INTRODUCTION
Age-related macular degeneration (AMD) is a chronic and progressive disease that causes significant blindness in the elderly population[1]. AMD can be classified as nonexudative (dry) or neovascular (wet). Neovascular AMD is characterized by the formation of abnormal blood vessels known as choroidal neovascularization (CNV), and the visual prognosis of neovascular AMD is poor if the condition is untreated[2].
Currently, the standard therapy for neovascular AMD is antivascular endothelial growth factor (VEGF) treatment[3].Ranibizumab (Lucentis, Novartis Pharma AG, Basel,Switzerland) was approved for treatment of neovascular AMD in the USA in 2006. The ANCHOR trial demonstrated the efficacy of ranibizumab, over for 24mo, in the treatment of (predominately classic) CNV. The MARINA trial demonstrated the efficacy of such treatment in minimally classic or occult CNV[4-5]. In the ANCHOR trial, 423 patients with predominantly classic CNV secondary to neovascular AMD were treated with either photodynamic therapy (PDT)every 3mo as needed, or with monthly intravitreal injections of either 0.3 or 0.5 mg ranibizumab. The results at the end of 2y showed that 89.9% of the 0.3-mg and 0.5-mg ranibizumab groups lost fewer than 15 letters of best-corrected visual acuity(BCVA) compared to 65.7% of the PDT-treated group. The mean BCVA improved by 8.1 and 10.7 letters in the 0.3-mg and 0.5-mg ranibizumab groups, respectively, while a 9.8-letter drop in vision was evident in the PDT-treated group[4].
In the MARINA trial, 716 patients were treated with either monthly placebo injections, or with 0.3-mg or 0.5-mg ranibizumab injections, over a period of 2y. The results showed that 90% of the 0.5-mg ranibizumab-treated eyes avoided visual loss ≥3 lines of vision, compared to 62.2% of the placebo injection group. A mean visual gain of 6.6 letters at 2y was evident in the 0.5-mg ranibizumab group, compared to a mean loss of 14.9 letters in the control group[5].
The long-term safety of ranibizumab used to treat AMD was assessed in the HORIZON trial, which was an open-labeled extension trial with patients who had completed the MARINA,ANCHOR, and FOCUS trials[6]. A slight mean decline in the visual acuity (VA) gain was observed in initial studies. With less frequent follow-up (a mean of 2mo) and a less rigorous injection schedule (a mean of four injections over 2y), a mean gain of 2.0 letters was evident in the ranibizumabtreated groups, and a mean loss of 11.8 letters in the pooled ranibizumab crossover and untreated groups, at 48mo. VA in the ranibizumab-treated group remained stable over 4y, while the control groups lost vision because of delays in their initial treatment. The study concluded that ranibizumab was safe long-term, but that an incremental decline in the VA gain was evident with a longer interval between follow-ups and less frequent dosing[6].
In the SEVEN-UP trial, performed approximately 7y after ranibizumab therapy in the ANCHOR or MARINA trials, onethird of patients exhibited good visual outcomes, whereas another third had poor outcomes. Compared to baseline, almost half of the eyes were stable, whereas one-third declined by 15 letters or more in terms of VA[7].
Because of lack of patient compliance, optimal treatment is usually not possible, and the mean periods between visits usually become gradually longer[8]. The objective of this study was therefore to analyze the results of intravitreal ranibizumab injection in real-life clinical practice, by retrospectively examining the visual and anatomical outcomes of intravitreal ranibizumab for neovascular AMD over a long period of time.
SUBJECTS AND METHODS
Patients treated with intravitreal ranibizumab for neovascular AMD at Hacettepe University Department of Ophthalmology between December 2006 and May 2013, and who were followed-up for at least 2y, were included in this study. In total, 74 eyes of 74 patients were analyzed. Each diagnosis of neovascular AMD was based on fundus examination, optical coherence tomography (OCT; Zeiss, Oberkochen, Germany),and fundus fluorescein angiography (FFA; Carl Zeiss Meditec AG, Jena, Germany).
Exclusion criteria included CNV attributable to other causes;previous intravitreal ranibizumab, bevacizumab, or pegaptanib monotherapy; previous intravitreal injections of other agents including triamcinolone acetonide; previous PDT; or fibrosis or atrophy at baseline.
