INTRODUCTION
Conjunctivochalasis (CCH) is presented for more than 70y, but scientists still haven’t found a recognized animal model. We think there are some reasons as follows.Firstly, CCH is not a blinding eye disease, so it is little emphasized by ophthalmologists; secondly, it is difficult to change the conjunctiva morphology of the eyeball by systemic administration. An epidemiological survey from China reported that the incidence of CCH on people over the age of 60 was 44.08%[1], and its severity increases with age[2]. With the aging of the global population, CCH will performance as a protruding health problem.
Because of the lack of relevant animal models, the normal animals were used to compare electrocoagulation with simple resection for the treatment of CCH[3]. Due to the difference between model animals and normal animals, the result is difficult to be recognized and promoted. Therefore, there is an urgent need for an animal model of CCH in the field of ophthalmology.
We hypothesized that it is possible to explore a feasible method for constructing animal models if the etiology and pathogenesis of CCH is replicated in the animal. The literature suggests that the most important factor associated with the development of CCH is aging[4]. Subcutaneously injection of D-galactose is one of the common methods of animal model of aging. In 1986, Meller and Tseng[5] hypothesized that the mechanism of CCH development is the accumulation of degrading enzymes which resulted in elastotic degeneration and collagenolysis of bulbar conjunctiva in the tears as a result of delayed tear clearance. Taking this hypothesis as the guiding principle, the later researchers made further study on the pathogenesis of CCH. Ward et al[6] found that all conjunctival resection specimens from the patients with CCH revealed marked staining for matrix metalloproteinase-3(MMP-3) and matrix metalloproteinase-9 (MMP-9). Xiang et al[7] reported that the expression of interleukin-6 (IL-6),interleukin-8 (IL-8), and tumor necrosis factor alpha (TNF-α)was up-regulated in tears of patients with CCH. Both studies demonstrated that CCH’s pathogenesis is closely related to MMPs and inflammatory factors. Based on these two studies,subconjunctivally injection of MMP-3 and eye drops of TNF-α were attempted to construct an animal model of CCH.
The specific steps of the experiment are: 1) the rabbits administered subcutaneous injection of D-galactose, subconjunctival injection of MMP-3 and eye drops of TNF-α respectively;2) anterior segment photography, light microscopy and transmission electron microscopy (TEM) was performed to evaluate the anatomical and histological features of the rabbits’conjunctiva at 12wk postoperatively; 3) the anatomical and histological features of conjunctiva were compared between the CCH findings in the literature and the rabbits; 4) the validity of the three methods was evaluated by the similarity of anatomical and histological features between the CCH findings in the literature and the rabbits. Our results showed that the anatomical and histological features of the conjunctiva in rabbits who administered subconjunctival injection of MMP-3 are most similar to those of CCH.
MATERIALS AND METHODS
Twelve New Zealand white rabbits aged between 12wk and 14wk and weighing 2.0-2.6 kg were used.
Procedures and Examination Animals were randomly divided into 4 groups to accept different interventions: the control group (n=3), one of them received no interventions and the others were underwent subconjunctival injection of sterile water 0.3 mL each eye per week; the MMPs group (n=3), each rabbit eye was given a subconjunctival injection of MMP-3 (Recombinant Human MMP-3, PerproTech, USA) 0.3 mL(1500 μg/L) per week; the aging group (n=3), 1 g/kg of body weight of D-galactose was injected subcutaneously into the back of each rabbit per day; the TNF solution group (n=3),each rabbit eye was given 0.05 mL (250 μg/L) of TNF-α(recombinant human TNF-α, PerproTech, USA) solution 3 times a day. The total intervention time was 12wk.
Anterior segment photography (Smartscope M5 OPTOMED,Finland) was performed preoperatively and 12wk postoperatively.At the 84th postoperative day, all the rabbits were sacrificed by intravenous injection of pentobarbital sodium. Each rabbit’s eyes were picked and the intact conjunctiva was preserved,and stored in 10% neutral formalin solution after the lens and vitreous body were removed. The tissue used for light microscopy was obtained from the inferior bulbar conjunctiva and preserved in 10% formaldehyde, embedded in paraffin,prepared for sequential 3-4 µm sections. Hematoxylin and eosin (HE) staining, trichrome staining, aldehyde-fuchsin staining were used to evaluate the general cellularity, collagen fibers and elastic fibers of the rabbits’ conjunctiva. The tissue used for TEM was obtained from the middle of the inferior bulbar conjunctiva of the rabbits’ right eyes and fixed in 2.5% glutaraldehyde in 0.1 mol/L phosphate buffer. After conventional dehydration, embedding, fixation, sectioning and staining, the fibroblasts of the tissue samples were observed and photographed by TEM (JEM 1200EX, Japan Electronics Co., Ltd.).
