INTRODUCTION
Keratoconus is a bilateral and progressive corneal disorder that affects one in 2000 individuals in the younger population[1]. It usually results in progressive corneal thinning,corneal deformation, and impaired vision.
At the University of Dresden, Spoerl and Seiler[2]developed photochemical corneal cross-linking (CXL) using ultraviolet A (UVA) (370 nm) light and riboflavin (vitamin B2). The basic mechanism of CXL is the formation of covalent bonds predominantly at the surface of collagen fibrils and in the protein network surrounding the collagen, which then results in a long-term increase in corneal biomechanical rigidity and improvement in its strength and stability[3-4]. Since its advent in the late 1990s, CXL has remained the primary treatment for corneal ectatic diseases worldwide. Standard epitheliumoff CXL (S-CXL) has been recommended as the gold standard of care for progressive keratoconus[5]. However, it is disadvantageous because it cannot be used in thin corneas,results in pain, temporarily reduces visual acuity, causes infections and a haze that is likely to persist for 1 to 12mo, it is also difficult to perform in children[6-10]. All of these defects have been shown to correlate with corneal epithelium removal.Therefore, the concept of epithelial-on cross-linking is more appealing.
Iontophoresis has been used for ophthalmological drug administration since 1908 and applied in various medical fields such as transdermal delivery of anti-inflammatory agents, local anesthetics or analgesics, and transmucosal antiviral administration[11-17]. More recently, it has been found to be efficacious for transcorneal drug delivery[18].Studies have demonstrated its safety and efficacy in drug penetration through the cornea and into other ocular tissues[19].Recently, iontophoresis and CXL have been combined to form iontophoresis-assisted epithelium-on CXL (I-CXL). But can I-CXL achieve the same therapeutic effects as S-CXL? In this article, we have reviewed the available literature concerning this topic. All articles used in this review were mainly retrieved from the PubMed database. Original articles and reviews were selected if they were related to the technique of I-CXL or related to a comparison between I-CXL and S-CXL. Letters and case reports were excluded.
FACTORS THAT INFLUENCE CORNEAL CROSSLINKING EFFICACY
Seiler and Hafezi[20]demonstrated that the depth of effective CXL treatment depends on UVA intensity and the concentration of corneal intrastromal riboflavin. Lombardo et al[21]showed that intact corneal epithelium filters out an average of 20% of the UVA radiation passing through the cornea in epithelium-on CXL. Bottos et al[22]reported that the reduced effects of conventional epithelium-on CXL (in comparison to S-CXL) was mainly the result of limited riboflavin penetration through the corneal epithelium and also demonstrated that it was not a barrier to UVA transmittance.The total UVA dose was consistent throughout different CXL protocols. Thus, corneal intrastromal riboflavin concentration appears to be the key factor limiting epithelium-on approaches.More recently, Richoz et al[23]reported that oxygen is also an essential element for photopolymerization in CXL in order for CXL to occur. Kamaev et al[24]hypothesized that oxygen plays a role in CXL via oxygen transformation into reactive oxygen species. Intact corneal epithelium may act as a barrier in order to reduce rapid oxygen diffusion into the stroma. However, whether this reduction leads to a decrease in photopolymerization in I-CXL has not yet been experimentally con firmed.
CORNEAL INTRASTROMAL RIBOFLAVIN CONCENTRATION
Several studies[25-28]have reported the differences in corneal intrastromal riboflavin concentrations after using three CXL imbibition techniques (S-CXL, conventional epithelium-on CXL, and I-CXL delivery) with a 0.1% riboflavin solution.These results demonstrated that I-CXL imbibition produced greater and deeper riboflavin saturation than the conventional epithelium-on technique but did not reach the level acquired with the S-CXL technique. All of the above studies used a 0.1% riboflavin solution plus enhancers. Novruzlu et al[29]obtained the same results after administration of a simple 0.2%riboflavin solution by iontophoresis without using penetration enhancers. In a rabbit model, Cassagne et al[30]compared the iontophoresis-riboflavin delivery technique using a charged riboflavin solution that contained 0.1% riboflavin, 0.1%ethylendiaminatetetraacetic acid, and 0.05% trometamol for I-CXL with S-CXL. Using a high-performance liquid chromatography analysis, they discovered that iontophoresis allowed riboflavin imbibition with two-fold less concentrations than the S-CXL technique (936.2±312.5 vs 1708±908.3 ng/mL,P<0.05).
Hayes et al[31]used spectrophotometry to indirectly measure and compare intrastromal riboflavin penetration in ex vivo porcine corneas following the standard protocol, clinically routine iontophoretic protocols, or two modified iontophoretic protocols. The St. Thomas’/Cardiff iontophoresis protocol B, which included two 5min iontophoresis-assisted deliveries of Ricrolin+ with a 15min soak time in between the two iontophoresis periods, still showed significantly less intrastromal riboflavin concentrations than just S-CXL.However, corneas treated with this protocol have a higher optical density than corneas treated with St. Thomas’/Cardiff iontophoresis protocol A (iontophoresis-assisted delivery for 5min with a 20min riboflavin soak) and routine iontophoretic protocols (iontophoresis-assisted delivery for 5min).