Institutional Ethics Board approval (LUT 14/365, dated June 25th, 2014) was obtained from the Hacettepe University Non-Interventional Clinical Research Ethics Board. After receiving approval from the Ethics Committee of the local institutional review board, a retrospective chart review was performed. All procedures were conducted in accordance with the Declaration of Helsinki.
Data on age, gender, the numbers of visits and injections,VA measured with ETDRS charts, and adverse events were extracted from the patient charts. The type, location, and size of CNV (using Zeiss Visupac software, version 4.3), and the development of fibrosis and/or atrophy were evaluated from fundus photos and FFA images, and OCT characteristics and central macular thicknesses (CMT) were retrieved from the OCT database.
A prorenata (PRN) treatment strategy was used, and patients were invited for monthly follow-up visits. Injections were repeated if ≥5 letters had been lost in the BCVA, or there was recurring or persisting intraretinal and/or subretinal fluid.
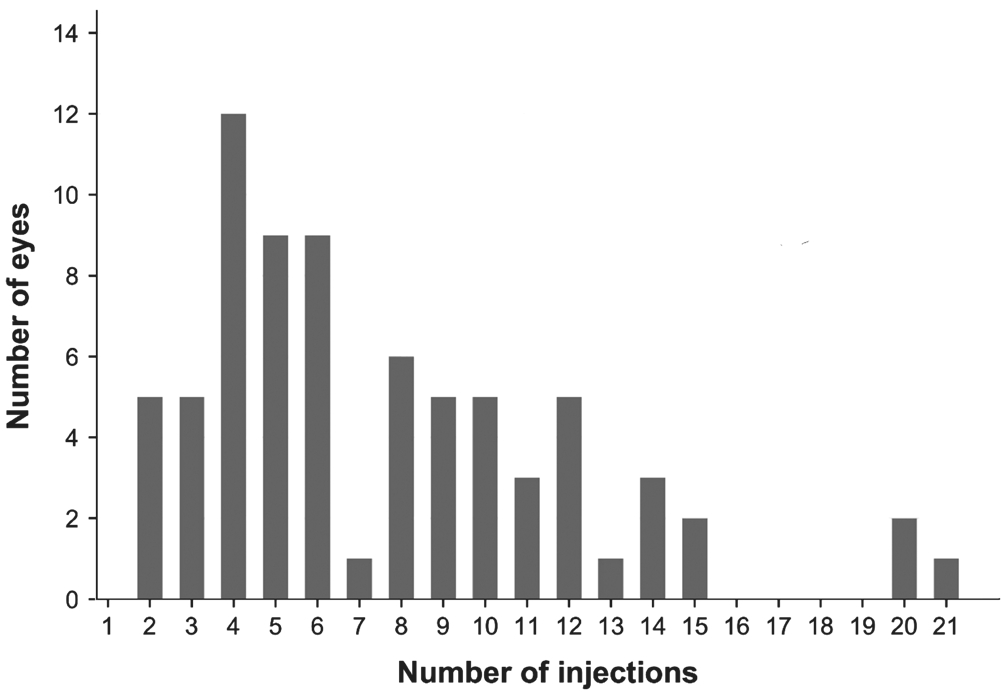
Figure 1 Number of injections administered to each eye.
Statistical analyses were performed using the Statistical Package for the Social Sciences, version 21.0 (IBM, Armonk,NY, USA). The Pearson’s Chi-square test and the Fisher’s exact test were used to compare categorical variables. Mean and standard deviation were used as the definitive statistics for digital variables in cases where the normal distribution assumption was verified, and median and inter-quartile distribution ranges were used in cases where such verification was not possible. Frequency and percentage were used as the definitive statistics for categorical variables. The level of significance was defined as 0.05.
RESULTS
A total of 74 eyes of 74 patients who were treated with intravitreal ranibizumab because of neovascular AMD were included in this study. In all, 40 patients (54%) were female and 34(46%) were male. The mean age at the time of diagnosis was 72.1±6.5 (range, 57-85)y, the mean follow-up period was 46.2±13.1 (range, 24-75)mo, and the mean number of visits 24.1±9.5 (range, 8-48). The mean period between two visits was 57±16d.