Ethics Statement All animal experimental process complies with the “ARVO Statement for the Use of Animals in Ophthalmic and Vision Research” and the “Guiding Opinions on the Treatment of Laboratory Animals” formulated by the Ministry of Science and Technology of the People’s Republic of China.
RESULTS
Anterior Segment Photography The inferior bulbar conjunctiva was close to the scleral surface and had no change under external force in the control group, the aging group and the TNF solution group. In contrast, a continuous wrinkle of the inferior bulbar conjunctiva was formed parallel to the eyelid under external force in the MMPs group (Figure 1).
Hematoxylin and Eosin Staining The conjunctival epithelium was a stratified epithelium of 2-4 layers, dilated lymphatic vessel without inflammatory cell in filtration (Figure 2). There were no significant differences among the groups.
Trichrome Staining The collagen fibers of the control group,the aging group, the TNF solution group were arranged in parallel but they were disordered, degenerated, destroyed in the MMPs group (Figure 3).
Aldehyde-fuchsin Staining The elastic fibers of the control group, the aging group, the TNF solution group were normal but they were fragmented in the MMPs group (Figure 4).
Transmission Electron Microscopy In the control group, the aging group and the TNF solution group, the fibroblasts were spindle-shaped and collagen fibers were densely arranged. In contrast, sparse collagen fibers and focal necrosis of fibroblasts were observed in MMPs group (Figure 5).
DISCUSSION
Three methods that subconjunctival injection of MMP-3,subcutaneous injection of D-galactose and eye drops of TNF-α were used to construct an animal model of CCH in our research. Among them, only subconjunctival injection of MMP-3 made the conjunctiva changes. The anatomical and histological changes of the conjunctiva of the MMPs group were formed wrinkles under external force, loose and disorderly collagen fibers, broken elastic fibers and focal necrosis of fibroblasts.
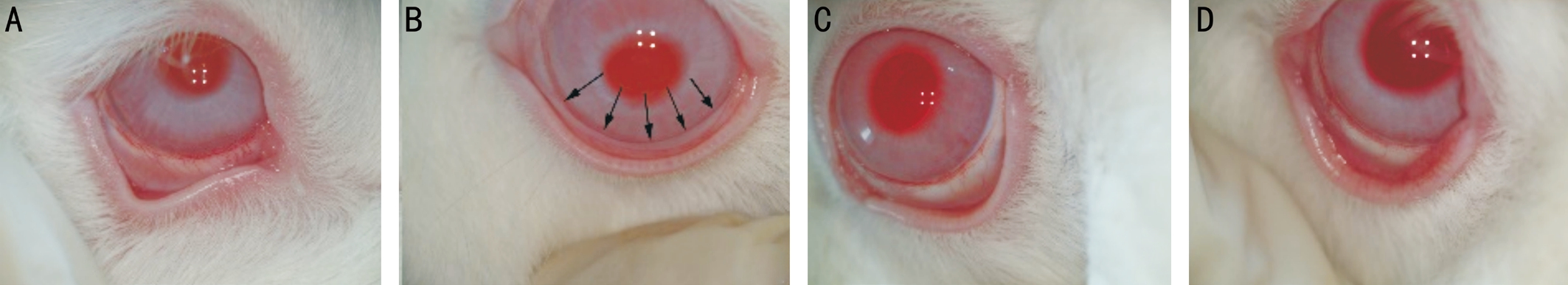
Figure 1 The comparison of anterior segment photography A: The control group; B: The MMPs group, arrows showed continuous wrinkles;C: The aging group; D: The TNF solution group.
