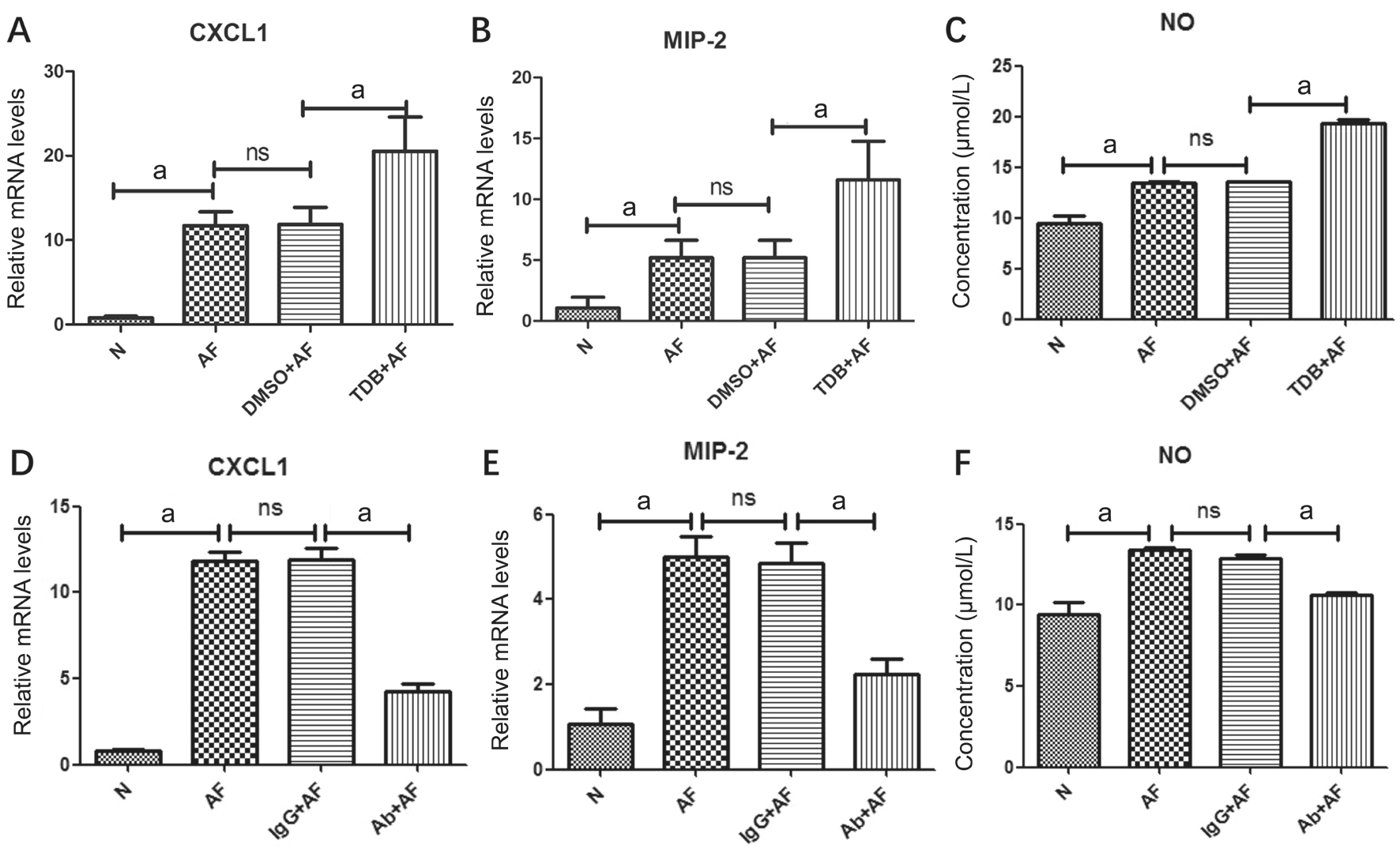
Figure 5 Effect of Mincle in expression of chemokines and nitric oxide Relative mRNA levels of CXCL1 and MIP-2 in corneas pretreated with TDB (A and B) or MincleAb (D and E) on 1dpi. The concentration of NO released by corneas in different mice groups on 1dpi(C and F).aP<0.01.