In addition, intrastromal riboflavin concentrations in different corneal segments after different delivery techniques exhibited obvious changes. It was reported that the intrastromal riboflavin concentration decreased in conjunction with depth enhancement, and the differences in the extent of decrease for the three delivery techniques were statistically significant. In a human cadaver corneal study[26]done with high-performance liquid chromatography, the mean riboflavin content in the superficial slice (0-150 μm) in the epithelium-off group was about 2-fold greater than that of the iontophoresis group(50.5±5.3 vs 23.6±2.5 mg/g) and 4-fold greater than that of the epithelium-on group (11.7±3.3 mg/g). Similar differences among three groups were observed for the intermediate and posterior stromal slices (150-300 μm and >300 μm,respectively), presenting an evident riboflavin concentration reduction with increasing depth in all groups. Using twophoton fluorescence microscopy, Gore et al[32]confirmed the depth-dependent riboflavin concentration differences and differences between conventional administration and iontophoresis with Ricrolin+ in fresh postmortem rabbit eyes. The epithelium-off and iontophoresis groups obtained peak riboflavin at concentrations of 0.09%±0.01% and 0.031%±0.003% within the most superficial stroma (0-10 μm),respectively. At a depth of 300 μm, the stromal riboflavin concentration was 0.075%±0.006% and 0.016%±0.002% in epithelium-off groups and iontophoresis groups, respectively.Using two-photon fluorescence microscopy, Gore et al[33]measured corneal intrastromal riboflavin concentrations in rabbits using different transepithelial iontophoresis protocols. The authors found that epithelium-on iontophoresis administration with higher-concentrations of riboflavin solutions, greater iontophoresis dosage, and longer solution contact times achieved greater intrastromal riboflavin penetration. A protocol utilizing 0.25% (wt/vol) riboflavin with benzalkonium chloride (BAC) 0.01% and two cycles of applied current and subsequent soaking (1 mA for 5min, soak for 5min;0.5 mA for 5min, soak for 5min) attained similar intrastromal riboflavin penetration to conventional epithelium-off protocol.The best-performing non-BAC containing protocol produced intrastromal riboflavin concentrations approximately 60% that of conventional epithelium-off protocol. Riboflavin solutions containing saline can lead to minimal stromal penetration.
Using Scheimpflug photography, Lombardo et al[34]analyzed corneal light backscattering before and after transepithelial I-CXL in donor eyes. Light backscattering significantly increased after iontophoresis and decreased significantly after I-CXL, approaching the baseline values in specimens with and without intact epithelium. After standard corneal soaking with riboflavin, a significant increase in corneal light backscattering was detected and remained unchanged up to 30min after S-CXL. The light backscattering increase after iontophoresis in corneas with epithelium was lower than that after standard soaking, whereas in corneas without epithelium it was similar to that after standard stromal soaking.
Hypoosmotic riboflavin saline buffer including enhancers were adopted in most of the above reports. Li et al[35]investigated the imbibition of 0.1% riboflavin-distilled water solution into corneal stromata by iontophoresis-assisted delivery at a current of 1 mA and duration of 10min; identical stromal yellow changes were observed when compared with the standard protocol. This suggested that iontophoresis using 0.1% riboflavin-distilled water solution yielded the same corneal intrastromal riboflavin concentrations as the standard protocol for two reasons: 1) fewer parasitic ions in the distilled water, thus resulting in less interference with riboflavin permeability; 2) a hypoosmotic pressure in the riboflavindistilled water solution that damaged the barrier function of the epithelium. However, these are qualitative observations.Whether this protocol can attain the same intrastromal riboflavin concentration as the standard technique needs further quantitative assessment.
Although the intrastromal riboflavin concentration obtained by iontophoresis did not reach the levels seen with the S-CXL imbibition technique, it was superior to the conventional epithelium-on protocol. It is not yet exactly clear which riboflavin concentration is necessary in the corneal stroma to achieve a sufficient cross-linking effect for corneal stabilization. Therefore, the I-CXL technique needs further clinical studies.
Corneal Biomechanics, Biomolecules and Morphology Mencucci et al[36]researched the I-CXL-induced early modifications in ex vivo human corneas. They found that biomolecular and morphological alterations of corneas treated with I-CXL at 10 mW/cm2for 9min were similar (but more superficial) than corneas treated with S-CXL. These alterations included maldistribution, decreaseD in quantity, and increased apoptosis of anterior stromal keratocytes in addition to reduced subepithelial interweaving of corneal collagen I fibers. Similarly, other reports showed that the apoptotic keratocyte effect following I-CXL was seen only at a depth of 210-230 μm, while it was 270-300 μm following the S-CXL procedure[37-39]. Bikbova and Bikbov[40]reported a similar outcome with 150-210 μm and 240-309 μm for the same set of parameters. These results implied that the efficacy of I-CXL may be inferior to that of S-CXL.
Cassagne et al[30]compared I-CXL with S-CXL in a rabbit model and found that I-CXL and S-CXL induced a similar increase in anterior and intermediate stromal collagen packing.The stress at 10% strain displayed comparable stiffness in I-CXL- and S-CXL-treated corneas. Furthermore, they observed an equal increase in resistance against corneal collagenase degradation after I-CXL and S-CXL. In addition,Lombardo et al[41]found Young’s modulus (E) of the anterior cornea increased by a mean of 1.8 times (from 1.6 to 2.9 MPa)and 1.9 times (from 1.3 to 2.5 MPa) after I-CXL and S-CXL in human donor eyes, respectively. Vinciguerra et al[27]also observed that stress-strain in human corneas did not show significant differences between I-CXL and S-CXL.