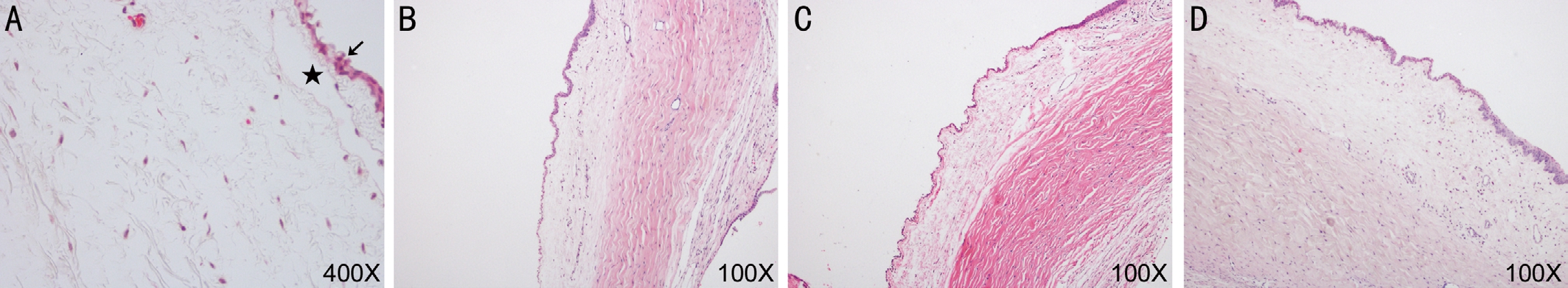
Figure 2 The comparison of HE staining A: The control group; B: The MMPs group; C: The aging group; D: The TNF solution group. Arrow:The conjunctival epithelium was a stratified epithelium of 2-4 layers; Pentagram: Dilated lymphatic vessel.
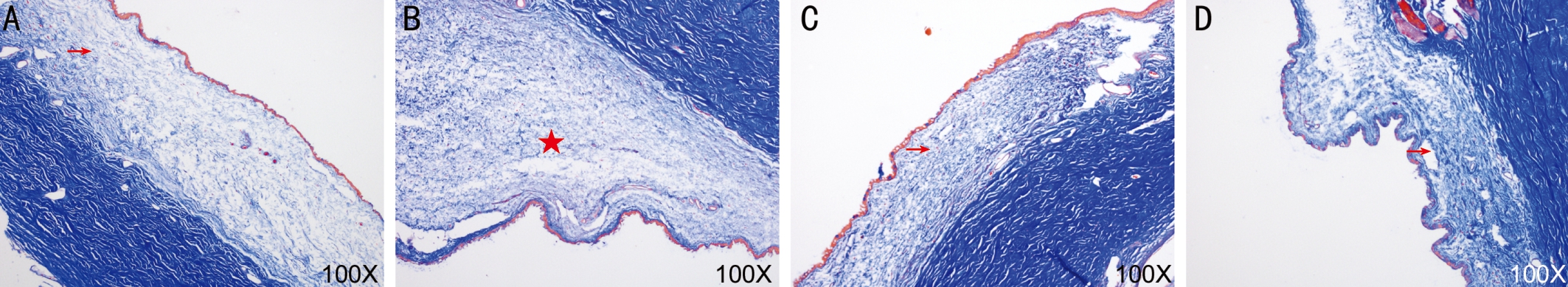
Figure 3 The comparison of trichrome staining A: The control group; B: The MMPs group; C: The aging group; D: The TNF solution group.Arrow: Arranged in parallel; Pentagram: Disordered in parallel.
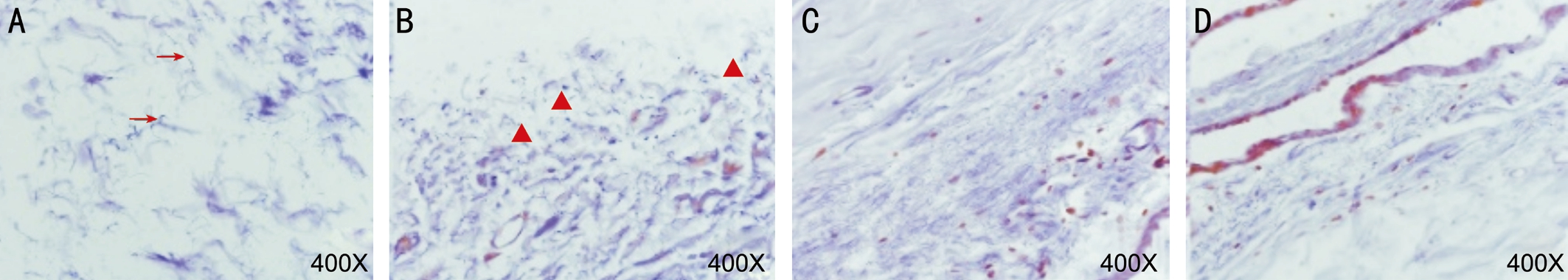
Figure 4 The comparison of aldehyde-fuchsin staining A: The control group; B: The MMPs group; C: The aging group; D: The TNF solution group. Arrow: Normal elastic fibers; Triangle: Fragmented elastic fibers.