FK is a serious eye disease all over the world. It has higher rate of blindness compared with other ocular disease[1]. The incidence is increasing in Asia and Africa where countries rely mainly on agriculture and eye trauma (especially plant injury) is common[5-8]. Fungal infection of cornea often leads to corneal ulcer, perforation or even loss of vision and eyeball.It is a serious problem affecting the agrarian poor and hence researches on strategies to improve the prognosis are of great importance[26].Mincle is a novel type II transmembrane protein consists of 219aa with a highly conserved C-type lectin domain. As a newly found CLR, Mincle has been proved to recognize various kinds of ligands such as mycobacteria, fungus,damaged cells and tumor. It was found to be expressed mainly on antigen presenting cells such as macrophages, neutrophils,dendritic cells, B cells and so on[27]. Recent studies also found Mincle expressed in non-immune cells. The expression of Mincle increased in neurons and endothelial cells after ischemic stroke in both mice and humans[20,28]. Miyake et al[22]reported that Mincle expression is very low in the steadystate condition but is strongly up-regulated when stimulated by different inflammatory signals. In our study, animal model of FK was built with A. fumigatus. Mincle mRNA and protein levels can be determined in normal corneas in a relative low degree. And these data were significantly increased 1d after infection, illustrating that Mincle is an important receptor to recognize A. fumigatus. Taking together with the result of clinical score, we can infer that Mincle plays a role in the process of FK in mice.
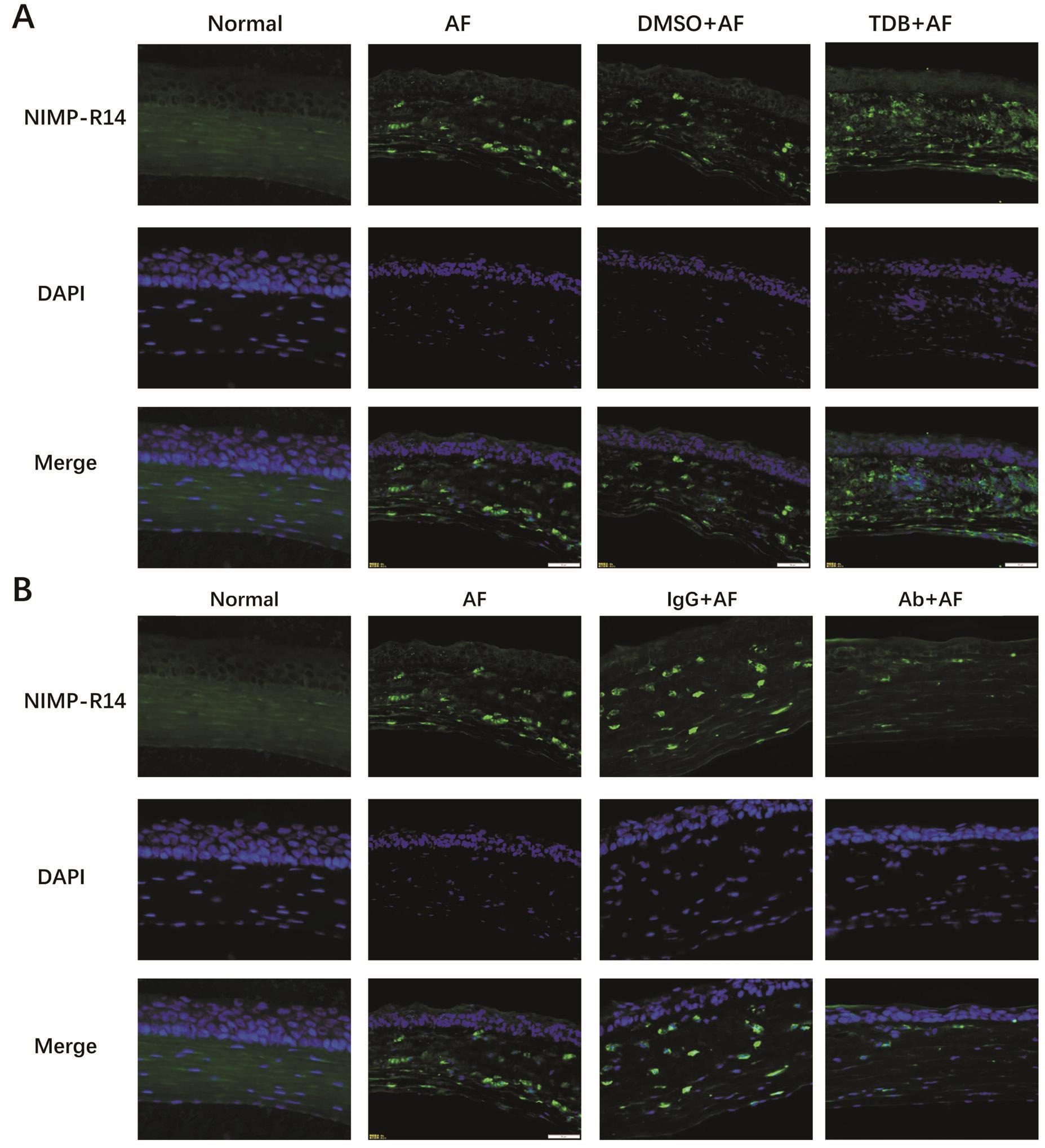
Figure 6 Mincle affected the degree of neutrophil in filtration Immunofluorescence images (×400) showing expression of neutrophils in the corneas of normal, AF, DMSO+AF, TDB+AF, IgG+AF and Ab+AF on 1dpi. Blue: Nuclear staining (DAPI); Green: Neutrophil (NIMP-R14)staining. Magnification: 40×.
