INTRODUCTION
Senescence marker protein 30 (SMP30) is a novel aging marker molecule and calcium-binding protein, and its content decreased with age[1]. In recent years, studies particularly in liver of rats and mice, have made it clear that SMP30 is significantly involved in a variety of biological regulation pathways: ascorbic acid (vitamin C) synthesis[2],cellular function regulation[3], intracellular oxidative stress level[4], cell apoptosis[5-6], cell proliferation[7], and calcium homeostasis[8].
Interestingly, intracellular damage of oxidative stress,apoptosis and calcium ion concentration changes have a close relationship with the formation of cataract[9-13]. Combined with the current studies of SMP30 in mouse liver, we hypothesize that SMP30 also plays an important role in lens, and involved in cataract etiology through regulating cell apoptosis, oxidation stress and calcium disorders. However, the regulation of SMP30 in human lens epithelial cells (HLEC) is rarely reported in domestic and international publications. Therefore,our previous experiments collected a large number of cataract patients with lens anterior capsule, and found that SMP30 mainly exists in cytoplasm and rarely in nucleus of HLEC in human lens anterior capsule[14]. What’s more, content of SMP30 is higher in cataract patient than normal and decreases in aging[15], this finding showed that SMP30 also decreases with age in the human lens, suggesting that SMP30 may be a protective factor or disease signal in cataract patients.
We therefore hypothesize that SMP30 is associated with apoptosis in cataract. Here, we set up to investigate the regulation of SMP30 in HLEC SRA01/04 cell proliferation and apoptosis. The study is composed of two parts: constructing SMP30 overexpressed and down-regulated HLEC (SRA01/04)by lentivirus transfection, and measuring cell proliferation and apoptosis.
MATERIALS AND METHODS
Cell Culture HLEC (SRA01/04) lines were obtained from Guangzhou Jennio Biotech Co., Ltd (China). Cells were cultured in a humidified atmosphere of 95% air, 5% CO2using RPMI 1640 media (Gibco, USA) and 10% fetal bovine serum(Gibco, USA).
Establishment of SMP30 Overexpressed and Knocked Down SRA01/04 Cell Lines To determine whether SRA01/04 is susceptible to lentivirus transfection, we carried out pre-experiment in which cells were transfected with empty lentiviral particles (Genechem, Shanghai, China). Cells were divided into four groups of different transfection conditions:Normal medium, Polybrene (5 μg/mL), Eni.s, Polybrene(5 μg/mL)+Eni.s, and divided into three groups of different multiplicity of infection (MOI): 1, 10, 100 to find out the best infection conditions.
Experimental group cells were infected with lentivirusregucalcin [LV-RGN (NM_152869)] (Genechem, Shanghai,China) in overexpression (OE) group, LV-RGN-RNAi(ACCTGAAGCTGGTGGAATT) (Genechem, Shanghai,China) in knock down (KD) group, and the control groups(NC-OE, NC-KD) were infected with the respective negative control virus.
Verify Transfection Efficiency by Real-time Polymerase Chain Reaction and Western Blot Total cellular RNA was extracted from SMP30 normal, OE (NC-OE), KD (NC-KD)group cells using the RNase kit from Axygen (USA) and was quantified spectrophotometrically by NanoDrop 2000 nucleic acid micro-detector (USA). A reverse transcription kit (Takara,Japan) was used to make complementary DNA (cDNA) and a real-time quantitative polymerase chain reaction (q-PCR)kit to carry out the PCR. For SMP30 (RGN), forward primer was 5’-GGTCGCTAGACCACAAAATCT-3’ and reverse primer was 5’-CTAAACGAATCACTCTTCCTCC-3’. For glyceraldehyde-3-phosphate dehydrogenase (GAPDH), forward primer was 5’-TGACTTCAACAGCGACACCCA-3’ and reverse primer was 5’-CACCCTGTTGCTGTAGCCAAA-3’(Takara, Japan). Real-time PCR was performed on a LightCycler®480 system real-time PCR instrument equipped with 8-strips reaction tube. All reactions were carried out in triplicate. The melting curve analyses were carried out to guarantee the specificity of the quantitative Real-time PCR reactions. The data analysis was carried out with the 2-△△ Ctmethod depicted previously.
We extracted the cell protein with lysis buffer (Beyotime,China). Expression of SMP30 in the established cells was analyzed by Western blot using a mouse monoclonal anti-regucalcin antibody (ab67336, Abcam) and a mouse monoclonal anti-GAPDH antibody (Santa Cruz, SC-32233).The secondary antibody was goat anti-mouse antibody (Santa Cruz, SC-2005).