At baseline, 74 eyes were evaluated. However, the number of evaluated eyes decreased during each year of follow-up. In years 1 and 2, 74 eyes were evaluated, whereas 53 eyes were evaluated in year 3, 30 in year 4, and 18 in year 5. The mean number of injections within year 1 was 4.5, 1.6 in year 2, 0.9 in year 3, 0.4 in year 4, and 0.1 in the following years. Overall,the mean number of injections was 7.6±4.4 (range, 2-21)(Figure 1).
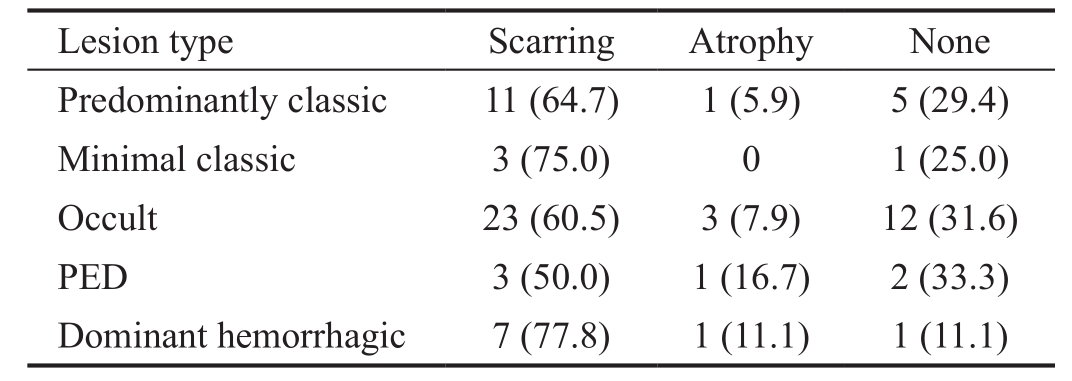
When eyes were evaluated in terms of lesion type at baseline,predominantly classic CNV was found in 17, minimally classic in 4, and occult CNV in 53 eyes (Table 1). Of the occult lesions, those of six eyes featured >50% pigment epithelial detachment (PED), and, in 9 eyes, more than 50% of the lesions were hemorrhagic. The baseline lesions were smallest in the predominantly classic type, and largest in the occult type with predominantly hemorrhagic characteristics (Figure 2).The mean BCVA was 48.1±15 (range, 15-76) letters at baseline,51.2±20 (range, 1-80) letters in year 1, 45.2±18 (range, 1-80)letters in year 2, 42.8±21 (range, 3-80) letters in year 3,46.6±24 (range, 5-80) letters in year 4, and 45.7±19 (range,7-75) letters in year 5 (Figure 3).
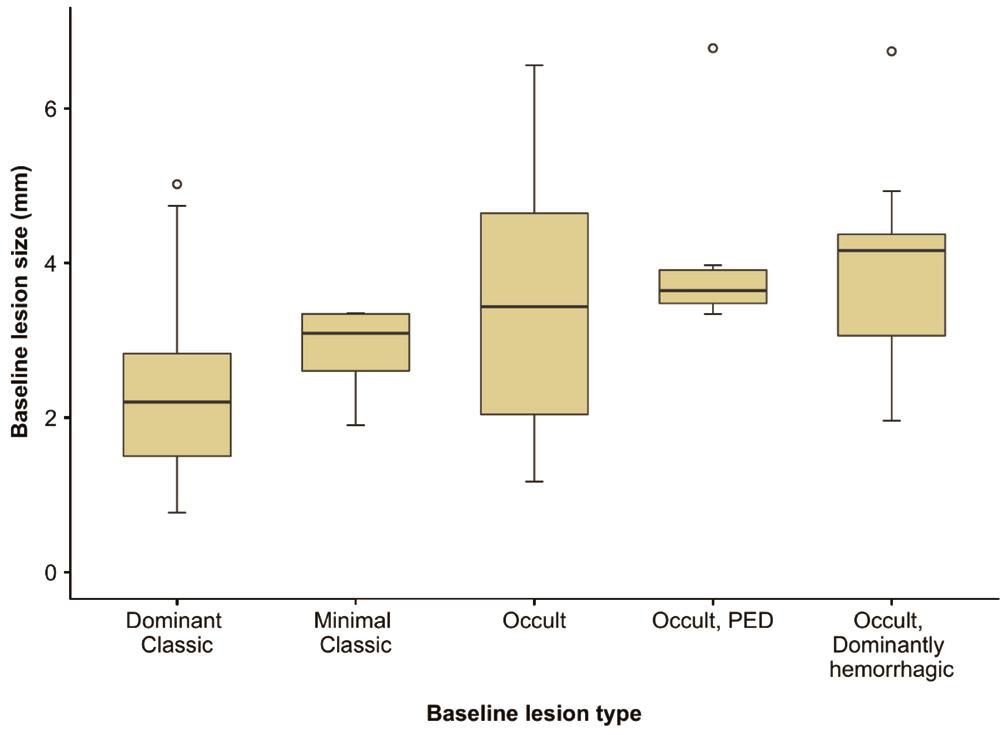
Figure 2 Distribution of baseline lesion sizes according to baseline lesion type.