Previous studies showed that Mincle recognized C. albicans and increased the production of TNF-α by macrophages. In addition, mice lacking of Mincle had a significantly increased susceptibility to systemic candidiasis[16]. According to the study of Kodar et al[29], GM-CSF derived bone marrow macrophages produced significant levels of the proinflammatory cytokines(IL-1β, IL-6, TNF-α) in a Mincle-dependent pathway. In our study, mRNA and protein levels of IL-1β, TNF-α and IL-6 of the cornea 1dpi post infection were detected by RT-PCR and ELISA respectively. From the data we can see that when up-regulated Mincle with TDB, cytokines above increased significantly. Meanwhile, when block Mincle with neutralizing antibody, the expression of these cytokines were suppressed.Based on the above, A. fumigatus can influence various kinds of cytokines through a Mincle-dependent pathway, thus regulate the immuno-inflammatory response in FK.
Recognition of foreign agent or invading organism by the PRRs is one of the major stimuli for activation of the immune response. When human body is infected by pathogens such as bacteria or fungi, this stimulation results in early arrival of neutrophils to the infected sites[30]. Neutrophils play a critical role in controlling infections in two ways, one is phagocytosis of the microorganisms and the other is releasing various kinds of mediators that draw other leukocytes into the injured tissue[31]. So it is vital to understand how these cells are recruited.MIP-2, also known as CXCL2 is one of the CXC chemokines.It serves as a potent chemotactic factor and affects neutrophil recruitment through the p38 MAPK-dependent signaling pathway[32]. CXCL1 and MIP-2 are important chemokines in neutrophil recruitment. We chose these two chemokines to investigate how Mincle influences the infiltration of neutrophils. Researches from Yamasaki et al[15]indicated that Malassezia-induced production of cytokines such as TNF-α,MIP-2 and CXCL1 was badly impaired in Mincle-deficient macrophages in vitro. In our experiment, CXCL1 and MIP-2 mRNA was significantly promoted in 1d after infection when pretreated with Mincle agonist TDB compared with DMSO+AF control group and decreased when pretreated with MincleAb compared with IgG. Immunostaining of neutrophils in the cornea also indicated that when Mincle was activated,there would be more neutrophils gathering around the wound tissue to fight against the pathogens. Correspondingly, when Mincle was blocked with neutralizing antibody, the amount of neutrophils would be decreased significantly.