Cell Viability Assay Cells in logarithmic growth phase were seeded at 2×103/well in 96-well plate and incubated for the cell attachment. Then cell viability was assessed by CCK-8 continuous detection (450 nm) in 1, 2, 3, 4, 5d and before measurement cells were incubated with 10 μl CCK8(DOJINDO, Japan) for 2h at 37℃. All samples were assayed in five copies and each experiment was repeated at least three times.
Cell Proliferation Assay Cells (2×103/well) were seeded in 96-well culture plates and Brdu kit (Roche, Switzerland)was tested on day 1 and 4. Totally 10 μL/well of diluted BrdU reagent was added 24h before test and incubated for 8h, and then proceed as follows (room temperature):FixDenat 200 μL/well at dark room for 30min; 5%-10%washing buffer closed for 30min; Anti-BrdU-POD working solution 100 μL/well in the dark room for 90min; Anti-BrdUPOD working solution washing buffer 200 μL/well; Substrate solution 100 μL/well in the dark for 5-30min until the reaction solution became blue; 50 μl/well 10% H2SO4was added to the culture wells and then single plate read at 450 nm (Tecan,Switzerland). All samples were assayed in triplicate and each experiment was repeated at least three times.
PI FACS Cell Cycle Detection Cells cultivated in 25 cm2bottle grown to about 80% coverage were digested with 0.25%trypsin (Gibco, USA) and resuspended (cell number ≥106) in 15 mL centrifuge tube (1300 rpm, 5min). Then discarded the supernatant and washed cell precipitation with 4℃ pre-cooling phosphate buffered saline (PBS) (pH=7.2-7.4) 1 times(1300 rpm, 5min). Cell precipitation were fixed with 4℃ precold 75% ethanol for at least 1h and centrifuged (1300 rpm,5min) to fix the solution, PBS washed cells precipitate once on the same step next. Cell staining: according to the amount of cells, added 0.6-1mL cell staining solution [PI (sigma),RNase (Fermentas)] to resuspend and analyzed through Flow Cytometry (BD Accuri).
Annexin V-APC Single Staining Cell Apoptosis Detection We washed the cell pellet with 4℃ pre-cooled PBS 1 time and 1×binding buffer once (1300 rmp, 3min centrifuged). Cell precipitation was resuspended by 200 μL 1×binding buffer and added 10 μL Annexin V-APC (eBioscience) staining for 15min at room temperature away from light. According to the amount of cells, added 400 μL 1×binding buffer for flow cytometry analysis.