CCH is defined as a loose inferior bulbar conjunctiva interposed between the ocular globe and the lower eyelid and affects the tear film stability and eyelid closure[4]. Histopathological manifestations of the loosen conjunctiva in patients with CCH have included hyperplasia of squamous epithelium with parakeratosis, infiltration of inflammatory cells, dilated lymphatic vessels, decreased collagen densities, degeneration of elastic fibers[8-12]. Observation by TEM, Li et al[13] reported that fibroblasts in CCH had decreased number, long spindle shape, and partially necrosis; collagen fibrils were dispersed and dissolved in some areas. This showed that the anatomical and histological features of the conjunctiva from CCH included the formation of conjunctival folds, hyperplasia of squamous epithelium, in filtration of inflammatory cells, dilated lymphatic vessels, decreased collagen densities, degeneration of elastic fibers; focal necrosis of fibroblasts.
The anatomical and histological features of the conjunctiva were compared between experimental animal and CCH. The results were shown in Table 1.
As the anatomical and histological features of the conjunctiva in rabbits who administered subconjunctival injection of MMP-3 are most similar to those of CCH, our findings suggest that subcutaneous injection of MMPs may be a viable approach to construct an animal model of CCH. The establishment of this animal model has important scientific and clinical significance to explore the occurrence and development regularity, from which we could find effective prevention methods and treatments of CCH. It will bring bene fits to patients with CCH, and further promote the development of ophthalmology.Primates, as distant relatives of humans, are most suited as experimental model animals, but our funds cannot afford such expensive animals. Mice are cheaper than primates, but their eyeballs are too small to collect enough conjunctival tissue. Based on the above two reasons, rabbits were selected as model animals. In theory, model animals should have all the clinical manifestations of CCH, including subjective symptoms and objective changes in the conjunctiva. In fact,subjective symptoms cannot be simulated in animals and only objective changes of the conjunctiva can be detected. So the anatomical and histological features of the conjunctiva are used to evaluate animal models. MMP family members have more than 20 species and MMP-1, MMP-2, MMP-3 and MMP-9 were related to CCH[14]. Because there is no animal model of CCH can be used for reference, it is difficult to choose the appropriate member of MMPs family as intervention drug and to confirm the appropriate concentration and the regimen.Li et al[15] reported that the content of MMP-1, MMP-3 in the conjunctiva from patients with CCH was 489.099±164.494 μg/L,1236.963±1419.531 μg/L respectively. MMP-3 was optionally chosen as intervention medication. In order to being operated easily, the concentration of MMP-3 was set to 1500 μg/L in our experiment. Rabbit’s conjunctiva swelled rapidly after subconjunctival injection and recovered to normal in 5-7d, so interval time of subconjunctival injection was set to a week.Totally 0.3 mL was found to be the maximum volume of subconjunctival injection in the trial. So that the final regimen was that each eye accepted a subconjunctival injection of MMP-3 0.3 mL (concentration was 1500 μg/L) per week.
Table 1 The comparison of anatomical and histological features of the conjunctiva between experimental animal and CCH

The anatomical and histological features of the conjunctiva in the MMPs group are most similar to those of CCH than the other groups. CCH:Conjunctivochalasis; MMPs: Matrix metalloproteinases; TNF-α: Tumor necrosis factor-α.

Figure 5 The comparison of TEM A1, A2: The control group; B1,B2: The MMPs group; C: The aging group; D: The TNF solution group.Pentagram: Sparse collagen fibers; Arrow: Focal necrosis of fibroblasts.
