INTRODUCTION
Meibomian gland dysfunction (MGD) is a chronic disorder of the mebomian gland usually due to compromised quality/quantity of glandular secretion and obstructive terminal duct[1]. MGD appears to affect the well-being and activities of routine life. The prevalence rate of MGD has been reported to be 3.5% to almost 70% worldwide[2-3]. Persistent MGD can lead to various clinical conditions such as altered tear film stability, dry eye symptoms, inflammation and ocular surface damage[1,4].
Demodex mites are microscopic ectoparasites harbour the human skin. Humans are infested by two distinct species namely: demodex folliculorum and demodex brevis. Demodex folliculorum infest the eyelash follicles, and demodex brevis are capable of burrowing deep into sebaceous glands and meibomian gland. Demodex is not only involved in the etiology of blepharitis, but also in other ocular surface manifestations such as superficial corneal neovascularization, marginal corneal infiltration, phlyctenule-like lesions, superficial corneal opacity, and nodular corneal scars, mainly in patients with ocular rosacea[5-9]. Until now, the role of demodex in the etiology of MGD related ocular surface damage remains elusive.
The purpose of this study was to investigate the relationship between ocular demodex folliculorum infestation and ocular surface manifestations in MGD.
SUBJECTS AND METHODS
Subjects The present study was performed according to the Helsinki Declaration of Human Studies and was approved by the Ethics Committee of He’s Eye Hospital. A total of 86 MGD patients were recruited from July 12, 2014 to January 15, 2015. MGD was diagnosed as previously reported, in brief[10]: 1) presence of ocular symptoms; 2) at least one lid margin abnormality including irregular lid margin, vascular engorgement, plugged meibomian gland orifices and anterior or posterior displacement of the mucocutaneous junction;and 3) impaired meibum expression. Ocular symptoms were determined by ocular surface disease index (OSDI), and subject with a score of >12 was considered abnormal. All participants were diagnosed as MGD at the Clinic of Shenyang He Eye Hospital. The exclusion criteria were: infectious keratitis or conjunctivitis, intraocular surgery within three months, ocular trauma within 6mo, and history of ocular burn,pterygium and serious systematic diseases.
Experimental Procedure All enrolled subjects were assessed in the following sequence: OSDI, slit-lamp biomicroscope examination, corneal surface regularity index (SRI) and surface asymmetry index (SAI), tear fluid collection, fluorescein tear film break-up time (F-BUT), corneal fluorescein staining(CFS), SchirmerI test (SIT) with anesthetic and finally demodex folliculorum counting. All examinations were assessed for one eye of each subject randomly.
Slit-lamp Biomicroscope Examination Lid margin abnormality observations include: lid margin irregularity,plugging of the meibomian orifices, lid margin vascular engorgement, and anterior or posterior replacement of mucocutaneous junction, giving a score of 0 to 4 as previously reported[11]. Meibum quality: each of the 8 glands of the upper and lower eyelid was graded on a scale from 0 to 3 as follows[11]: grade 0, clear; grade 1, cloudy; grade 2, cloudy with granular debris; grade 3, thick, like toothpaste. The scores of the 8 glands were summed to obtain a total score (maximum score for each eye, 24). Meibum expressibility: the degree of meibum expressibility was assessed using firm digital pressure applied over central 5 upper lid glands or lower lid glands as follows[11]: grade 0, all glands expressible; grade 1, 3-4 glands expressible; grade 2, 1-2 glands expressible; grade 3, no glands expressible. The scores of the 5 glands were summed to obtain a total score (maximum score for each eye, 15).
Videokeratoscopy Videokeratoscopic examination (TMS-4;Japan) was performed to test corneal SRI and SAI. The patients placed the head against the support rest of the topograph, and look straight ahead. The subject was then asked to make a complete blink and subsequently to keep the eyes open. At the same time, acquisition of video images was initiated. The measurement was repeated for thrice, and the mean value was calculated.
Sampling and Counting Demodex Counting of parasite was performed as described previously[5]. In brief, under a slit lamp microscope, two lashes, one from each half of each lid, were removed by fine forceps and placed separately on each end of glass slides. Total 20 μL of 100% alcohol (If the eyelash covered with dandruff, 70% glycerinum was used) was added and mounted with coverslip. After 10min, the samples were analysed under a light microscope. The demodex folliculorum mites were recognized according to its morphology and peculiar movement.