Expression of Caspase-3 Protein The expression of caspase-3 protein was analyzed by Western blot analysis using a rabbit monoclonal anti-caspase-3 antibody (ab32351, Abcam) and a secondary antibody of goat anti-rabbit antibody (Santa Cruz, SC-2004). For GAPDH: a mouse monoclonal GAPDH antibody (Santa Cruz, SC-32233) and a secondary antibody of goat anti-mouse antibody (Santa Cruz, SC-2005).
Statistical Analysis Data are expressed as mean±SD.Experimental group and control group data will first analyzed by the F-test (i.e., homogeneity of variance test). If the F-test value>0.05, the t-test value is obtained by equal variance double sample test; If the F-test value<0.05, the t-test value is obtained by the heteroskedastic two-sample test. P<0.05 was considered statistically significant. SPSS for Windows (version 17.0, SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
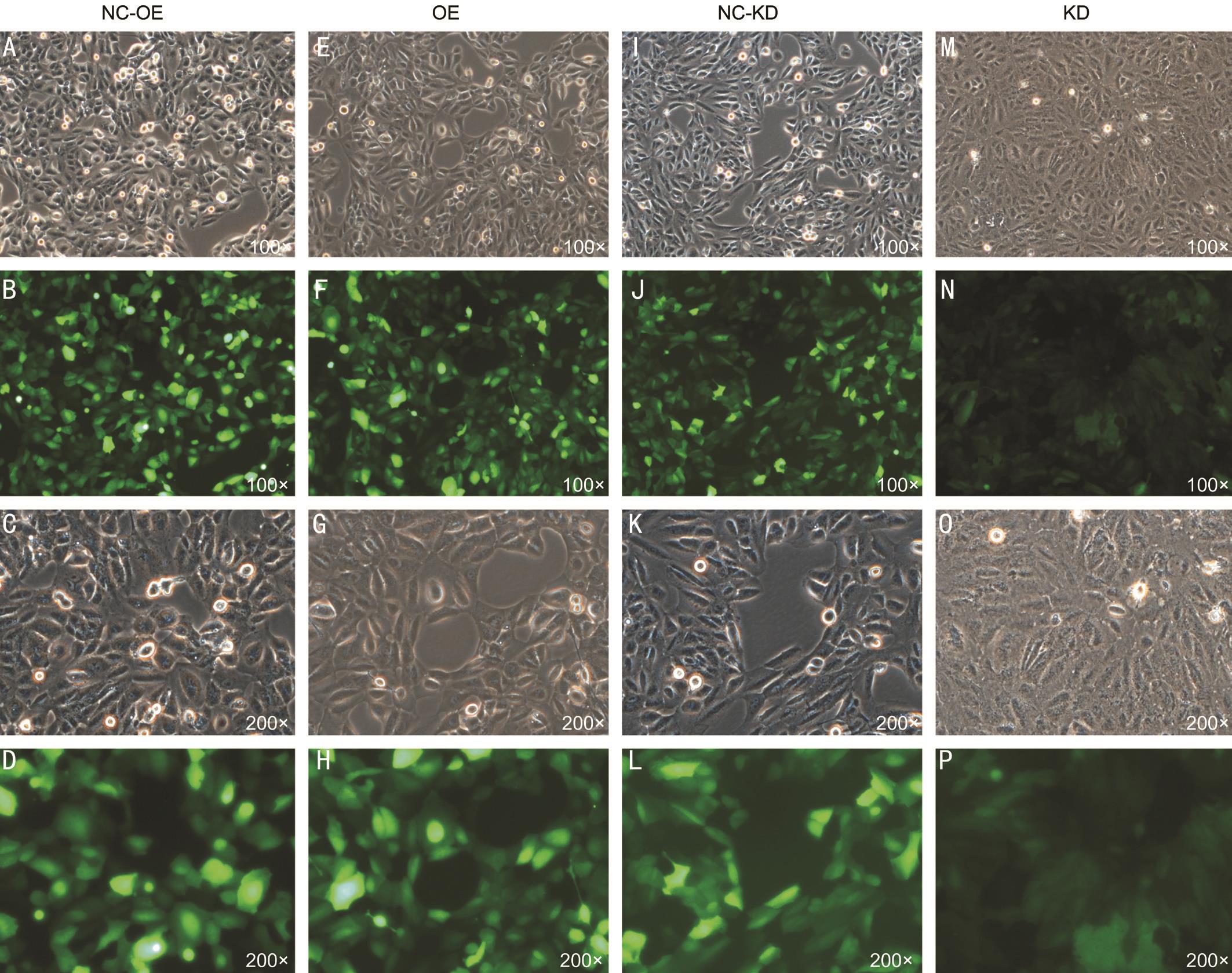
Figure 1 Transfected cells under light field and green fluorescence field microscopy A, B, C, D: SMP30 overexpression negative control group (NC-OE); E, F, G, H: SMP30 overexpression group (OE); I, J, K, L: SMP30 knock down negative control group (NC-KD); M, N, O, P:SMP30 knock down group (KD). Magnification: 100× and 200×.
RESULTS
Susceptibility of SRA01/04 Cells in Lentivirus Infection We determined the optimal infection condition was Normal+Polybrene (5 μg/mL) and the MOI=5 by pre-infection experiments. In the formal infection experiment, cells in the 6-well plate grown to about 20% were added to each experimental and control group virus. After 24h, the cells were changed to normal complete medium and the fluorescence efficiency was observed at 72h. When the cells were grown to about 90%, 2 μg/mL of puromycin was added to remove uninfected cells, and then 1 μg/mL of puromycin was maintained in subcultured cells to obtain stably transfected cells (Figure 1).
Stable Transfection of SMP30 in SRA01/04 Cells We confirmed that the abundance of RGN gene expression in OE group was 19.373 times higher than that of SMP30 overexpression negative control group (NC-OE) (P<0.01) and the knock down rate of KD group was 96% compared with SMP30 knock down negative control group (NC-KD) (P<0.01)by q-PCR assay (Figure 2A). The expression of SMP30 proteins were significantly higher in OE group and lower in KD group respectively compared to the control group through Western Blot assay (Figure 2B).