Mastropasqua et al[42]measured the deformation amplitude index in human cadaver corneas after different cross-linking protocols. The deformation amplitude index is a biomarker indicating corneal biomechanical rigidity. The results showed that the trend in deformation amplitude index reduction was more evident in corneas treated with I-CXL (10 mW/cm2,9min) than S-CXL and I-CXL (3 mW/cm2, 30min) even if this difference was not statistically significant. Lanzini et al[43]reported that the stress values for 10% strain and the Young’s modulus after I-CXL (10 mW/cm2, 9min) were higher than those after S-CXL and I-CXL (3 mW/cm2, 30min) in human cadaver corneas. This suggested that I-CXL (10 mW/cm2,9min) produced the greatest mechanical resistance.
However, the corneas analyzed in the above studies were healthy, not keratoconic. Moreover, explanted fresh corneas may be slightly different from in vivo corneas. In addition,corneas were analyzed immediately after I-CXL in the aforementioned studies. Manetti et al[44]evaluated type I collagen fiber and keratocyte distribution in the corneal stroma of in vivo I-CXL in a patient with advanced keratoconus.The corneal tissue was collected six months after I-CXL at 10 mW/cm2, and the authors indicated an attempt to restore parallel distribution of type I collagen fibers even when fiber interweaving appeared less organized than in healthy corneas and S-CXL-treated keratoconic corneas. In the above study, I-CXL improved the distribution of CD34-positive keratocytes in keratoconic corneas although scattered CD34 immunoreactivity was still evident in the subepithelial stroma.It has previously been demonstrated that CD34-positive keratocytes are responsible for corneal matrix synthesis and maintenance[39].
Aldahlawi et al[45]reported that in porcine eyes, S-CXL-treated keratocytes showed greater enzymatic resistance to pepsin digestion than I-CXL. This result indicated that S-CXL may be more effective than I-CXL in preventing keratoconus progression.
CLINICAL STUDIES OF IONTOPHORESIS-ASSISTED EPITHELIUM-ON CORNEAL CROSS-LINKING
Several clinical studies about I-CXL for keratoconus have been reported[1,40,46-60]. The results of these studies are promising(Table 1).
Case Series In the first prospective clinical study, Bikbova and Bikbov[46]used I-CXL (iontophoresis: 0.2-1.0 mA,10min; radiation: 3 mW/cm2, 30min) in 19 patients (22 eyes)with progressive keratoconus. They found decreases in the average keratometry values and stabilization of uncorrected visual acuity (UCVA), corrected distance visual acuity(CDVA), corneal astigmatism, and endothelial cell density with a 1-year follow-up. Thereafter, several other clinical reports followed. Lin et al[47]displayed similar outcomes as Bikbova and Bikbov[46]in a prospective nonrandomized trial in which the maximum keratometric reading (Kmax) values showed a statistically significant decrease and UCVA, CDVA,and endothelial cell density remained stable after I-CXL(iontophoresis: 1.0 mA, 5min; radiation: 9 mW/cm2, 10min) in 23 patients (23 eyes) with progressive keratoconus. However,Kmax values showed no statistically significant decreases and remained stable[1,48-52], but CDVA improved significantly in some studies[49,51]and was stabilized in other reports[50,52]. Jia et al[57]reported a 24-month follow-up clinical observation of I-CXL (iontophoresis: 1.0 mA, 5min; radiation: 3 mW/cm2, 30min)in 75 patients (94 eyes) with 0.1% riboflavin in distilled water for progressive keratoconus. The authors showed that Kmax and average keratometry had significantly decreased, and CDVA had significantly improved.
Table 1 Results reported in literature for I-CXL procedures