The expression of inflammatory factors such as TNF-α, IL-6 and IL-8 were up-regulated in tears of patients with CCH,the concentration of TNF-α, IL-6 and IL-8 was 19.05±6.35 ng/mL,13.16±8.97 pg/mL and 34.40±20.73 pg/mL respectively[7].TNF-α was chosen as the intervention medication optionally among inflammatory factors. Taking account eye drops of TNF-α solution will rapidly be diluted and cleared by tears,based on the data of above literature, its concentration of our experiment was increased to 250 μg/L. In order to maintain the concentration of TNF-α in tears, so the dosage was three times per day. And 0.05 mL was the maximum volume of single conjunctival sac volume on rabbit. The final regimen was that each rabbit eye was given 0.05 mL (250 μg/L) of TNF-α solution 3 times a day.
Several clinical epidemiological data show that the incidence of CCH increases with age[16]. Related literature shows that senescence can increase the activity of MMPs[17] and reduce the secretion of extracellular matrix[18]. Based on the above data, the aging group was set up in our experiment. The natural aging model is most similar for human physiological aging, but the natural life of New Zealand rabbits is 8y, so the natural aging method is not desirable. D-galactose-induced aging model can mimic the natural senescence in the aspects of morphology, biochemical index detection and molecular biology, so it was selected. The dosage of D-galactose used in animal model was from 30 mg/kg to 1000 mg/kg and the time of administration was 6wk[19-20]. Finally, the regimen was that 1 g/kg of body weight of D-galactose was injected subcutaneously into the back of each rabbit per day. Worried about the failure of establishment on animal model, so the time of administration of D-galactose was extended from 6wk in the literature to 12wk in our experiment. In order to manage and operate conveniently, the time of administration of the MMPs group and the TNF-α group was as the same as those of the aging group.
The pathogenesis of CCH can be summarized as follows:various etiologies[4] induce the increase of inflammatory factors[7] such as ILs, TNF, which resulting in imbalance of MMPs[6] and tissue inhibitor of metalloproteinases, and eventually leads to degradation of elastic fibers. The reduction of elastic fibers makes the bulbar conjunctiva less elastic and cannot adhere to the sclera, forming wrinkles under repeated compression by inferior palpebral margin. Conjunctival folds delay the removal of tears, and delayed tear clearance leads to accumulation of inflammatory factors in the tears, which in turn promotes progression of the disease. From the mechanism for human CCH, all three methods should be effective.
Unfortunately, subcutaneous injection of D-galactose and eye drops of TNF-α did not work as expected in the experiment.The specific reasons were unknown. It should not be negated the idea of using aging and inflammatory factors to construct animal model of CCH. Perhaps the changes in the route of administration from eye drops to subconjunctival injection will help TNF-α (inflammatory factor) to take effect in the trial. In addition, the combination between D-galactose-induced aging model and other methods probably bring unexpected results.
There were some limitations in this study. First, the number of rabbits used in our study was too small to carry out statistical analysis, so the conclusion has some limitations. Second, the conjunctival performance of animals of the aging group was evaluated, but the aging model itself was not assessed. Third,the pictures we took were not the same position of the anterior eye in the rabbits. Fourth, on the experiment plan the choice of intervention medication and the confirmation of the dosage look like not rigorous. Finally, using the anatomical and histological changes of the conjunctiva to evaluate the animal model of CCH may not appropriate.
In conclusion, subconjunctival injection of MMPs may be a feasible method for the establishment of an animal model of CCH. In the future we will try to choose the other MMPs family members as intervention medication, to explore an optimal dosage and time on intervention medication, to probe an appropriate method for evaluating the animal model of CCH.
ACKNOWLEDGEMENTS
Thanks for the support from the Laboratory Animal Center and the Pathology Department.
Foundations: Supported by the Key Medical Discipline Project of Shanghai Municipal Health Bureau-Ophthalmology(No.ZK2015A20); the Health System Independent Innovation Science Foundation of Shanghai Putuo District(No.2015PTKW001); Plateau Science, Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine.
Conflicts of Interest: Gan JY, None; Li QS, None; Zhou HM, None; Zhang W, None; Lian LZ, None; Yu Z, None;Zhang ZY, None.
REFERENCES
1 Zhang X, Li Q, Zou H, Peng J, Shi C, Zhou H, Zhang G, Xiang M, Li Y. Assessing the severity of conjunctivochalasis in a senile population: a community-based epidemiology study in Shanghai, China. BMC Public Health 2011;11:198.
2 Gumus K, Pflugfelder SC. Increasing prevalence and severity of conjunctivochalasis with aging detected by anterior segment optical coherence tomography. Am J Ophthalmol 2013;155(2):238-242.e2.