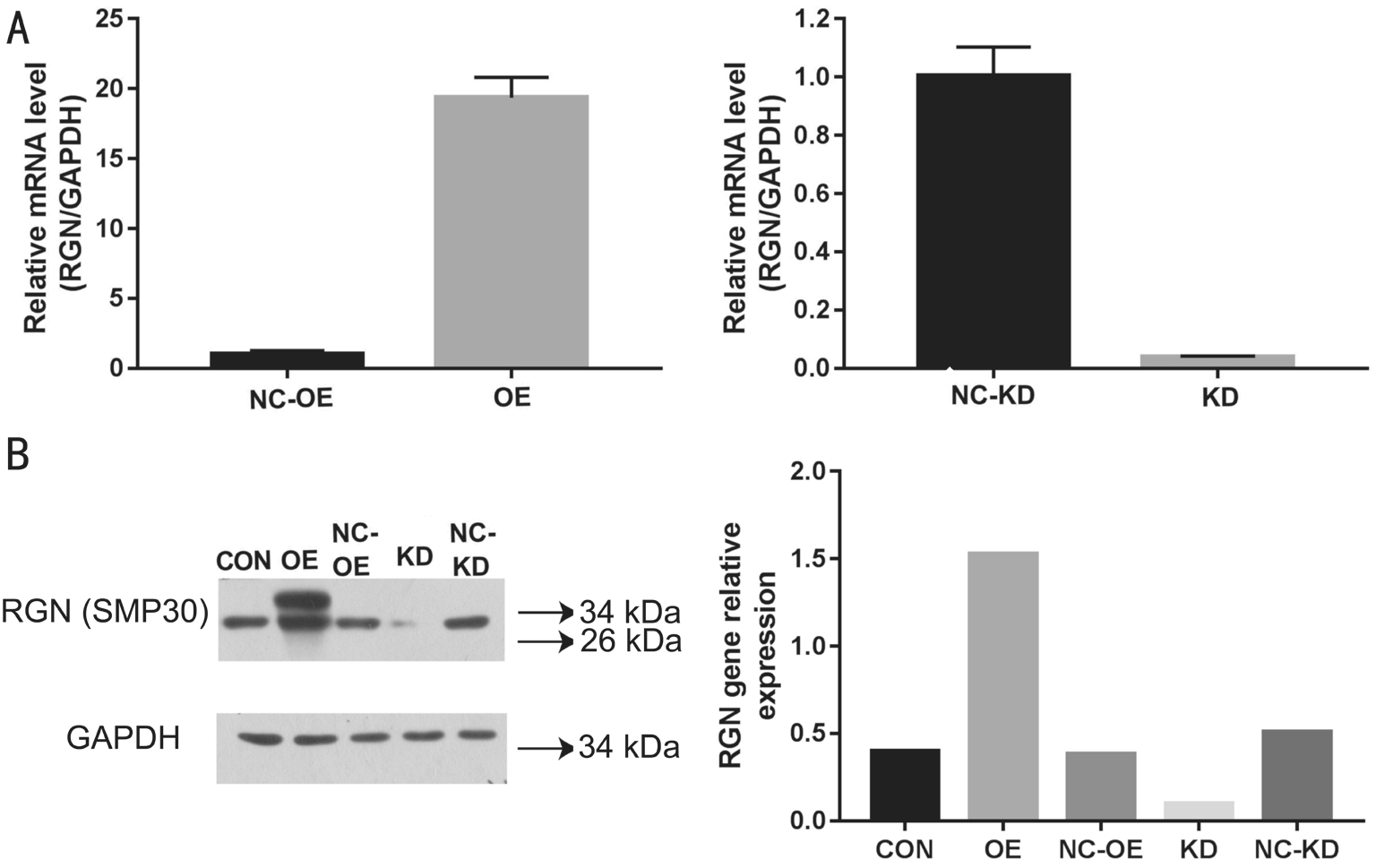
Figure 2 Expression of SMP30 (RGN) mRNA and protein in transfected cells A: RGN mRNA levels were determined by q-PCR; B:Detection of SMP30 protein by Western blot. CON: Control group; OE: SMP30 overexpression group; NC-OE: SMP30 overexpression negative control group; KD: SMP30 knock down group; NC-KD: SMP30 knock down negative control group.
Promotion of SMP30 Overexpression in Cell Proliferation of SRA01/04 After measurement of CCK8 assay on the 1, 2, 3, 4, 5d, we observed the proliferation of KD group was decreased compared with the NC-KD group (P<0.05).However, there was no difference between OE and NCOE group (Figure 3A). By Brdu proliferation experiment,we found that the proliferation of OE group was promoted compared with NC-OE group (P<0.01). However, there was no difference between KD and NC-KD group (Figure 3B). Using PI FACS cell cycle assay, we discovered that compared with NC-KD group, the cells in the KD group were decreased in the S phase (P<0.01), and increased in the G1 phase (P<0.01), and there was no significant change in the G2/M phase (P>0.05);Compared with NC-OE group, the cells in the OE group were decreased in the S phase (P<0.01), and increased in the G1 and G2/M phase (P<0.01, P<0.01, respectively) (Figure 3C, 3D).Suppression of SRA01/04 Cells Apoptosis by SMP30 Overexpressing The results of Annexin V APC signal staining detection indicated that the cell apoptosis rate was higher than NC-KD group in KD group (P<0.01), but lower than NCOE group in OE group (P<0.05) (Figure 4A, 4B). Compared with respective control group, the expression of caspase-3 was down-regulated in OE group through Western blot assay and up-regulated in KD group compared with NC-KD group(Figure 4C).