Tear Fluid Collection Tears was collected as previously reported[12]. Briefly, tears was collected with a 0.5-μL glass capillary tube (Drummond Scientific, Broomall, PA, USA)from the inferior tear meniscus of each eye. Tear samples from both eyes (1 μL total) were eluted into a sterile tube containing 9 μL of PBS and 0.1% bovine serum albumin. The tubes were immediately stored at -80℃.
Tear Matrix Metalloproteinase-9 Activity Matrix metalloproteinase (MMP)-9 enzyme activity was tested as previously reported[12]. MMP-9 enzyme activity was tested with a MMP activity assay kit (Biotrak; Amersham Biosciences,Piscataway, NJ, USA) according to the manufacturer’s protocol.Statistical Analysis The data was analyzed using SPSS 17.0 (Inc., Chicago, Illinois, USA). Data was expressed as mean±standard deviations. Except of OSDI and CFS scores in which the Mann-Whitney U test was used, unpared t-tests were performed to determine the differences of variables between the two groups. P<0.05 was considered significant.
RESULTS
Prevalence of Ocular Demodex Folliculorum Mites in Meibomian Gland Dysfunction Patients Ocular demodex folliculorum mite was found in 40 of 86 MGD patients(46.5%). The number of mite count was 2.4±0.33/8 lashes.No significant difference was found in age (P=0.46) or gender(P=0.82) between the ocular demodex folliculorum-positive group (26 females and 14 males; aged 36.5±9.6y) and ocular demodex folliculorum-negative group (28 females and 18 males; aged 38.2±11.2y).
Ocular Surface Manifestation of Demodex Folliculorum Infection in Meibomian Gland Dysfunction Statistical difference was noted in OSDI, lid margin abnormality and CFS between the two groups (P<0.05 for each; Table 1). Based on the data of OSDI, the power is 0.83 under the type I error rate of 0.05. No significant difference was found in meibum quality and expressibility, SRI, SAI, F-BUT, and SIT between the two groups (P>0.05 for each; Table 2).