Table 1 Baseline lesion characteristics
At 1y following the initial injection, there was an increase of 15 letters or more in 9 (12.16%) eyes, and after 2y an increase was seen in 9 (12.16%) eyes. At the end of year 1, a decrease of ≥30 letters was observed in 1 eye and after 2y, a decrease was seen in 6 eyes. The mean CMT was 303±78 (range, 178-552) microns at baseline, 272±83 (range, 166-647) microns in year 1, 280±93 (range, 131-666) microns in year 2, 269±106(range, 142-734) microns in year 3, 266±87 (range, 160-587) microns in year 4, and 251±51 (range, 138-359) microns in year 5 (Figure 4). Correlations between VA and macular thickness changes were evaluated by drawing scatter plots. No definite correlation was observed between the VA and changes in macular thicknesses.
A macular scar developed in 47 (63.5%) eyes at the end of the follow-up period, and atrophy was evident in 6 (8.1%). No scarring or atrophy developed in 21 eyes (28.4%) (Table 2,Figure 5). Scarring or atrophy developed in 70.6% of eyes that had a baseline lesion classified as predominantly classic, in 75% of lesions that were initially classified as minimal classic,in 68.4% of occult lesions, in 66.6% of occult PED lesions,and in 88.8% of predominantly hemorrhagic occult lesions. No significant among-group difference was apparent.
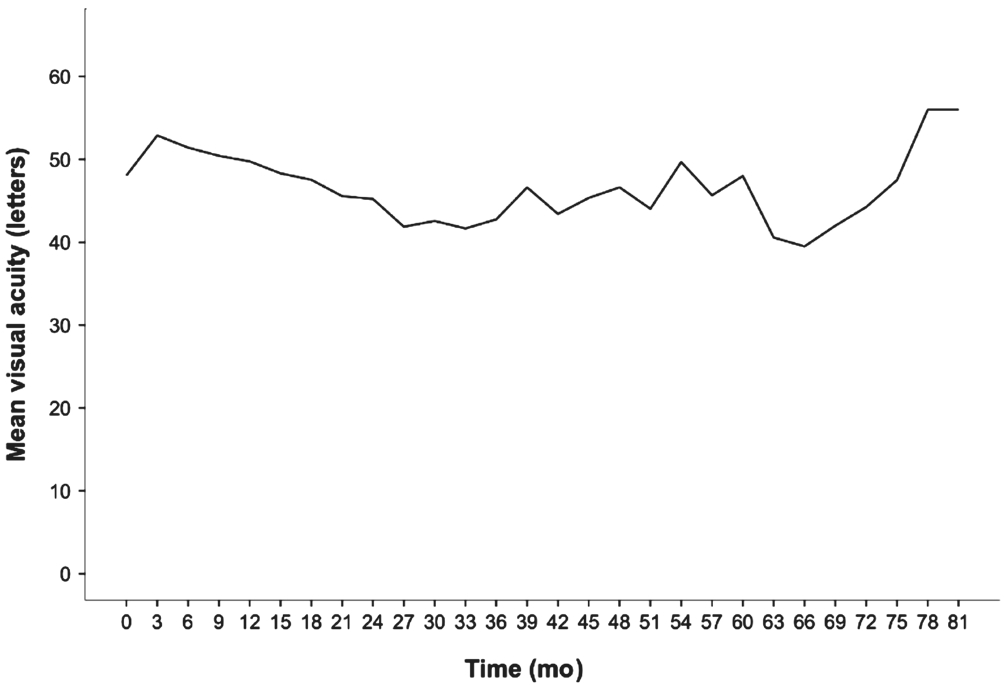
Figure 3 Change in mean visual acuity.