DISCUSSION
In this study, we found that SMP30 inhibited the apoptosis of HLEC SRA01/04 and promoted its proliferation. This finding suggests that SMP30 may reduce apoptosis in human lens and regulate the occurrence of cataract caused by cell apoptosis.
Cataract is the most frequently reason of blindness in the world today[16], and the only treatment is the surgery[17]. However,there are many complications of the surgery, such as corneal edema, a shallow anterior chamber[18], capsular rupture and even loss of the eye due to endophthalmitis[19]. What’s more,in developing countries, the important obstacles to cataract surgery include the cost of surgery and IOL, lack of awareness,poor service, long distances from surgical centers[20]. Therefore,researches for cataract pathogenesis, the corresponding drug treatments and prevention methods are imminent.
Previous studies of SMP30 mainly focused on the liver, and found that SMP30 can regulate liver cell regeneration, rescue cell death by enhancing plasma membrane Ca2+-pumping activity[21], and may be applied to clinical tumor markers[22].Studies have shown that SMP30 can slightly inhibit hepatocyte proliferation[23-24], this is contrary to our result that SMP30 promoted SRA01/04 proliferation, we think the possible causes are differences in cell types and culture conditions. Study that hepatocyte of SMP30 gene knock out (KO) mice was more susceptible to apoptosis[25]indicates SMP30 is a protective factor for cell apoptosis, this is consistent with our result in this study.