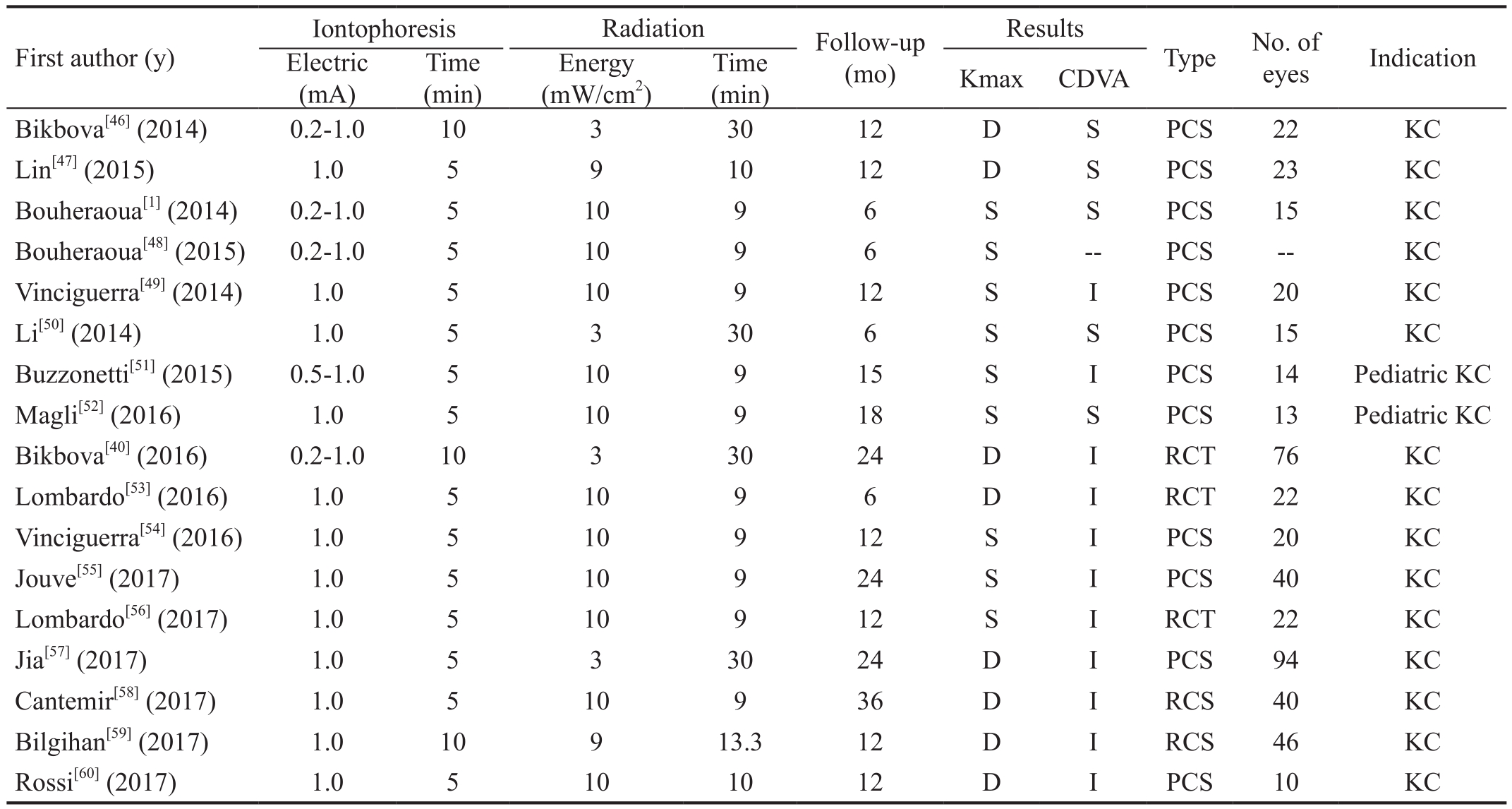
CDVA: Corrected distance visual acuity; D: Dcreased; S: Stabilized; I: Improved; PCS: Prospective clinical study; RCT: Randomized controlled trial; RCS: Retrospective clinical study; KC: Keratoconus; Kmax: Maximum keratometric reading. The 0.2 (0.5)-1.0 mA indicated the electric intensity was initially 0.2 (0.5) mA and gradually increased to 1.0 mA.
Vinciguerra et al[49]enrolled patients with advanced keratoconus with a Kmax value of up to 64 D. Using I-CXL, Buzzonetti et al[51]and Magli et al[52]treated pediatric patients who had progressive keratoconus. The progression of pediatric keratoconus is usually faster than adult keratoconus, treatment compliance is poor, permanent complications appear easily,and the risk of requiring keratoplasty is high[61-62]. However,even in these advanced keratoconus or pediatric patients,I-CXL safely and effectively halted keratoconus progression.
Comparative, Non-randomized Trials Several prospective,comparative, non-randomized clinical studies have been published. Vinciguerra et al[54]have shown a stable Kmax in I-CXL (20 eyes; iontophoresis: 1.0 mA, 5min; radiation:10 mW/cm2, 9min), a statistically significant reduction of Kmax in S-CXL (20 eyes), and a statistically significant improvement in CDVA in both groups at the 1-year follow up for progressive keratoconus. However, CDVA showed a quicker improvement in the I-CXL group compared to the S-CXL group. Spherical equivalent refraction reduced significantly at all postoperative fol low-up times in the I-CXL group. Conversely, it was stable after S-CXL. Jouve et al[55]reported identical outcomes in comparative 24-month followup studies between I-CXL (40 eyes; iontophoresis: 1.0 mA,5min; radiation: 10 mW/cm2, 9min) and S-CXL (40 eyes) in terms of Kmax and CDVA.
Recently, Rossi et al[60]evaluated the efficacy differences between S-CXL (10 eyes) and I-CXL (10 eyes; iontophoresis:1 mA, 5min; radiation: 10 mW/cm2, 10min) with the differences between 12mo and baseline data. The differences of UDVA, CDVA, spherical error, aberrometric outcomes,corneal astigmatism, and flat, mean, and apex keratometries were not statistically significant. However, the differences in spherical equivalent, steep keratometry, and superiorinferior symmetry index were statistically significant. The improvement in these three parameters in the S-CXL group was superior to that in the I-CXL group.
In a retrospective study, Cantemir et al[58]compared the 3-year I-CXL (40 eyes; iontophoresis: 1.0 mA, 5min; radiation:10 mW/cm2, 9min) outcomes with S-CXL (40 eyes) for early stages of keratokonus. Visual acuity significantly improved at month 36 in both groups. In the I-CXL group,UDVA recovered more rapidly than S-CXL after 3mo. The trend in CDVA improvement in the S-CXL group was more favorable than that in the I-CXL group. Kmax values showed a significant reduction by 0.9 D in the I-CXL group and by 1.2 D in the S-CXL group after 36mo. Spherical equivalent did not experience statistically significant changes in both groups throughout 36mo follow-up.
In another retrospective trial comparing S-CXL (47 eyes) and diluted alcohol and iontophoresis assisted CXL (DAI-CXL)(46 eyes; iontophoresis: 1.0 mA, 10min; radiation: 9 mW/cm2,13.3min) for keratoconus, Bilgihan et al[59]used a 10%alcohol solution as an enhancer, enhancer-free 0.2% riboflavin solution as a photosensitizer, and a total dose of 7.2 J/cm2for UVA radiation. In this study, CDVA improved at months 3 and 6 after DAI-CXL and S-CXL, respectively. Higherorder aberrations, coma, and spherical aberrations improved significantly at month 12 in both groups. Kmax values decreased in both groups at month 6.