Tear Matrix Metalloproteinase-9 Activity In demodex folliculorum-positive group, the level of tear MMP-9 activity was significantly increased compared with demodex folliculorum-negative group (102.9±32.4 ng/mL versus 46.2±19.2 ng/mL, P=0.03). The level of tear MMP-9 activity in demodex folliculorum-positive group was significantly higher than that in demodex-free group (P=0.03).
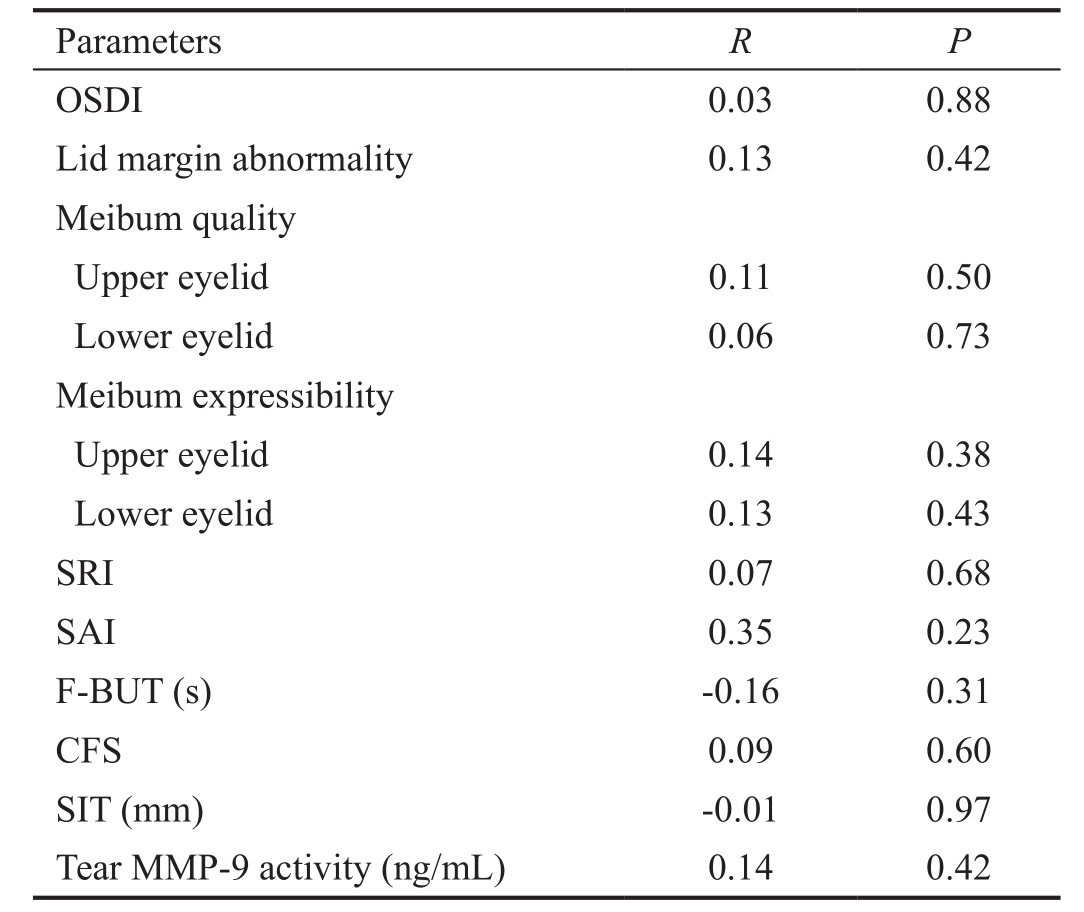
The Association Between the Number of Demodex and Ocular Surface Parameters in Demodex Folliculorum positive Meibomian Gland Dysfunction No significant correlation was noted between the number of demodex and ocular surface parameters in demodex-positive MGD group(P>0.05 for each). No significant correlation was noted between the number of demodex and the level of tear MMP-9 activity in demodex-positive MGD group (P>0.05; Table 2).
Table 1 Ocular surface manifestation of demodex infection in MGD
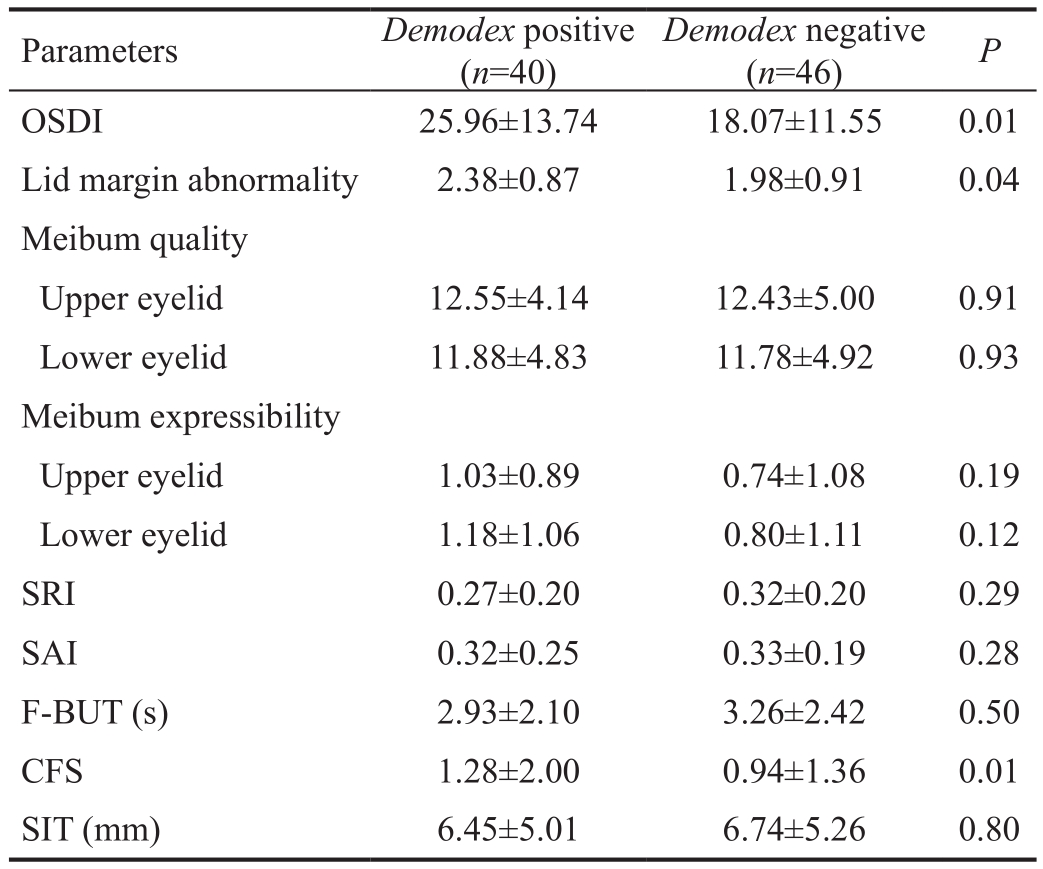
Table 2 The association between the number of demodex and ocular surface parameters in demodex-positive MGD
DISCUSSION
Though the role of ocular demodex infestation in the etiology of blepharitis has been debated since decades, it has been reported the incidence of demodex infestation is relatively higher in blepharitis patients compared to the patients without ophthalmic diseases[13]. Studies have also proved that the corneal and conjunctival pathologies were significantly reduced after demodex eradication[6]. In the present study,ocular demodex folliculorum infestation in MGD patients is related to ocular discomfort, lid margin abnormality and corneal epithelial barrier disruption in MGD. The evidence above mentioned, taken together, implied that demodex folliculorum may be related to ocular surface disorders.