3 Kim KH, Ko AY, Ryu JS, Kim MK, Wee WR. Effect of electrocauterization on the inflammation of the conjunctiva in experimental animal model.Korean J Ophthalmol 2013;27(4):282-287.
4 Hughes WL. Conjunctivochalasis. Am J Ophthalmol 1942;25(1):48-51.
5 Meller D, Tseng SC. Conjunctivochalasis: literature review and possible pathophysiology. Surv Ophthalmol 1998;43(3):225-232.
6 Ward SK, Wakamatsu TH, Dogru M, Ibrahim OM, Kaido M, Ogawa Y,Matsumoto Y, Igarashi A, Ishida R, Shimazaki J, Schnider C, Negishi K,Katakami C, Tsubota K. The role of oxidative stress and inflammation in conjunctivochalasis. Invest Ophthalmol Vis Sci 2010;51(4):1994-2002.
7 Xiang MH, Zhang XR, Zhang XY, Zhuang FJ, Li L, Li QS, Fan J.Tears cytokines in conjunctivochalasis. Guoji Yanke Zazhi (Int Eye Sci)2010;10(9):1702-1703.
8 Zhang XR, Zhang ZY, Hoffman MR, Li QS, Liu B, Zhou HM. The effect of age and conjunctivochalasis on conjunctival thickness. Curr Eye Res 2013;38(3):331-334.
9 Bae JB, Park WC. Histopathologic characteristics of conjunctivochalasis.J Korean Ophthalmol Soc 2013;54(8):1165-1174.
10 Yu XY, Jian ZY, Wu W, Lu XH. Simultaneous treatment of pterygium complicated with conjunctivochalasis: analysis of pterygium excision and conjunctival autotransplantation combined with sclera fixation. BMC Ophthalmol 2015;15:100.
11 Kantaputra PN, Kaewgahya M, Wiwatwongwana A, Wiwatwongwana D, Sittiwangkul R, Iamaroon A, Dejkhamron P. Cutis laxa with pulmonary emphysema, conjunctivochalasis, nasolacrimal duct obstruction, abnormal hair, and a novel FBLN5 mutation. Am J Med Genet A 2014;164A(9):2370-2377.
12 Francis IC, Chan DG, Kim P, Wilcsek G, Filipic M, Yong J, Coroneo MT.Case-controlled clinical and histopathological study ofconjunctivochalasis.Br J Ophthalmol 2005;89(3):302-305.
13 Li QS, Yu Z, Xiang MH, Zhang XR, Li YJ. Ultrastructure change of conjunctiva and fascia tissue of conjunctivochalasis. Chin J Exp Ophthalmol 2012;30(7):638-640.
14 Li DQ, Meller D, Liu Y, Tseng SC. Overexpression of MMP-1 and MMP-3 by cultured conjunctivochalasis fibroblasts. Invest Ophthalmol Vis Sci 2000;41(2):404-410.
15 Li QS, Zhang XR, Xiang MH, Zhang XY, Ni ZH, Zhang L, Shen JF,Zhou HM, Shi CC, Li YJ. Expression of matrix metalloproteinases in conjunctival tissues of the patients with conjunctivochalasis. Chin J Exp Ophthalmol 2010;46(9):838-840.
16 Mimura T, Yamagami S, Usui T, Funatsu H, Mimura Y, Noma H,Honda N, Amano S. Changes of conjunctivochalasis with age in a hospital-based study. Am J Ophthalmol 2009;147(1):171-177.e1.
17 Kostrominova TY, Brooks SV. Age-related changes in structure and extracellular matrix protein expression levels in rat tendons. Age (Dordr)2013;35(6):2203-2214.
18 Wang M, Kim SH, Monticone RE, Lakatta EG. Matrix metalloproteinases promote arterial remodeling in aging, hypertension, and atherosclerosis.Hypertension 2015;65(4):698-703.
19 Wang HL, Wu T, Qian JB, Wu J, Quo QJ. Effect of D-galactose on parameters for skin aging in murine model. Chin J Geriatr 2004;23(8):566-568.
20 Zhang WL, Li RH, Wang SM, Mu FZ, Jia P. Fuyuan capsule downregulates uPA,uPAR and NF-κB while upregulates PAI in knee cartilage of osteoarthritis rabbits. Third Mil Med Univ 2011;33(20):2172-2176.