Apoptosis is the common basis for the development of human and animal non congenital cataracts, transforming growth factor (TGF)-beta and ultraviolet radiation type B (UVB)irradiation-induced apoptosis can cause cataract[26-27]. Studies of the anterior capsule of different types of age-related cataract(ARC) patients have shown that apoptosis and expression of SMP30 are present in nuclear and cortical ARC[28-29], this finding indicate that SMP30 expression and cell apoptosis may related to cataract formation. Besides, a recent study showed that SMP30 (KO) mice are more susceptible to develop UVB induced cataracts because vitamin C can’t be synthesized[30].This indicated that SMP30 may protect lens from radiation damage by producing sufficient vitamin C, which is a critical factor that prevent photo-oxidative stress and lens protein damage[31]. In conclusion, SMP30 appears to be associated with apoptosis and oxidative stress in the lens, but the regulatory mechanism remains unclear.
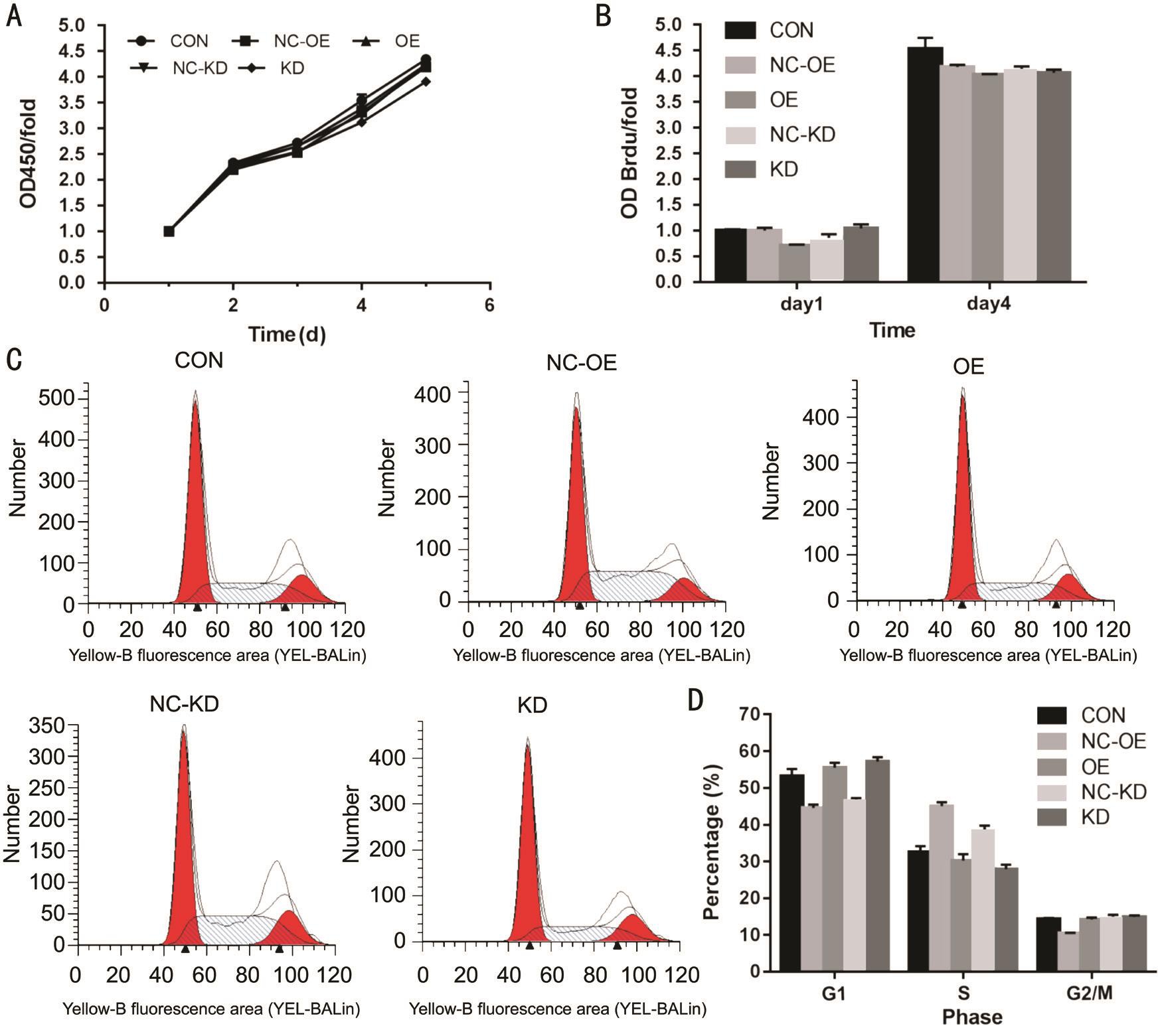
Figure 3 Effect of SMP30 content on SRA01/04 proliferation A: Cell proliferation was observed by CCK-8 assay on day 1, 2, 3, 4, 5; B:Cell proliferation was tested by Brdu assay; C, D: Effect of SMP30 on cell cycle by PI FACS cell cycle assay. CON: Control group; OE: SMP30 overexpression group; NC-OE: SMP30 overexpression negative control group; KD: SMP30 knock down group; NC-KD: SMP30 knock down negative control group.
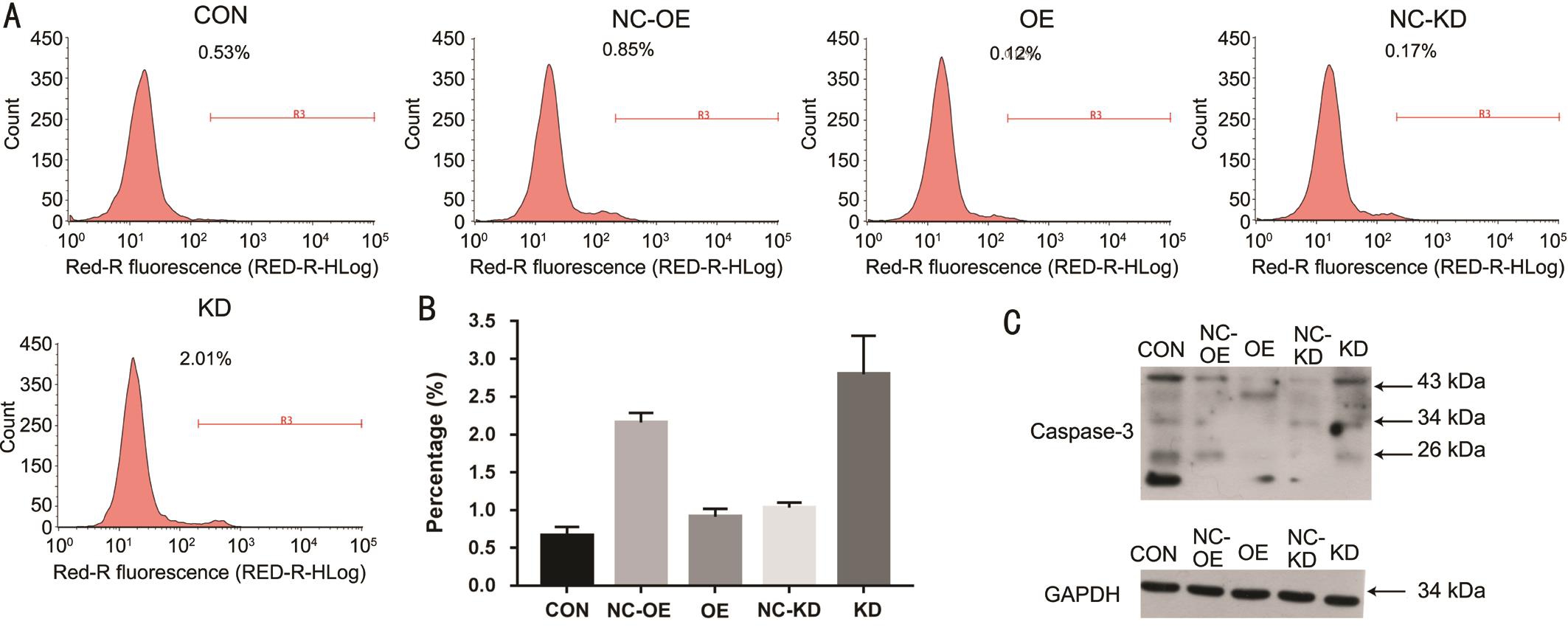
Figure 4 Detection of cell apoptosis A, B: Cell apoptosis was tested by Annexin V-APC signal staining detection; C: Cell apoptosis was reflected by caspase-3 protein content. CON: Control group; OE: SMP30 overexpression group; NC-OE: SMP30 overexpression negative control group; KD: SMP30 knock down group; NC-KD: SMP30 knock down negative control group.