In this study, the lid margin abnormality was more severe in demodex-infested MGD patients than in demodex-negative MGD patients. Though the definite mechanism of lid margin disorders exacerbated by demodex folliculorum infestation remain elusive, we speculate that the host inflammatory responses elicited by demodex mites in lid margin could be the possible mechanism. Studies have proved that demodex folliculorum infestation can directly damage the epithelial cells of the eye lash follicle and are capable to induce delayed type hypersensitivity in the host meibomian gland, lid margin and ocular surface[6-8]. Indeed, lid margin inflammation shown by crusting and reddening of lid margin has been noted in the demodex-infected patients[9]. In addition, the association between lid margin inflammation and facial rosacea also suggests that demodex infestation may result in lid margin inflammation which eventually results in exacerbated MGD[9].Lee et al[14]has shown that the number of demodex folliculorum was proportional to the severity levels of ocular discomfort and the OSDI score. Similarly, in our study, the OSDI was higher in the demodex folliculorum-positive MGD patients compared to demodex folliculorum-negative MGD patients. Taken together, these evidences suggests that eradication of demodex folliculorum mites may be beneficial in relieving ocular surface discomfort related to MGD.
In our study, CFS was more severe in demodex folliculoruminfested MGD patients compared to demodex folliculorumnegative MGD patients. Fluorescein staining is an important indicator of the integrity of the corneal epithelial barrier function. Increased fluorescene staining is usually observed during the disruption of the superficial epithelial cell-cell junctions and superficial corneal epithelial cells damage[15].Interleukin (IL)-17/MMP-9 signaling has been found to be the key factor in disrupting corneal epithelial barrier function in ocular surface diseases, such as dry eye[16-20]. MMP-9 can rapidly degrade the corneal epithelial basement membrane components and tight-junction proteins such as Zonula occludens-1 and occludin that are involved in maintaining the corneal epithelial barrier function[17]. Studies show that MMP-9-deficient mice were resistant to corneal epithelial barrier disruption in experimental dry eye[16]. In addition, it has been proved that T helper-17 cytokine, IL-17, can disrupt the corneal barrier via upregulating the expression of MMP-9[18-20]. Previously, it has been confirmed the association between tear IL-17 level and demodex folliculorum infection as eradication of demodex folliculorum significantly decreased tear IL-17 level[8,21]. In our study, demodex-positive group showed significantly increased levels of tear MMP-9 activity compared to demodex-negative group. These results suggest that demodex folliculorum may exacerbate corneal epithelial barrier function via activation IL-17/MMP-9 signaling.
In our study, no significant difference was noted in meibum quality and expressibility between the two groups, suggesting demodex folliculorum infection has no obvious effect on meibum quality and expressibility. Further studies are required to investigate the effect of demodex brevis infection in meibomian gland on meibum quality and expressibility asthey are capable of borrowing deep into the meibomian gland.Significant difference in F-BUT and SIT values were not observed between the two groups in our study, indicating that demodex folliculorum infection has no obvious effect on tear film stability and tear production. The SRI represents surface regularity within the central area, and the SAI is a measure of central corneal asymmetry. No significant difference was observed in SRI and SAI values between the two groups,indicating that demodex folliculorum infection has no obvious effect on the irregularity and asymmetry of corneal surface in MGD patients.
Our study focused on the relationship between demodex folliculorum infestation and ocular surface damge in MGD,and found that ocular demodex folliculorum infestation is related to ocular discomfort, lid margin abnormality, corneal epithelial barrier disruption, and tear MMP-9 activity. Our findings suggest that treatment of demodex folliculorum is necessary and critical in patients with MGD.
ACKNOWLEDGEMENTS
We thank Dr. Xin Di and Dr. Tao Yao for their insightful comments regarding this manuscript.
Foundation:Supported by the National Natural Science Foundation of China (No.81500693).