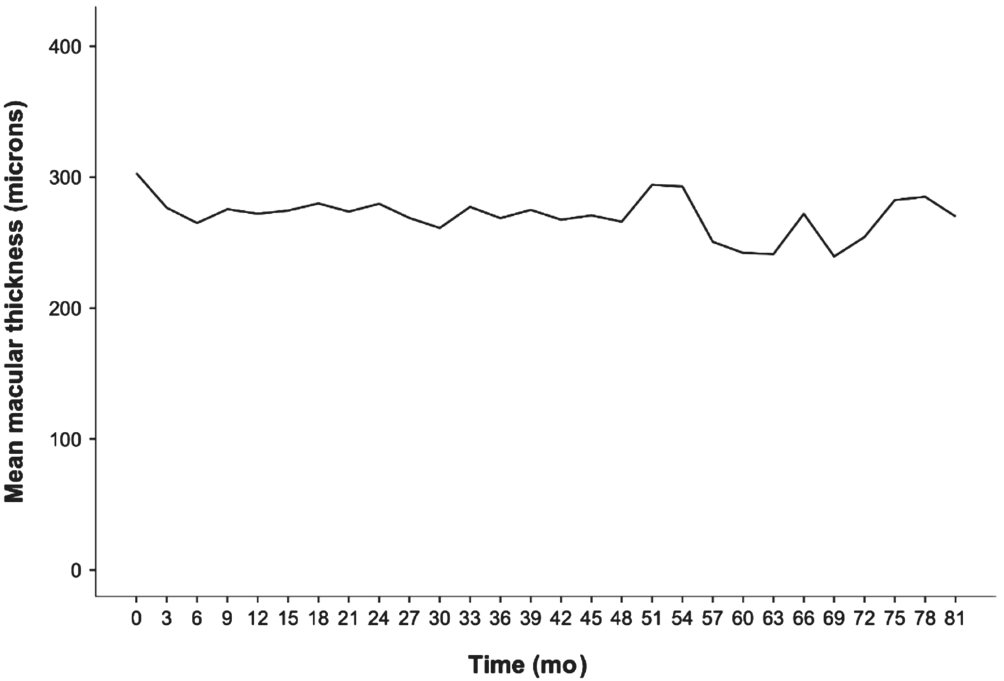
Figure 4 Change in macular thickness.
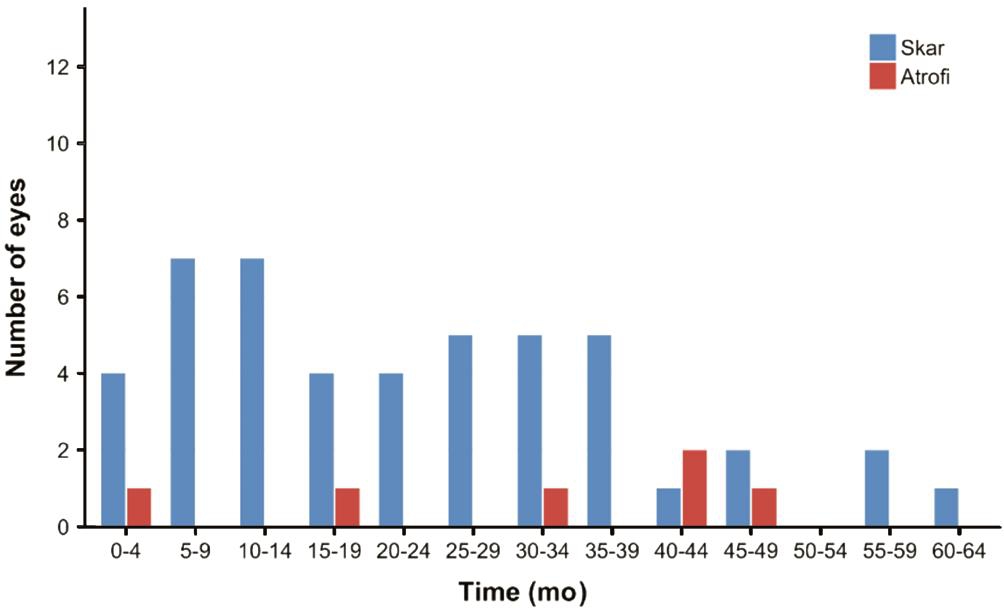
Figure 5 Scar and atrophy formation times.