At present, the studies about anti-fungal mechanism are mainly focused on the immuno-inflammatory response. However,the direct killing effect to fungi is of equal importance. NO is an unstable, short-lived and potential toxic radical. The sources of NO in ocular surface tissue primarily included corneal epithelium, fibroblasts, endothelium and inflammatory cells[33]. NO contributes to the acute immune response by two pathways, one is toxic effect against infectious organisms and the second is regulation of immune cell function[34-35].Recent studies show that NO may act as a double edged sword depending on its concentration degree. According to Kim et al[36],NO may induce tissue damage in ocular surface disease when the concentration ratio is 3-10 folds higher than normal functional NO level. However, if the concentration ratio of NO is 1.5-2.5 folds higher, it may play a defensive role in contrary.Studies from Lee et al[37]showed that Mincle enhanced the translation of key enzyme required for NO synthesis via p38-dependent eIF5A hypusination. In our work, the NO concentration is approximately 2 folds higher in corneas treated with Mincle agonist TDB before infection compared with normal state. And the NO concentration is lower than 1.5 folds in DMSO control group. From these data we can infer that Mincle plays a defective role in the early stage of mice FK by regulating the NO production to a proper degree.
In summary, our data provide evidence that Mincle was expressed in C57BL/6 mice corneas, and it increased after stimulation of A. fumigatus in vivo experiments. In addition, we confirmed that expression of IL-1β, TNF-α, IL-6, CXCL1 and MIP-2 as well as NO concentration in infected corneas were all positively associated with Mincle expression, and the degree of neutrophil aggregation also have a positive correlation with Mincle. These data suggest that Mincle plays an important role by regulating the immuno-inflammatory response during FK.Further research of the regulatory mechanisms of Mincle in FK may be helpful to explore the subtle strategies of eye barrier to prevent cornea inflammation.
ACKNOWLEDGEMENTS
Foundations:Supported by the National Natural Science Foundation of China (No.81470609; No.81500695).
Conflicts of Interest:Yu GR, None; Lin J, None; Zhang J,None; Che CY, None; Peng XD, None; Li C, None; Hu LT,None; Zhu GQ, None; He K, None; Zhao GQ, None.
REFERENCES
1 Keay LJ, Gower EW, Iovieno A, Oechsler RA, Alfonso EC, Matoba A, Colby K, Tuli SS, Hammersmith K, Cavanagh D, Lee SM, Irvine J, Stulting RD, Mauger TF, Schein OD. Clinical and microbiological characteristics of FK in the United States, 2001-2007: a multicenter study.Ophthalmology 2011;118(5):920-926.
2 Mellado F, Rojas T, Cumsille C. FK: review of diagnosis and treatment.Arq Bras Oftalmol 2013;76(1):52-56.
3 Ansari Z, Miller D, Galor A. Current thoughts in FK: diagnosis and Treatment. Curr Fungal Infect Rep 2013;7(3):209-218.
4 Tanure MA, Cohen EJ, Sudesh S, Rapuano CJ, Laibson PR. Spectrum of FK at Wills Eye Hospital, Philadelphia, Pennsylvania. Cornea 2000;19(3):307-312.
5 Leck AK, Thomas PA, Hagan M, Kaliamurthy J, Ackuaku E, John M, Newman MJ, Codjoe FS, Opintan JA, Kalavathy CM, Essuman V,Jesudasan CA, Johnson GJ. Aetiology of suppurative corneal ulcers in Ghana and south India, and epidemiology of FK. Br J Ophthalmol 2002;86(11):1211-1215.
6 Ghosh AK, Gupta A, Rudramurthy SM, Paul S, Hallur VK, Chakrabarti A. FK in North India: spectrum of agents, risk factors and treatment.Mycopathologia 2016;181(11-12):843-850.