Conflicts of Interest:Zhang XB, None; Ding YH, None;He W, None.
REFERENCES
1 Nelson JD, Shimazaki J, Benitez-del-Castillo JM, Craig JP, McCulley JP, Den S, Foulks GN. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee.Invest Ophthalmol Vis Sci 2011;52(4):1930-1937.
2 Schaumberg DA, Nichols JJ, Papas EB, Tong L, Uchino M, Nichols KK. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Invest Ophthalmol Vis Sci 2011;52(4):1994-2005.
3 Amano S, Inoue K. Estimation of prevalence of meibomian gland dysfunction in Japan. Cornea 2017;36(6):684-688.
4 Wu H, Lin Z, Yang F, Fang X, Dong N, Luo S, Shang X, Li W, Liu Z.Meibomian gland dysfunction correlates to the tear film instability and ocular discomfort in patients with pterygium. Sci Rep 2017;7:45115.
5 Gao YY, Di Pascuale MA, Elizondo A, Tseng SC. Clinical treatment of ocular demodecosis by lid scrub with tea tree oil. Cornea 2007;26(2):136-143.
6 Kheirkhah A, Casas V, Li W, Raju VK, Tseng SC. Corneal manifestations of ocular demodex infestation. Am J Ophthalmol 2007;143(5):743-749.
7 Liu J, Sheha H, Tseng SC. Pathogenic role of demodex mites in blepharitis. Curr Opin Allergy Clin Immunol 2010;10(5):505-510.
8 Kim JT, Lee SH, Chun YS, Kim JC. Tear cytokines and chemokines in patients with demodex blepharitis. Cytokine 2011;53(1):94-99.
9 Cheng AM, Sheha H, Tseng SC. Recent advances on ocular demodex infestation. Curr Opin Ophthalmol 2015;26(4):295-300.
10 Arita R, Morishige N, Shirakawa R, Sato Y, Amano S. Effects of eyelid warming devices on tear film parameters in normal subjects and patients with meibomian gland dysfunction. Ocul Surf 2015;13(4):321-330.
11 Lee H, Min K, Kim EK, Kim TI. Minocycline controls clinical outcomes and in flammatory cytokines in moderate and severe meibomian gland dysfunction. Am J Ophthalmol 2012;154(6):949-957.e1.
12 Chotikavanich S, de Paiva CS, Li de Q, Chen JJ, Bian F, Farley WJ,Pflugfelder SC. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci 2009;50(7):3203-3209.
13 Gao YY, Di Pascuale MA, Li W, Liu DT, Baradaran-Rafii A, Elizondo A, Kawakita T, Raju VK, Tseng SC. High prevalence of demodex in eyelashes with cylindrical dandruff. Invest Ophthalmol Vis Sci 2005;46(9):3089-3094.
14 Lee SH, Chun YS, Kim JH, Kim ES, Kim JC. The relationship between demodex and ocular discomfort. Invest Ophthalmol Vis Sci 2010;51(6):2906-2911.
15 Norn MS. Micropunctate fluorescein vital staining of the cornea. Acta Ophthalmol (Copenh) 1970;48(1):108-118.
16 De Paiva CS, Corrales RM, Villarreal AL, Farley WJ, Li DQ, Stern ME, Pflugfelder SC. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res 2006;83(3):526-535.
17 P flugfelder SC, Farley W, Luo L, Chen LZ, de Paiva CS, Olmos LC,Li DQ, Fini ME. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol 2005;166(1):61-71.
18 P flugfelder SC, Corrales RM, de Paiva CS. T helper cytokines in dry eye disease. Exp Eye Res 2013;117:118-125.
19 Zhang X, Volpe EA, Gandhi NB, Schaumburg CS, Siemasko KF,Pangelinan SB, Kelly SD, Hayday AC, Li DQ, Stern ME, P flugfelder SC,de Paiva CS. NK cells promote Th-17 mediated corneal barrier disruption in dry eye. PLoS One 2012;7(5):e36822.
20 Zhang X, Schaumburg CS, Coursey TG, Siemasko KF, Volpe EA,Gandhi NB, Li DQ, Niederkorn JY, Stern ME, P flugfelder SC, de Paiva CS. CD8+cells regulate the T helper-17 response in an experimental murine model of Sjögren syndrome. Mucosal Immunol 2014;7(2):417-427.
21 Kim JH, Chun YS, Kim JC. Clinical and immunological responses in ocular demodecosis. J Korean Med Sci 2011;26(9):1231-1237.