In this study, we found that SMP30 inhibited the apoptosis of HLEC and promoted its proliferation, which laid a foundation for the follow-up study on the regulation of SMP30 in HLEC cell function and also provided new directions and ideas for the study of cataract prevention mechanism. The disadvantage is that it does not explore the impact of SMP30 on oxidative stress and calcium disorders, so in the follow-up study we will continue to study and build animal models. In summary,SMP30 may be expected to be an important molecule for the treatment of diseases caused by apoptosis, increasing its expression or affecting its regulatory pathway may prevent the occurrence of cataracts. In the future, it may be possible to find a biological monomer, through eye drops, oral drugs and other ways to prevent cataracts from SMP30 anti-aging mechanism.
ACKNOWLEDGEMENTS
Foundation:Supported by the National Natural Science Foundation of China (No.81360146).
Conflicts of Interest:Chen X, None; Li SM, None; Li YW,None; Han ZH, None; Liang H, None.
REFERENCES
1 Nishijima K, Ohno T, Amano A, Kishimoto Y, Kondo Y, Ishigami A,Tanaka S. Bone degeneration and its recovery in SMP30/GNL-knockout mice. J Nutr Health Aging 2017,21(5):573-578.
2 Arun P, Aleti V, Parikh K, Manne V, Chilukuri N. Senescence marker protein 30 (SMP30) expression in eukaryotic cells: existence of multiple species and membrane localization. PLoS One 2011;6(2):e16545.
3 Kondo Y, Ishigami A, Kubo S, Handa S, Gomi K, Hirokawa K,Kajiyama N, Chiba T, Shimokado K, Maruyama N. Senescence marker protein-30 is a unique enzyme that hydrolyzes diisopropyl phosphoro fluoridate in the liver. FEBS Lett 2004;570(1-3):57-62.
4 Kadowaki S, Shishido T, Sasaki T, Sugai T, Narumi T, Honda Y, Otaki Y, Kinoshita D, Takahashi T, Nishiyama S, Takahashi H, Arimoto T,Miyamoto T, Watanabe T, Ishigami A, Takeishi Y, Kubota I. Deficiency of senescence marker protein 30 exacerbates cardiac injury after ischemia/reperfusion. Int J Mol Sci 2016;17(4):542.
5 Goo MJ, Park JK, Hong IH, Kim AY, Lee EM, Lee EJ, Hwang M, Jeong KS. Increased susceptibility of radiation-induced intestinal apoptosis in SMP30 KO mice. Int J Mol Sci 2013;14(6):11084-11095.
6 Mizukami H, Saitoh S, Machii H, Yamada S, Hoshino Y, Misaka T, Ishigami A, Takeishi Y. Senescence marker protein-30 (SMP30)deficiency impairs myocardium-induced dilation of coronary arterioles associated with reactive oxygen species. Int J Mol Sci 2013;14(5):9408-9423.
7 Zhang SC, Liang MK, Huang GL, Jiang K, Zhou SF, Zhao S. Inhibition of SMP30 gene expression influences the biological characteristics of human Hep G2 cells. Asian Pac J Cancer Prev 2014;15(3):1193-1196.
8 Lin PH, Jian CY, Chou JC, Chen CW, Chen CC, Soong C, Hu S, Lieu FK, Wang PS, Wang SW. Induction of renal senescence marker protein-30(SMP30) expression by testosterone and its contribution to urinary calcium absorption in male rats. Sci Rep 2016;6:32085.
9 Iezhitsa I, Agarwal R, Saad SD, Zakaria FK, Agarwal P, Krasilnikova A,Rahman TH, Rozali KN, Spasov A, Ozerov A, Alyautdin R, Ismail NM.Mechanism of the anticataract effect of liposomal MgT in galactose-fed rats. Mol Vis 2016;22:734-747.