Randomized Controlled Trials Bikbova and Bikbov[40]published the first prospective randomized controlled trial(RCT) comparing I-CXL (iontophoresis: 0.2-1.0 mA, 10min;radiation: 3 mW/cm2, 30min) with S-CXL. They enrolled 119 patients (149 eyes) with Amsler classification of keratoconus I-II (73 and 76 eyes in the S-CXL and in I-CXL groups,respectively) and found statistically significant differences in CDVA between the two groups with a better result in the I-CXL group after 6mo; however, no significant differences were observed 24mo after the procedures. Keratometric value stabilization and decrease were obtained in both groups, but S-CXL was more effective after 24mo of follow-up.
Another RCT was performed by Lombardo et al[53]. They enrolled 34 eyes of 25 patients, including 12 eyes with S-CXL and 22 eyes with I-CXL (iontophoresis: 1.0 mA, 5min;radiation: 10 mW/cm2, 9min). A significant decrease in Kmax and a significant improvement CDVA were observed in both groups at 6mo. However, the manifest spherical equivalent refraction changed on average by 0.65±1.20 D (P=0.02)and 0.24±0.77 D (P=0.32) in the I-CXL and S-CXL groups,respectively. Changes in these three parameters between the two groups did not show statistical significance. The contrast sensitivity function recovery was slower in S-CXL than in I-CXL. At 6mo, no significant central corneal thickness differences were found in I-CXL, whereas it was seen in S-CXL. In this trial, I-CXL showed comparable results with S-CXL with respect to halting keratoconus progression during a 6mo follow-up.
When the follow-up period was extended to 12mo in the above RCT, the decrease of Kmax was not significant and CDVA had significantly improved[56]. The change of Kmax from baseline in I-CXL was not statistically significant; however,it was opposite if participants <24 years old were removed from the analysis. The change in Kmax from baseline was statistically significant in S-CXL. CDVA, manifest spherical equivalent refraction, and contrast sensitivity function improved significantly in the I-CXL group; however, they changed slightly in the S-CXL group. The changes in Kmax,CDVA, manifest spherical equivalent refraction, and contrast sensitivity function from baseline between the two groups did not show statistical significance. The central corneal thickness measures did not change significantly in both groups at 12mo.Most of the above clinical studies adopted a hypoosmolar 0.1% riboflavin solution enriched with penetration enhancers(trometamol and ethylenediaminetetraacetic acid) without dextran or sodium chloride, specifically formulated to facilitate quick penetration via corneal iontophoresis into the corneal stroma through an intact epithelium. Nevertheless, Bouheraoua et al[48]used hypoosmolar 0.1% riboflavin without dextran,Jia et al[57]and Li et al[50]both used 0.1% riboflavin-distilled water solution only. In all of the above reports, corneal endothelial cell density was not significantly reduced, and no severe postoperative complications were found. These findings confirmed I-CXL safety.
Corneal Stromal Demarcation Line Lin et al[47]reported that within one postoperative month, I-CXL-treated keratoconus corneas exhibited a demarcation line in the anterior stroma with maximal depth of about 133 μm, whereas Bouheraoua et al[48]showed that the demarcation line was not clearly measurable at a mean depth of 214 μm in 46.5% of the patients treated with I-CXL. Similarly, in several other studies, the demarcation line after I-CXL appeared to be shallower and less easily distinguishable than after S-CXL; however, it exhibited features more similar to those observed after S-CXL with respect to depth and visualization when compared with enhancer-assisted transepithelial CXL[1,37,49,63]. Even using iontophoresis at 1.0 mA and for 10min (usually it was 5min)in a prospective RCT, Bikbova and Bikbov[40]only observed a corneal demarcation line with mean depths of 172 μm and 292 μm in the I-CXL and S-CXL groups, respectively. Until now,the deepest demarcation line after I-CXL was observed by Jia et al[57]who reported a mean depth of 298.95 μm at one month postoperatively after using a 0.1% riboflavin-distilled water solution.
In Bikbova and Bikbov’s[40]RCT, the depth of the corneal stromal demarcation line paralleled the clinical efficacy between I-CXL and S-CXL. In other words, the corneal stromal demarcation line in the I-CXL group was shallower than that in the S-CXL group (172 μm vs 292 μm), and the efficacy of the I-CXL group was also less than that of the S-CXL group. However, until now, whether the depth of corneal stroma demarcation line indicates the effectiveness of CXL treatment has not been con firmed.
Corneal Anterior Stromal Keratocyte Bouheraoua et al[1]found that the mean corneal anterior stromal keratocyte densities were significantly lower at 6mo in the S-CXL group when compared to preoperative values, whereas they returned to preoperative values in the I-CXL group by in vivo real-time confocal microscopy scans. In another prospective in vivo confocal microscopy study in S-CXL, Jordan et al[64]revealed a significant decrease in the mean anterior keratocyte density at 1, 3 and 6mo postoperatively with return to the baseline values at 12mo postoperatively. Similarly, Touboul et al[65]also confirmed the decrease of corneal anterior stromal keratocyte within six months postoperatively in S-CXL. These findings suggested that corneal anterior stromal keratocyte in I-CXL recovered faster than in S-CXL.
CONCLUSION
I-CXL has the major advantage of retaining the corneal epithelium and avoiding de-epithelization-related complications such as early postoperative pain, vision impairment, and infection risks[66].Moreover, it has the advantage of shortening riboflavin delivery time from 30 to 5min. Clinical studies have confirmed the safety and efficacy of I-CXL for halting the progression of keratoconus. There are several advantages of clinical results in I-CXL compared to S-CXL: 1) I-CXL had significantly faster recovery of visual acuity and contrast sensitivity function than S-CXL; and 2) spherical equivalent refraction was significantly less myopic 6 and 12mo after I-CXL than that after S-CXL.However, I-CXL is inferior to S-CXL with respect to many factors that affect efficacy such as corneal intrastromal riboflavin concentration and transmissivity of UVA. Long-term I-CXL effectiveness needs to be further observed.
