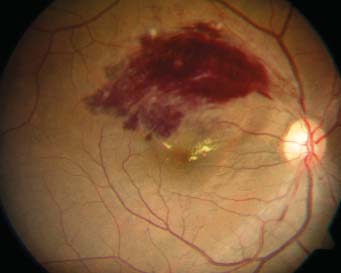
Figure 1 Fundus photograph of initial presentation Fundus photo of the patient’s right eye, illustrating a hemorrhagic BRVO along the superior arcade and macula.
We read with interest your recent article on the impact of combined oral contraceptives on ocular tissues[1].We report a case of a branch retinal vein occlusion (BRVO)associated with use of an etonogestrel/ethinyl estradiol vaginal ring (NuvaRing), an implantable combined hormonal contraceptive, in a young healthy female with no other identifiable risk factors.
A 34-year-old Hispanic female presented with 2mo of decreased vision and metamorphopsia in the right eye. Two months prior, she had started using an etonogestrel/ethinyl estradiol vaginal ring when she developed visual changes; she
self-discontinued the vaginal ring one week later but her visual symptoms did not resolve. She denied a history of diabetes,hypertension, thrombotic disorders, smoking, or any prior oral contraceptive use. There is no known family history of any thrombotic disorders.
Visual acuity was 20/40 in the right eye and 20/20-1 in the left.Intraocular pressures were 12 mm Hg in the right eye and 13 mm Hg in the left. Anterior segment examination of both eyes was unremarkable and within normal limits. Dilated retinal examination revealed a hemorrhagic superotemporal BRVO in the right eye with associated macular edema (Figure 1),which was confirmed on fluorescein angiography (Heidelberg Engineering, Heidelberg, Germany). Optical coherence tomography (OCT) of the macula (Heidelberg Engineering,Heidelberg, Germany) demonstrated both intraretinal hemorrhage and fluid, in addition to a serous detachment under the fovea (Figure 2A). The left fundus was normal.Laboratory evaluation of homocysteine levels, activated protein C resistance, protein C, protein S and antithrombin III activity, and antiphospholipid and anticardiolipin antibodies,was negative. Workup was also negative for diabetes or hypertension.
After informed consent was obtained, the patient received intravitreal bevacizumab 1.25 mg/0.05 mL. One month later the patient’s vision was 20/30, metamorphopsia had resolved,and OCT demonstrated resolution of the subretinal fluid with a minimal amount of residual intraretinal fluid (Figure 2B).She received a second intravitreal injection of bevacizumab at that time. Subsequently at 3mo after initial presentation,OCT showed complete resolution of both intraretinal and subretinal fluid, with a vision of 20/20-1 (Figure 2C). On followup 2mo later, vision was 20/20 with minimal retinal pigment epithelium changes around the superotemporal retinal vascular arcade.
A review of adverse events associated with Nuva Ring in the Food and Drug Administration (FDA) adverse event reporting system from its initial FDA approval in October 2001 through the end of December 2016 was done. Among a total of 15 526 reported adverse events associated with etonogestrel/ethinyl estradiol, there were 10 retinal vein occlusions, 2 combined retinal vein and retinal artery occlusions, and 3 retinal artery occlusions.
Since this database relied on voluntary reporting, it is difficult to extrapolate the total number of retinal vascular events associated with etonogestrel/ethinyl estradiol. However,given that it constitutes a small proportion of all adverse events associated with Nuva Ring, retinal vascular occlusions associated with using this vaginal ring seem to be quite rare,just as it is rare to find retinal vascular occlusions caused by oral contraceptive use[2].

Figure 1 Fundus photograph of initial presentation Fundus photo of the patient’s right eye, illustrating a hemorrhagic BRVO along the superior arcade and macula.

Figure 2 Macular OCT of the right eye A: At initial presentation,demonstrating both intraretinal and subretinal fluid in her macula; B:At 1mo after intravitreal bevacizumab injection, showing resolution of subretinal fluid with a trace amount of intraretinal edema; C: At 3mo after initial presentation, status post 2 injections of intravitreal bevacizumab, showing complete resolution.