7 Xie L, Zhong W, Shi W, Sun S. Spectrum of FK in north China.Ophthalmology 2006;113(11):1943-1948.
8 Furlanetto RL, Andreo EG, Finotti IG, Arcieri ES, Ferreira MA,Rocha FJ. Epidemiology and etiologic diagnosis of infectious keratitis in Uberlandia, Brazil. Eur J Ophthalmol 2010;20(3):498-503.
9 Gower EW, Keay LJ, Oechsler RA, Iovieno A, Alfonso EC, Jones DB,Colby K, Tuli SS, Patel SR, Lee SM,Irvine J, Stulting RD, Mauger TF,Schein OD. Trends in FK in the United States, 2001 to 2007. Ophthalmology 2010;117(12):2263-2267.
10 Jurkunas U, Behlau I, Colby K. FK: changing pathogens and risk factors. Cornea 2009;28(6):638-643.
11 Lee EJ, Brown BR, Vance EE, Snow PE, Silver PB, Heinrichs D, Lin X, Iwakura Y, Wells CA, Caspi RR, Rosenzweig HL. Mincle activation and the Syk/Card9 signaling axis are central to the development of autoimmune disease of the eye. J Immunol 2016;196(7):3148-3158.
12 Ishikawa T, Itoh F, Yoshida S, Saijo S, Matsuzawa T, Gonoi T, Saito T, Okawa Y, Shibata N, Miyamoto T, Yamasaki S. Identification of distinct ligands for the C-type lectin receptors Mincle and Dectin-2 in the pathogenic fungus Malassezia. Cell Host Microbe 2013;13(4):477-488.
13 Greco SH, Torres-Hernandez A, Kalabin A, Whiteman C, Rokosh R, Ravirala S, Ochi A, Gutierrez J, Salyana MA, Mani VR, Nagaraj SV, Deutsch M, Seifert L, Daley D, Barilla R, Hundeyin M, Nikifrov Y,Tejada K, Gelb BE, Katz SC, Miller G. Mincle signaling promotes con a hepatitis. J Immunol 2016;197(7):2816-2827.
14 Shah S, Nagata M, Yamasaki S, Williams SJ. Total synthesis of a cyclopropane-fatty acid α-glucosyl diglyceride from Lactobacillus plantarum and identification of its ability to signal through Mincle. Chem Commun (Camb) 2016;52(72):10902-10905.
15 Yamasaki S, Matsumoto M, Takeuchi O, Matsuzawa T, Ishikawa E,Sakuma M, Tateno H, Uno J, Hirabayashi J,Mikami Y, Takeda K, Akira S, Saito T. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc Natl Acad Sci U S A 2009;106(6):1897-1902.
16 Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S, Murray RZ, Beckhouse AG, Lo YL, Manzanero S,Cobbold C, Schroder K, Ma B,Orr S, Stewart L, Lebus D, Sobieszczuk P, Hume DA, Stow J, Blanchard H, Ashman RB. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol 2008;180(11):7404-7413.
17 Rabes A, Zimmermann S, Reppe K, Lang R, Seeberger PH, Suttorp N, Witzenrath M, Lepenies B, Opitz B. The C-type lectin receptor Mincle binds to Streptococcus pneumoniae but plays a limited role in the antipneumococcal innate immune response. PLoS One 2015;10(2):e0117022.
18 Kiyotake R, Oh-Hora M, Ishikawa E, Miyamoto T, Ishibashi T,Yamasaki S. Human mincle binds to cholesterol crystals and triggers innate immune responses. J Biol Chem 2015;290(42):25322-25332.
19 Tanaka M, Ikeda K, Suganami T, Komiya C, Ochi K, Shirakawa I,Hamaguchi M, Nishimura S, Manabe I, Matsuda T, Kimura K, Inoue H,Inagaki Y, Aoe S, Yamasaki S, Ogawa Y. Macrophage-inducible C-type lectin underlies obesity-induced adipose tissue fibrosis. Nat Commun 2014;5:4982.