ACKNOWLEDGEMENTS
Foundation:Supported by Beijing Municipal Science and Technology Commission (No.Z151100004015217).
Conflicts of Interest:Jia HZ, None; Peng XJ, None.
REFERENCES
1 Bouheraoua N, Jouve L, El Sanharawi M, Sandali O, Temstet C,Loriaut P, Basli E, Borderie V, Laroche L. Optical coherence tomography and confocal microscopy following three different protocols of corneal collagen-crosslinking in keratoconus. Invest Ophthalmol Vis Sci 2014;55(11):7601-7609.
2 Spoerl E, Seiler T. Techniques for stiffening the cornea. J Refract Surg 1999;15(6):711-713.
3 Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-A-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol 2003;135(5):620-627.
4 Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue.Exp Eye Res 1998;66(1):97-103.
5 Shalchi Z, Wang X, Nanavaty MA. Safety and efficacy of epithelium removal and transepithelial corneal collagen crosslinking for keratoconus.Eye (Lond) 2015;29(1):15-29.
6 Mazzotta C, Balestrazzi A, Baiocchi S, Traversi C, Caporossi A.Stromal haze after combined riboflavin-UVA corneal collagen crosslinking in keratoconus: in vivo confocal microscopic evaluation. Clin Exp Ophthalmol 2007;35(6):580-582.
7 Greenstein SA, Fry KL, Bhatt J, Hersh PS. Natural history of corneal haze after collagen crosslinking for keratoconus and corneal ectasia:Scheimp flug and biomicroscopic analysis. J Cataract Refract Surg 2010;36(12):2105-2114.
8 Rama P, Di Matteo F, Matuska S, Paganoni G, Spinelli A.Acanthamoeba keratitis with perforation after corneal crosslinking and bandage contact lens use. J Cataract Refract Surg 2009;35(4):788-791.
9 Pollhammer M, Cursiefen C. Bacterial keratitis early after corneal crosslinking with riboflavin and ultraviolet-A. J Cataract Refract Surg 2009;35(3):588-589.
10 Sharma N, Maharana P, Singh G, Titiyal JS. Pseudomonas keratitis after collagen crosslinking for keratoconus: case report and review of literature. J Cataract Refract Surg 2010;36(3):517-520.
11 Eljarrat-Binstock E, Domb AJ. Iontophoresis: a non-invasive ocular drug delivery. J Control Release 2006;110(3):479-489.
12 Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular drug delivery. AAPS J 2010;12(3):348-360.
13 Yasukawa T, Ogura Y, Tabata Y, Kimura H, Wiedemann P, Honda Y. Drug delivery systems for vitreoretinal diseases. Prog Retin Eye Res 2004;23(3):253-281.
14 Tyle P. Iontophoretic devices for drug delivery. Pharm Res 1986;3(6):318-326.
15 Prasad R, Koul V. Transdermal delivery of methotrexate: past, present and future prospects. Ther Deliv 2012;3(3):315-325.
16 Dubinsky RM, Kabbani H, El-Chami Z, Boutwell C, Ali H. Practice parameter: treatment of postherpetic neuralgia: an evidence-based report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2004;63(6):959-965.
17 Gomez I, Szabo A, Pap L Jr, Pap L, Boda K, Szekanecz Z. In vivo calcium and phosphate iontophoresis for the topical treatment of osteoporosis. Phys Ther 2012;92(2):289-297.
18 Rootman DS, Jantzen JA, Gonzalez JR, Fischer MJ, Beuerman R,Hill JM. Pharmacokinetics and safety of transcorneal iontophoresis of tobramycin in the rabbit. Invest Ophthalmol Vis Sci 1988;29(9):1397-1401.19 Zhang X, Tao XC, Zhang J, Li ZW, Xu YY, Wang YM, Zhang CX, Mu GY. A review of collagen cross-linking in cornea and sclera. J Ophthalmol 2015;2015:289467.
20 Seiler T, Hafezi F. Corneal cross-linking-induced stromal demarcation line. Cornea 2006;25(9):1057-1059.
21 Lombardo M, Pucci G, Barberi R, Lombardo G. Interaction of ultraviolet light with the cornea: clinical implications for corneal crosslinking. J Cataract Refract Surg 2015;41(2):446-459.
22 Bottos KM, Schor P, Dreyfuss JL, Nader HB, Chamon W. Effect of corneal epithelium on ultraviolet-A and riboflavin absorption. Arq Bras Oftalmol 2011;74(5):348-351.
23 Richoz O, Hammer A, Tabibian D, Gatzioufas Z, Hafezi F. The biomechanical effect of corneal collagen cross-linking (CXL) with riboflavin and UV-A is oxygen dependent. Transl Vis Sci Technol 2013;2(7):6.
24 Kamaev P, Friedman MD, Sherr E, Muller D. Photochemical kinetics of corneal cross-linking with riboflavin. Invest Ophthalmol Vis Sci 2012;53(4):2360-2367.
25 Franch A, Birattari F, Dal Mas G, Luznik Z, Parekh M, Ferrari S,Ponzin D. Evaluation of intrastromal riboflavin concentration in human corneas after three corneal cross-linking imbibition procedures: a pilot study. J Ophthalmol 2015;2015:794256.