10 Beebe DC, Holekamp NM, Shui YB. Oxidative damage and the prevention of age-related cataracts. Ophthalmic Res 2010;44(3):155-165.
11 Palsamy P, Bidasee KR, Shinohara T. Selenite cataracts: activation of endoplasmic reticulum stress and loss of Nrf2/Keap1-dependent stress protection. Biochim Biophys Acta 2014;1842(9):1794-1805.
12 Asbell PA, Dualan I, Mindel J, Brocks D, Ahmad M, Epstein S. Agerelated cataract. Lancet 2005;365(9459):599-609.
13 Xu GX, Hu JZ, Lin H, Zhou LY, Wang TT, Heng XW. Ultrastructural study of the lens epithelial cells in the human age-related cata ract. Guoji Yanke Zazhi (Int Eye Sci) 2004;4(4):631-632.
14 Hu MX, Luo GR, Zhou SF, Mo FR, Xie XX, Liang H. Expression and significance of senescence marker protein-30 in human lens epithelial cells. Rec Adv Ophthalmol 2008;28(1):13-15.
15 Lai WX, Tan SJ, Li X, Zou WJ, Jiang LZ, Liang H. Expression of calmodulin in lens epithelial cells of normal and cataract patients with different age. Rec Adv Ophthalmol 2014;32(6):521-524.
16 Foster A, Resnikoff S. The impact of Vision 2020 on global blindness.Eye (Lond) 2005;19(10):1133-1135.
17 Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010.Br J Ophthalmol 2012;96(5):614-618.
18 Limbu B, Jha HC. Intraoperative complications of high volume sutureless cataract surgery in Nepal: a prospective study. Kathmandu Univ Med J (KUMJ) 2014;12(47):194-197.
19 David Y. Cataract complications. Community Eye Health 2008;21(65):1-3.
20 Yorston D. High-volume surgery in developing countries. Eye (Lond)2005;19(10):1083-1089.
21 Jung KJ, Ishigami A, Maruyama N, Takahashi R, Goto S, Yu BP,Chung HY. Modulation of gene expression of SMP-30 by LPS and calorie restriction during aging process. Exp Gerontol 2004;39(8):1169-1177.
22 Li XL, Kang FK, Lei M, Jiang LY, Lin CQ, Zhou SF. Expression of mRNA of senescence marker protein 30 on human tumor cell lines.Letters in Biotechnology 2010;21(3): 332-335.
23 Son TG, Kim SJ, Kim K, Kim MS, Chung HY, Lee J. Cytoprotective roles of senescence marker protein 30 against intracellular calcium elevation and oxidative stress. Arch Pharm Res 2008;31(7):872-877.
24 Ishigami T, Fujita T, Simbula G, Columbano A, Kikuchi K, Ishigami A, Shimosawa T, Arakawa Y, Maruyama N. Regulatory effects of senescence marker protein 30 on the proliferation of hepatocytes. Pathol Int 2001;51(7):491-497.
25 Maruyama N, Ishigami A, Kondo Y. Pathophysiological significance of senescence marker protein-30. Geriatr Gerontol Int 2010;10(Suppl 1):S88-S98.
26 Maruno KA, Lovicu FJ, Chamberlain CG, McAvoy JW. Apoptosis is a feature of TGF beta-induced cataract. Clin Exp Optom 2002;85(2):76-82.
27 Wang D, Guo D, Bi H, Wu Q, Tian Q, Du Y. Zinc oxide nanoparticles inhibit Ca2+-ATPase expression in human lens epithelial cells under UVB irradiation. Toxicol In Vitro 2013;27(8):2117-2126.
28 Liu L, Cai XJ, Yu AH, Song YW, Wang HT, Liu Y, Jiao F. The expression of senescenee markern protein-30 in different types of agerelated cataracts and its relation to apoptosis of lens epithelial cell.Chinese Journal of Experimental Ophthalmology 2012;30(6):529-533.
29 Zhou D, Yin D, Xiao F, Hao J. Expressions of senescence-associated β-galactosidase and senescence marker protein-30 are associated with lens epithelial cell apoptosis. Med Sci Monit 2015;21:3728-3735.
30 Ishikawa Y, Hashizume K, Kishimoto S, Tezuka Y, Nishigori H,Yamamoto N, Kondo Y, Maruyama N, Ishigami A, Kurosaka D. Effect of vitamin C depletion on UVR-B induced cataract in SMP30/GNL knockout mice. Exp Eye Res 2012;94(1):85-89.
31 Varma SD, Kumar S, Richards RD. Light-induced damage to ocular lens cation pump: prevention by vitamin C. Proc Natl Acad Sci U S A 1979;76(7):3504-3506.