Table 2 Relationship between type of baseline lesion and the development of scarring and atrophy n (%)
PED: Pigment epithelial detachment.
Endophthalmitis developed in 1 patient of 74, 2d after the ninth injection. The patient recovered after vitrectomy and treatment with intravitreal antibiotics. At baseline, the BCVA of that patients was 45 letters; was 60 letters just before the last injection prior to endophthalmitis; and was 80 letters at the last visit 2y after vitrectomy. No other significant ocular sideeffects were observed. Rhegmatogenous retinal detachment was observed in 1 patient. The retina was re-attached using pneumatic retinopexy and 360° laser photocoagulation.However, after the next injection for disease treatment, the retina redetached and further surgery was needed. During follow-up, one patient developed breast cancer and another an acute myocardial infarction.
DISCUSSION
Wet AMD is one of the most significant causes of visual loss in people over 60 years of age in industrialized countries[9].Currently anti-VEGF treatment is used to treat subfoveal lesions,and the short-term results of monthly or PRN ranibizumab are well-established.
The results afforded by ranibizumab in actual practice are not as good as those of randomized clinical trials, possibly because of the lax inclusion, exclusion, and follow-up criteria used in clinical trials. In the LUMIERE study, patients received an average of 5.1 injections, and the mean change in VA from baseline to month 12 was 3.2 letters[10]. Another retrospective study conducted in France showed that ranibizumab was associated with stabilization of VA, but not with visual improvement[11].
In the WAVE study, patients received a mean of 4.34 ranibizumab injections over 12mo, and the mean change in VA from baseline to month 12 was +0.02 logarithm of the minimal angle of resolution (logMAR). The majority of patients (n=2605,75.1%) were treatment-naïve, and 11.7% (n=405) had already undergone prior intravitreal treatments. Of cases classified as CNV, most were occult (n=2092, 63.9%), followed by classic(n=801, 24.5%) and minimally classic (n=381, 11.6%). Central retinal thickness exhibited a significant mean reduction from baseline (mean=349.4 μm) to the first follow-up (-98.6 μm) and the final follow-up (-78.9 μm)[12-13]. In our present study, the baseline CNV lesion was found to be of the occult type in 53 eyes (71.6%), predominantly classic in 17 (23.0%), and minimally classic in 4 (5.4%). At the end of 1y, the extent of CMT reduction was approximately -30.9 μm from baseline.
In a retrospective review of 50 consecutive United Kingdom patients with neovascular AMD receiving ranibizumab on a PRN basis, patients were seen an average of 11 times during a mean follow-up period of 13.6mo. The VA at the last visit had improved by 4.6 letters from baseline, achieved by giving a mean of 4.7 injections per 12-month period. Notably, 42 patients (84%) lost less than 15 letters (three lines) of VA and 13 patients (26%) gained 15 letters or more at the end of follow-up. The reasons for the vision losses of 15 letters or more in eight patients were development of subfoveal/subretinal fibrosis in four, and geographic atrophy in two patients. The reasons for vision loss in the remaining two patients were not recorded[8].
In the CATT study, VA scores were reported for 1030 (93.0%)of 1107 patients 2y after enrollment. At the end of 2y, the mean increase in VA letters from baseline was 8.8 in the group receiving monthly ranibizumab treatment, 7.8 in the group receiving monthly bevacizumab, 6.7 in the group receiving ranibizumab as needed, and 5.0 in group receiving bevacizumab as needed. Furthermore, geographic atrophy developed in 109 patients (10.6%) at the end of 1y and in 187 patients (18.3%) at the end of 2y[14].