26 Mastropasqua L, Nubile M, Calienno R, Mattei PA, Pedrotti E, Salgari N, Mastropasqua R, Lanzini M. Corneal cross-linking: intrastromal riboflavin concentration in iontophoresis-assisted imbibition versus traditional and transepithelial techniques. Am J Ophthalmol 2014;157(3):e1623-e1630.
27 Vinciguerra P, Mencucci R, Romano V, Spoerl E, Camesasca FI,Favuzza E, Azzolini C, Mastropasqua R, Vinciguerra R. Imaging mass spectrometry by matrix-assisted laser desorption/ionization and stressstrain measurements in iontophoresis transepithelial corneal collagen cross-linking. BioMed Res Int 2014;2014:1-12.
28 Arboleda A, Kowalczuk L, Savoldelli M, Klein C, Ladraa S, Naud MC, Aguilar MC, Parel JM, Behar-Cohen F. Evaluating in vivo delivery of riboflavin with coulomb-controlled iontophoresis for corneal collagen cross-linking: a pilot study. Invest Ophthalmol Vis Sci 2014;55(4):2731-2738.
29 Novruzlu S, Turkcu UO, Kvrak I, Kvrak S, Yuksel E, Deniz NG,Bilgihan A, Bingihan K. Can Riboflavin Penetrate stroma without disrupting integrity of corneal epithelium in rabbits? Iontophoresis and ultraperformance liquid chromatography with electrospray ionization tandem mass spectrometry. Cornea 2015;34(8):932-936.
30 Cassagne M, Laurent C, Rodrigues M, Galinier A, Spoerl E, Galiacy SD, Soler V, Fournie P, Malecaze F. Iontophoresis transcorneal delivery technique for transepithelial corneal collagen crosslinking with riboflavin in a rabbit model. Invest Ophthalmol Vis Sci 2016;57(2):594-603.
31 Hayes S, Morgan SR, O’Brart DP, O’Brart N, Meek KM. A study of stromal riboflavin absorption in ex vivo porcine corneas using new and existing delivery protocols for corneal cross-linking. Acta Ophthalmol 2016;94(2):e109-e117.
32 Gore DM, O’Brart D, French P, Dunsby C, Allan BD. Transepithelial riboflavin absorption in an ex vivo rabbit corneal model. Invest Ophthalmol Vis Sci 2015;56(8):5006-5011.
33 Gore DM, O’Brart DP, French P, Dunsby C, Allan BD. A comparison of different corneal iontophoresis protocols for promoting transepithelial riboflavin penetration. Invest Ophthalmol Vis Sci 2015;56(13):7908-7914.
34 Lombardo M, Serrao S, Carbone G, Lombardo G. Corneal light backscattering after transepithelial corneal crosslinking using iontophoresis in donor human corneal tissue. J Cataract Refract Surg 2015;41(3):635-643.
35 Li N, Peng X, Fan Z, Xia Y. Iontophoretic delivery of riboflavin into the rabbit cornea: a primary study. Eye Sci 2014;29(1):30-35.
36 Mencucci R, Ambrosini S, Paladini I, Favuzza E, Boccalini C, Raugei G, Vannelli GB, Marini M. Early effects of corneal collagen cross-linking by iontophoresis in ex vivo human corneas. Graefes Arch Clin Exp Ophthalmol 2015;253(2):277-286.
37 Wollensak G, Iomdina E. Biomechanical and histological changes after corneal crosslinking with and without epithelial debridement. J Cataract Refract Surg 2009;35(3):540-546.
38 Wollensak G. Crosslinking treatment of progressive keratoconus: new hope. Curr Opin Ophthalmol 2006;17(4):356-360.
39 Mencucci R, Marini M, Paladini I, Sarchielli E, Sgambati E, Menchini U, Vannelli GB. Effects of riboflavin/UVA corneal cross-linking on keratocytes and collagen fibres in human cornea. Clin Exp Ophthalmol 2010;38(1):49-56.
40 Bikbova G, Bikbov M. Standard corneal collagen crosslinking versus transepithelial iontophoresis-assisted corneal crosslinking, 24 months follow-up: randomized control trial. Acta Ophthalmol 2016;94(7):e600-e605.
41 Lombardo M, Serrao S, Rosati M, Ducoli P, Lombardo G.Biomechanical changes in the human cornea after transepithelial corneal crosslinking using iontophoresis. J Cataract Refract Surg 2014;40(10):1706-1715.
42 Mastropasqua L, Lanzini M, Curcio C, Calienno R, Mastropasqua R, Colasante M, Mastropasqua A, Nubile M. Structural modifications and tissue response after standard epi-off and iontophoretic corneal crosslinking with different irradiation procedures. Invest Ophthalmol Vis Sci 2014;55(4):2526-2533.
43 Lanzini M, Curcio C, Spoerl E, Calienno R, Mastropasqua A,Colasante M, Mastropasqua R, Nubile M, Mastropasqua L. Confocal microscopy evaluation of stromal fluorescence intensity after standard and accelerated iontophoresis-assisted corneal cross-linking. Int Ophthalmol 2017;37(1):235-243.
44 Manetti M, Favuzza E, Sgambati E, Mencucci R, Marini M. A case of in vivo iontophoresis-assisted corneal collagen cross-linking for keratoconus: an immunohistochemical study. Acta Histochem 2017;119(3):343-347.
45 Aldahlawi NH, Hayes S, O’Brart DP, O’Brart ND, Meek KM. An investigation into corneal enzymatic resistance following epitheliumoff and epithelium-on corneal cross-linking protocols. Exp Eye Res 2016;153:141-151.