Retinal vein occlusions are a potential cause of visual loss through subsequent macular edema or is chemia and have been associated with hypercoagulable states[3], including oral contraceptive use[4]. Oral contraceptives contain as little as 20 μg to as much as 50 μg of estrogen[5]. NuvaRing uses a sustained release mechanism that dispenses 120 μg etonogestrel and 15 μg ethinyl estradiol daily over a 3wk period, with 15 μg of estrogen daily considered a low dose[6].Nevertheless, a review of the literature found that the risk of thromboembolism with NuvaRing is similar to that of combined oral contraceptives[7-8]. Specific instances of venous thromboembolic events associated with Nuva Ring use include deep venous thrombosis and pulmonary embolism[9-10], cerebral venous sinus thrombosis[11-13], as well as arterial strokes[11].Given the BRVO occurred in this patient after her brief use of this implanted combined hormonal contraceptive without other risk factors in an otherwise healthy woman, the role of estrogen must be considered.
To our knowledge, this represents the first published case associating an implanted combined hormonal contraceptive device with retinal vascular complications, though several more have been reported to the FDA. We acknowledge the limitations in drawing conclusions from a single case report of a retinal vascular occlusion occurring in association with NuvaRing use. At the same time, we feel that this case is reportable because it raises an important question of whether a lower dose of estrogen than typical oral contraceptives can still be associated with increased hypercoagulability and adverse sequelae, such as in the eye. The relative paucity of retinal vascular occlusions among the entire database of adverse effects of NuvaRing reported to the FDA over its 15y on the market suggests that the incidence is quite rare, but further studies are needed to verify an association between implantable combined hormonal contraceptives and ocular vascular events.
The authors thank Juliza Manrique for her technical assistance in obtaining the clinical images presented in this letter.
Conflicts of Interest: Li AS, None; Naysan J, None;Lieberman RM, None.
REFERENCES
1 Moschos MM, Nitoda E. The impact of combined oral contraceptives on ocular tissues: a review of ocular effects. Int J Ophthalmol 2017;10(10):1604-1610.
2 Fong AC, Schatz H. Central retinal vein occlusion in young adults. Surv Ophthalmol 1993;37(6):393-417.
3 Sandset PM. Mechanisms of hormonal therapy related thrombosis.Thromb Res 2013;131 Suppl 1:S4-S7.
4 Vessey MP, Hannaford P, Mant J, Painter R, Frith P, Chappel D. Oral contraception and eye disease: findings in two large cohort studies. Br J Ophthalmol 1998;82(5):538-542.
5 Petitti DB. Clinical practice. Combination estrogen-progestin oral contraceptives. N Engl J Med 2003;349(15):1443-1450.
6 Roumen FJ, Mishell DR Jr. The contraceptive vaginal ring, NuvaRing(®), a decade after its introduction. Eur J Contracept Reprod Health Care 2012;17(6):415-427.
7 Nguyen BT, Jensen JT. Evaluating the efficacy and safety of a progestin and estrogen-releasing ethylene vinyl acetate copolymer contraceptive vaginal ring. Expert Opin Drug Saf 2014;13(10):1423-1430.
8 Rott H. Contraception, venous thrombosis and biological plausability.Minerva Med 2013;104(2):161-167.
9 Paresi RJ Jr, Myers RS, Matarasso A. Contraceptive vaginal rings:do they pose an increased risk of venous thromboembolism in aesthetic surgery? Aesthet Surg J 2015;35(6):721-727.
10 Spinner T, Segerer M, Holub A, Spes C, Mudra H. A young woman with fulminant pulmonary embolism. Internist (Berl) 2012;53(8):985-989.
11 Selvan P, Piran P, Balucani C, Tark B, Adler Z, Levine SR. Stroke and etonogestrel/ethinyl estradiol ring (Nuvaring): clinical, radiological, and prognostic features. J Stroke Cerebrovasc Dis 2017;26(3):608-617.
12 Vo TL, Cook RM, Rondina MT, Kaplan D. Cerebral venous sinus thrombosis in the setting of combined vaginal contraception. Blood Coagul Fibrinolysis 2014;25(2):183-185.
13 Kolacki C, Rocco V. The combined vaginal contraceptive ring,nuvaring, and cerebral venous sinus thrombosis: a case report and review of the literature. J Emerg Med 2012;42(4):413-416.
Received:2018-01-02
Accepted:2018-03-13
DOl:10.18240/ijo.2018.05.28
Citation: Li AS, Naysan J, Lieberman RM. Retinal vein occlusion associated with combined hormonal contraceptive vaginal ring use.Int J Ophthalmol 2018;11(5):891-892