In the IVAN study, 610 patients with untreated wet AMD were randomly assigned to receive intravitreal injections of ranibizumab or bevacizumab monthly, or as needed. A noninferiority comparison was made between bevacizumab and ranibizumab (in terms of visual change) at the end of 2y.The “as needed” group received an average of 13 injections over the 2-year period compared to 23 for those treated monthly. However, continuous treatment was associated with more geographic atrophy[15]; data on the safety of long-term ranibizumab use to treat wet AMD are still lacking.
In the SEVEN-UP study that reported the 7-year follow-up results of patients who had received ranibizumab therapy for neovascular AMD in the ANCHOR and MARINA studies,65 patients were followed-up for 7.3y on average (range, 6.3-8.5y). In our study, the mean follow-up time was 3.9y (range,2.0-6.25y). In this study, 18% of patients received 1 to 5 injections, 18% received 6 to 10 injections, 23% received 11 or more injections, and 41% of the study eyes received no injections.
In our study, 41.9% (31 eyes) of eyes were injected 1 to 5 times, 35.1% (26 eyes) were injected 6-10 times, and 23.0%(17 eyes) received 11 injections or more. The mean letter loss in the SEVEN-UP study within the 7-year period was 8.6.The mean change in VA was a gain of 11.2 letters at the end of year 2 and a gain of 1.7 letters at the end of year 4. In our study, the VA of patients was stable for 4y. The longer followup period in the SEVEN-UP study and the decreasing number of patients over the time period within our study may explain this difference. In the SEVEN-UP study, ≥15 letters were lost by the end of 7y in 33.8% of patients. However, in our study,when the patients’ data were evaluated at year 4, we observed that ≥15 letters were lost in 35.1%, and ≥15 letters were gained in 14.9%. In the SEVEN-UP study, fibrosis was reported in 26 (42%) eyes and geographic atrophy in 34 (54%)[7]. In our study, fibrosis developed in 47 (63.5%) eyes, and geographic atrophy in 6 (8.1%). Longer periods between visits, and fewer injections, may explain the greater number of fibrosis cases and the lower extent of geographic atrophy.
In a previous study performed in Germany, there was a mean letter gain of 1.1±15.7 and a mean letter loss of 0.8±17.2 at the end of year 2[16]. In this study, 4.3±1.9 anti-VEGF injections were administered to patients in year 1, and 1.3±2.2 injections in year 2. The mean period between two injections was 47.7 ±36.7d. Occult CNV was reported in 40.3% of patients,and classic CNV in 27.3%. In a similar manner in our study,a mean gain of 2.8 letters was seen at the end of year 1, and a loss of 2.5 letters was seen at the end of year 2. In our study,4.5±1.7 injections on average were given to patients in year 1,and 1.6±1.8 injections in year 2. The mean period between two visits was 57±16d.
In a multicentric study evaluating the safety of ranibizumab in 4444 patients with wet AMD, the number of injections given during year 1 ranged between 4.3 and 5.5[17]. In another multicenter study, the mean change in VA in patients was a gain of 2.4 letters at the end of year 1 and a gain of 0.6 letters at the end of year 2. In year 1, 5.0 injections were given to patients on average, and 2.2 injections were given in year 2. Patients had a mean of 8.6 visits within year 1, while this number decreased to 4.9 in year 2[18]. In this trial, more frequent visits and injections were associated with greater improvements in VA. In our study, the mean period between two visits was longer, and the numbers of injections less, than in the cited trial, which may explain the poorer VA results of our study.
Our study had some limitations, including being retrospective,being from a single-center, and involving a decreasing patient number over the 5-year period. We report more fibrosis and less geographic atrophy than in SEVEN-UP study, macular thickness does not change much with increased fibrosis and anatomical results with more fibrosis can be explained by the lower rate of injections in current study
In conclusion, ranibizumab monotherapy can provide stabilization of VA for a mean period of 4y in wet AMD. We gave fewer injections compared to other studies, and observed less improvement in VA and macular thickness.
ACKNOWLEDGEMENTS
We want to thank for statistical analysis to Dincer Goksuluk.
Conflicts of Interest:Küçük B, None; Kadayıfçılar S, None;Eldem B, None.