46 Bikbova G, Bikbov M. Transepithelial corneal collagen cross-linking by iontophoresis of riboflavin. Acta Ophthalmol 2014;92(1):e30-e34.
47 Lin Z, Wu H, Luo S, Liu Z, Dong N, Shang X, Li X. Transepithelial iontophoresis corneal collagen cross-linking for progressive keratoconus:one year results. Zhonghua Yan Ke Za Zhi 2015;51(9):677-682.
48 Bouheraoua N, Jouve L, Borderie V, Laroche L. Three different protocols of corneal collagen crosslinking in keratoconus: conventional,accelerated and iontophoresis. J Vis Exp 2015;(105).
49 Vinciguerra P, Randleman JB, Romano V, Legrottaglie EF, Rosetta P, Camesasca FI, Piscopo R, Azzolini C, Vinciguerra R. Transepithelial iontophoresis corneal collagen cross-linking for progressive keratoconus:initial clinical outcomes. J Refract Sur 2014;30(11):746-753.
50 Li N, Fan Z, Peng X, Pang X, Tian C. Clinical observation of transepithelial corneal collagen cross-linking by lontophoresis of riboflavin in treatment of keratoconus. Eye Sci 2014;29(3):160-164.
51 Buzzonetti L, Petrocelli G, Valente P, Iarossi G, Ardia R, Petroni S.Iontophoretic transepithelial corneal cross-linking to halt keratoconus in pediatric cases: 15-month follow-up. Cornea 2015;34(5):512-515.
52 Magli A, Chiariello Vecchio E, Carelli R, Piozzi E, Di Landro F, Troisi S. Pediatric keratoconus and iontophoretic corneal crosslinking: refractive and topographic evidence in patients underwent general and topical anesthesia, 18 months of follow-up. Int Ophthalmol 2016;36(4):585-590.
53 Lombardo M, Serrao S, Raffa P, Rosati M, Lombardo G. Novel technique of transepithelial corneal cross-linking using iontophoresis in progressive keratoconus. J Ophthalmol 2016;2016:7472542.
54 Vinciguerra P, Romano V, Rosetta P, Legrottaglie EF, Piscopo R,Fabiani C, Azzolini C, Vinciguerra R. Transepithelial iontophoresis versus standard corneal collagen cross-linking: 1-year results of a prospective clinical study. J Refract Surg 2016;32(10):672-678.
55 Jouve L, Borderie V, Sandali O, Temstet C, Basli E, Laroche L,Bouheraoua N. Conventional and iontophoresis corneal cross-linking for keratoconus: efficacy and assessment by optical coherence tomography and confocal microscopy. Cornea 2017;36(2):153-162.
56 Lombardo M, Giannini D, Lombardo G, Serrao S. Randomized controlled trial comparing transepithelial corneal cross-linking using iontophoresis with the Dresden protocol in progressive keratoconus.Ophthalmology 2017;124(6):804-812.
57 Jia HZ, Pang X, Fan ZJ, Li N, Li G, Peng XJ. Iontophoresis-assisted corneal crosslinking using 0.1% riboflavin for progressive keratoconus.Int J Ophthalmol 2017;10(5):717-722.
58 Cantemir A, Alexa AI, Galan BG, Anton N, Ciuntu RE, Danielescu C, Chiselita D, Costin D. Iontophoretic collagen cross-linking versus epithelium-off collagen cross-linking for early stage of progressive keratoconus-3 years follow-up study. Acta Ophthalmol 2017;95(7):e649-e655.
59 Bilgihan K, Yesilirmak N, Altay Y, Yuvarlak A, Ozdemir HB.Conventional corneal collagen cross-linking versus transepithelial diluted alcohol and iontophoresis-assisted corneal cross-linking in progressive keratoconus. Cornea 2017;36(12):1492-1497.
60 Rossi S, Santamaria C, Boccia R, De Rosa L, D’Alterio FM, Simonelli F, De Rosa G. Standard, transepithelial and iontophoresis corneal crosslinking: clinical analysis of three surgical techniques. Int Ophthalmol 2017.
61 Reeves SW, Stinnett S, Adelman RA, Afshari NA. Risk factors for progression to penetrating keratoplasty in patients with keratoconus. Am J Ophthalmol 2005;140(4):607-611.
62 Kankariya VP, Kymionis GD, Diakonis VF, Yoo SH. Management of pediatric keratoconus-evolving role of corneal collagen cross-linking: an update. Indian J Ophthalmol 2013;61(8):435-440.
63 Bonnel S, Berguiga M, De Rivoyre B, Bedubourg G, Sendon D,Froussart-Maille F, Rigal-Sastourne JC. Demarcation line evaluation of iontophoresis-assisted transepithelial corneal collagen cross-linking for keratoconus. J Refract Surg 2015;31(1):36-40.
64 Jordan C, Patel DV, Abeysekera N, McGhee CN. In vivo confocal microscopy analyses of corneal microstructural changes in a prospective study of collagen cross-linking in keratoconus. Ophthalmol 2014;121(2):469-474.
65 Touboul D, Efron N, Smadja D, Praud D, Malet F, Colin J. Corneal confocal microscopy following conventional, transepithelial, and accelerated corneal collagen cross-linking procedures for keratoconus. J Refract Surg 2012;28(11):769-776.
66 Rama P, Di Matteo F, Matuska S, Insacco C, Paganoni G. Severe keratitis following corneal cross-linking for keratoconus. Acta Ophthalmol 2011;89(8):e658-e659.