20 de Rivero Vaccari JC, Brand FJ 3rd, Berti AF, Alonso OF, Bullock MR,de Rivero Vaccari JP. Mincle signaling in the innate immune response after traumatic brain injury. J Neurotrauma 2015;32(4):228-236.
21 Roperto S, Russo V, Esposito I, Ceccarelli DM, Paciello O, Avallone L,Capparelli R, Roperto F. Mincle, an innate immune receptor, is expressed in urothelial cancer cells of papillomavirus-associated urothelial tumors of cattle. PLoS One 2015;10(10):e0141624.
22 Miyake Y, Ishikawa E, Ishikawa T, Yamasaki S. Self and nonself recognition through C-type lectin receptor, Mincle. Self Nonself 2010;1(4):310-313.
23 Kerscher B, Dambuza IM, ChristofiM, Reid DM, Yamasaki S,Willment JA, Brown GD. Signalling through MyD88 drives surface expression of the mycobacterial receptors MCL (Clecsf8, Clec4d)and Mincle (Clec4e) following microbial stimulation. Microbes Infect 2016;18(7-8):505-509.
24 Wu TG, Wilhelmus KR, Mitchell BM. Experimental keratomycosis in a mouse model. Invest Ophthalmol Vis Sci 2003;44(1):210-216.
25 Lin J, He K, Zhao G, Li C, Hu L, Zhu G, Niu Y, Hao G. Mincle inhibits neutrophils and macrophages apoptosis in A. fumigatus keratitis.Int Immunopharmacol 2017;52:101-109.
26 Prajna VN, Prajna L, Muthiah S. FK: The Aravind experience. Indian J Ophthalmol 2017;65(10):912-919.
27 Matsumoto M, Tanaka T, Kaisho T, Sanjo H, Copeland NG,Gilbert DJ, Jenkins NA, Akira S. A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J Immunol 1999;163(9):5039-5048.
28 He Y, Xu L, Li B, Guo ZN, Hu Q, Guo Z, Tang J, Chen Y, Zhang Y,Tang J, Zhang JH. Macrophage-inducible c-type lectin/spleen tyrosine kinase signaling pathway contributes to neuroinflammation after subarachnoid hemorrhage in rats. Stroke 2015;46(8):2277-2286.
29 Kodar K, Harper JL, McConnell MJ, Timmer MSM, Stocker BL. The Mincle ligand trehalose dibehenate differentially modulates M1-like and M2-like macrophage phenotype and function via Syk signaling. Immun Inflamm Dis 2017;5(4):503-514.
30 De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N,Hartmann K, Gunzer M, Roers A, Hogg N. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 2013;121(24):4930-4937.
31 Nathan C. Neutrophils and immunity: challenges and opportunities.Nature Reviews Immunology 2006;6(3):173-182.
32 Qin CC, Liu YN, Hu Y, Yang Y, Chen Z. Macrophage inflammatory protein-2 as mediator of inflammation in acute liver injury. World J Gastroenterol 2017;23(17):3043-3052.
33 Jeng BH, Meisler DM, Holly field JG, Connor JT, Aulak KS, Stuehr DJ. Nitric oxide generated by corneas in corneal storage media. Cornea 2002;21(4):410-414.
34 Park GS, Kwon NS, Kim YM, Kim JC. The role of nitric oxide in ocular surface diseases. Korean J Ophthalmol 2001;15(2):59-66.
35 Erdinest N, Shohat N, Moallem E, Yahalom C, Mechoulam H, Anteby I, Ovadia H, Solomon A. Nitric oxide secretion in human conjunctival fibroblasts is inhibited by alpha linolenic acid. J Inflamm (Lond) 2015;12:59.
36 Kim JC, Park GS, Kim JK, Kim YM. The role of nitric oxide in ocular surface cells. J Korean Med Sci 2002;17(3):389-394.
37 Lee WB, Kang JS, Choi WY, Zhang Q, Kim CH, Choi UY, Kim-Ha J, Kim YJ. Mincle-mediated translational regulation is required for strong nitric oxide production and inflammation resolution. Nat Commun 2016;7:11322.