REFERENCES
1 Smith W, Assink J, Klein R, Mitchell P, Klaver CC, Klein BE, Hofman A, Jensen S, Wang JJ, de Jong PT. Risk factors for age-related macular degeneration: pooled findings from three continents. Ophthalmology 2001;108(4):697-704.
2 Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE.Fifteen-year cumulative incidence of age-related macular degeneration:the Beaver Dam Eye Study. Ophthalmology 2007;114(2):253-262.
3 Freund KB, Mrejen S, Gallego-Pinazo R. An update on the pharmacotherapy of neovascular age-related macular degeneration. Expert Opin Pharmacother 2013;14(8):1017-1028.
4 Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T.Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR Study. Ophthalmology 2009;116(1):57-65.
5 Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY,Kim RY; MARINA Study Group. Ranibizumab for neovascular agerelated macular degeneration. N Engl J Med 2006;355(14):1419-1431.
6 Singer MA, Awh CC, Sadda S, Freeman WR, Antoszyk AN, Wong P, Tuomi L. HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology 2012;119(6):1175-1183.
7 Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K; SEVENUP Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study(SEVEN-UP). Ophthalmology 2013;120(11):2292-2299.
8 Rotsos T, Patel PJ, Chen FK, Tufail A. Initial clinical experience of ranibizumab therapy for neovascular age-related macular degeneration.Clin Ophthalmol 2010;4:1271-1275.
9 Kulkarni AD, Kuppermann BD. Wet age-related macular degeneration.Adv Drug Deliv Rev 2005;57(14):1994-2009.
10 Cohen SY, Mimoun G, Oubraham H, Zourdani A, Malbrel C, Queré S, Schneider V; LUMIERE Study Group. Changes in visual acuity in patients with wet age-related macular degeneration treated with intravitreal ranibizumab in daily clinical practice: the LUMIERE Study.Retina 2013;33(3):474-481.
11 Cohen SY, Dubois L, Tadayoni R, Fajnkuchen F, Nghiem-Buffet S, Delahaye-Mazza C, Guiberteau B, Quentel G. Results of oneyear’s treatment with ranibizumab for exudative age-related macular degeneration in a clinical setting. Am J Ophthalmol 2009;148(3):409-413.
12 Finger RP, Wiedemann P, Blumhagen F, Pohl K, Holz FG. Treatment patterns, visual acuity and quality-of-life outcomes of the WAVE study-a noninterventional study of ranibizumab treatment for neovascular agerelated macular degeneration in Germany. Acta Ophthalmol 2013;91(9):540-546.
13 Finger RP, Holz FG. Access to healthcare services for elderly patients with neovascular age-related macular degeneration. Ophthalmologe 2012;109(5):474-478.
14 Comparison of Age-related Macular Degeneration Treatments Trials(CATT) Research Group, Martin DF, Maguire MG, Fine SL, Ying GS,Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL3rd. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 2012;119(7):1388-1398.
15 Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Culliford LA, Reeves BC; IVAN study investigators. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation:2-year findings of the IVAN randomised controlled trial. Lancet 2013;382(9900):1258-1267.
16 Ziemssen F, Eter N, Fauser S, Bopp S, Radermacher M, Hasanbasic Z, Holz FG; AURA-Studiengruppe. Retrospective investigation of anti-VEGF treatment reality and effectiveness in patients with neovascular age-related macular degeneration (AMD) in Germany: treatment reality of ranibizumab for neovascular AMD in Germany. Ophthalmologe 2015;112(3):246-254.
17 Holz FG, Bandello F, Gillies M, Mitchell P, Osborne A, Sheidow T, Souied E, Figueroa MS; LUMINOUS Steering Committee. Safety of ranibizumab in routine clinical practice: 1-year retrospective pooled analysis of four European neovascular AMD registries within the LUMINOUS programme. Br J Ophthalmol 2013;97(9):1161-1167.
18 Holz FG, Tadayoni R, Beatty S, Berger A, Cereda MG, Cortez R, Hoyng CB, Hykin P, Staurenghi G, Heldner S, Bogumil T, Heah T, Sivaprasad S. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration.Br J Ophthalmol 2015;99(2):220-226.