INTRODUCTION
Fungal keratitis is an infectious corneal disease common in agricultural areas that can cause visual impairment and blindness[1-3]. Pattern recognition receptors (PRRs) are triggered by pathogen-associated molecular patterns (PAMPs)derived from microorganisms, leading to the production of many pro/anti-inflammatory cytokines[4-5]. The immune response protects the host from pathogenic microorganisms,but the strict regulation of the induced response is a necessary condition for limiting bystander damage[6]. But how to strike a balance between infection/inflammation and bystander damage?Interleukin-10 (IL-10) is a general anti-inflammatory cytokine,it mediates anti-inflammatory response and limits bystander damage[6-7]. Previous studies have shown that the recognition of PAMPs by Toll-like receptors (TLRs) induces IL-10 production in macrophages[8-9] and B cells[10-11] and dendritic cells (DCs)[12]. Dectin-1 is one of C-type lectin receptors(CLRs) that participates in many activities in protection against fungi by inducing cytokine production, reactive oxygen species[13-14]. Lipoxygenase-1 (LOX-1) is expressed in many types of cells, and our previous studies have proved that LOX-1 was expressed in human corneal epithelial cells and was up-regulated after Aspergillus fumigatus (A. fumigatus)stimulation[15]. What’s more, LOX-1 was involved in production of pro-inflammatory cytokines in mice with fungal keratitis[3,16].However, whether or not LOX-1 and Dectin-1 are involved in the regulation of IL-10 production in mouse with A. fumigatus keratitis is rarely studied. Our study showed that IL-10 was upregulated upon fungal stimulation and was regulated by LOX-1 and Dectin-1 in A. fumigatus keratitis.
MATERIALS AND METHODS
Mice and Corneal Infection Eight-week C57BL/6 female mice(Changzhou Cavens Laboratory Animal Ltd., Jiangsu, China)were used in experiments. A. fumigatus strain (No.3.0772)was provided by the China General Microbiological Culture Collection Center (Beijing, China). After anesthesia, 2 μL conidial suspension (5.0×104 conidia/μL PBS) was given into the mice corneal stroma with a No.33 Hamilton syringe. All animal experiments were in accordance with Statement on the Use of Animals in Ophthalmic and Vision Research announced by Association for Research in Vision and Ophthalmology(ARVO).
Down-regulation of Lipoxygenase-1 and Dectin-1 A total of 5 μL LOX-1 inhibitor Poly(I) (2 μg/5 μL; Sigma) or sterile water was given into the left eyes of C57BL/6 mice (n=6/group/time)by subconjunctival injection 1d before or at the time of A. fumigatus infection. The 5-μL Dectin-1 siRNA (40 μmol/L; Santa Cruz) or scrambled siRNA (Santa Cruz) was given into the left eyes of C57BL/6 mice (n=6/group/time) by subconjunctival injection 1d before or at the time of A. fumigatus infection.
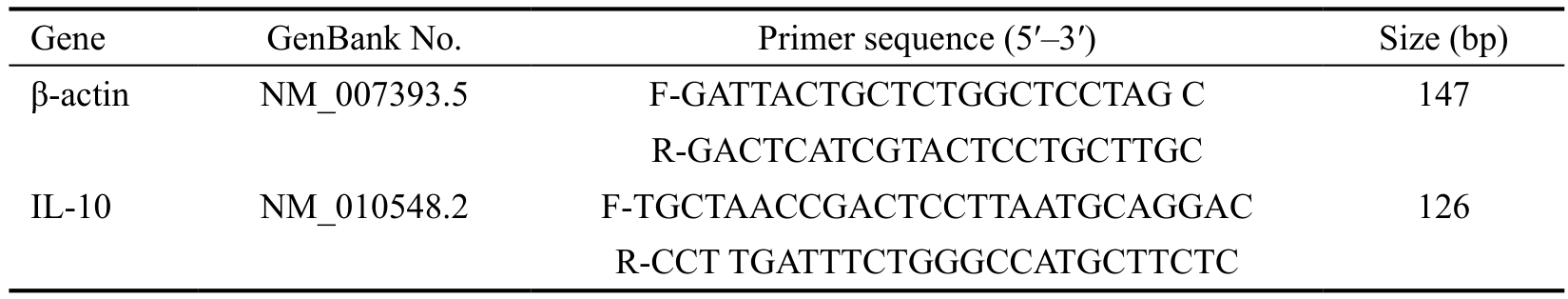
Table 1 Nucleotide sequences of mouse primers for real-time RT-PCR
RT-PCR: Reverse transcription-polymerase chain reaction; IL-10: Interleukin-10.
Real-time Reverse Transcription-polymerase Chain Reaction After sacrifice, the corneas of C57BL/6 mice were removed. Total RNA was extracted using RNAiso plus reagent(TaKaRa) and rapidly detected using spectrophotometry(Eppendorf). Of 2 μg total RNA was reverse transcribed using the PrimeScript RT Reagent Kit (TaKaRa). Real-time PCR was carried out using SYBR green and Eppendorf Mastercycler.All values obtained were normalized to β-actin. The sequences of oligonucleotide primers are listed in Table 1.
Western Blotting Radioimmunoprecipitation assay (RIPA;Solarbio) mixed with PMSF (Solarbio) and phosphatase inhibitor cocktail (MCE) (100:1:1) was used to extract total protein from the corneas of C57BL/6 mice. Total protein was run on 8%-16% SDS-PAGE gels (GenScript, China)and transferred to PVDF membrane (Millipore). Membranes were blocked with blocking buffer (Beyotime, China) at room temperature for 2h and incubated with IL-10 primary antibody at 4℃ overnight. Horse radish peroxidase-linked antimouse was used for secondary antibodies. Electrochemical luminescence reagents (BIO-RAD) were used to detect the signal of the bands.
Statistical Analysis Data are expressed as mean±SD and were analyzed by GraphPad 5.0 software (USA). Statistical significance of real-time RT-PCR, Western blotting data was determined by unpaired, two-tailed Student’s t-test. Differences were considered significant at P≤0.05.
RESULTS
Increase of IL-10 in mouse A. fumigatus keratitis In order to facilitate the follow-up study, the time-dependent expression of IL-10 was examined in mice with A. fumigatus keratitis.PCR and Western blot were done to examine the expressions of IL-10 in normal and A. fumigatus infected mouse corneas.Compared with the normal control, infection of mice corneas by A. fumigatus significantly increased the mRNA level of IL-10 at 12h (P<0.001; Figure 1A), 1d (P<0.001; Figure 1A) and 2d (P<0.001; Figure 1A). IL-10 mRNA expression increased gradually from 12h after A. fumigatus infection (Figure 1A).
The result of Western Blot showed that there is no significant difference in IL-10 protein expression level between the normal control and 12h p.i (Figure 1B), but it was significantly increased at 1d (P<0.001; Figure 1B) and 2d (P<0.001; Figure 1B).
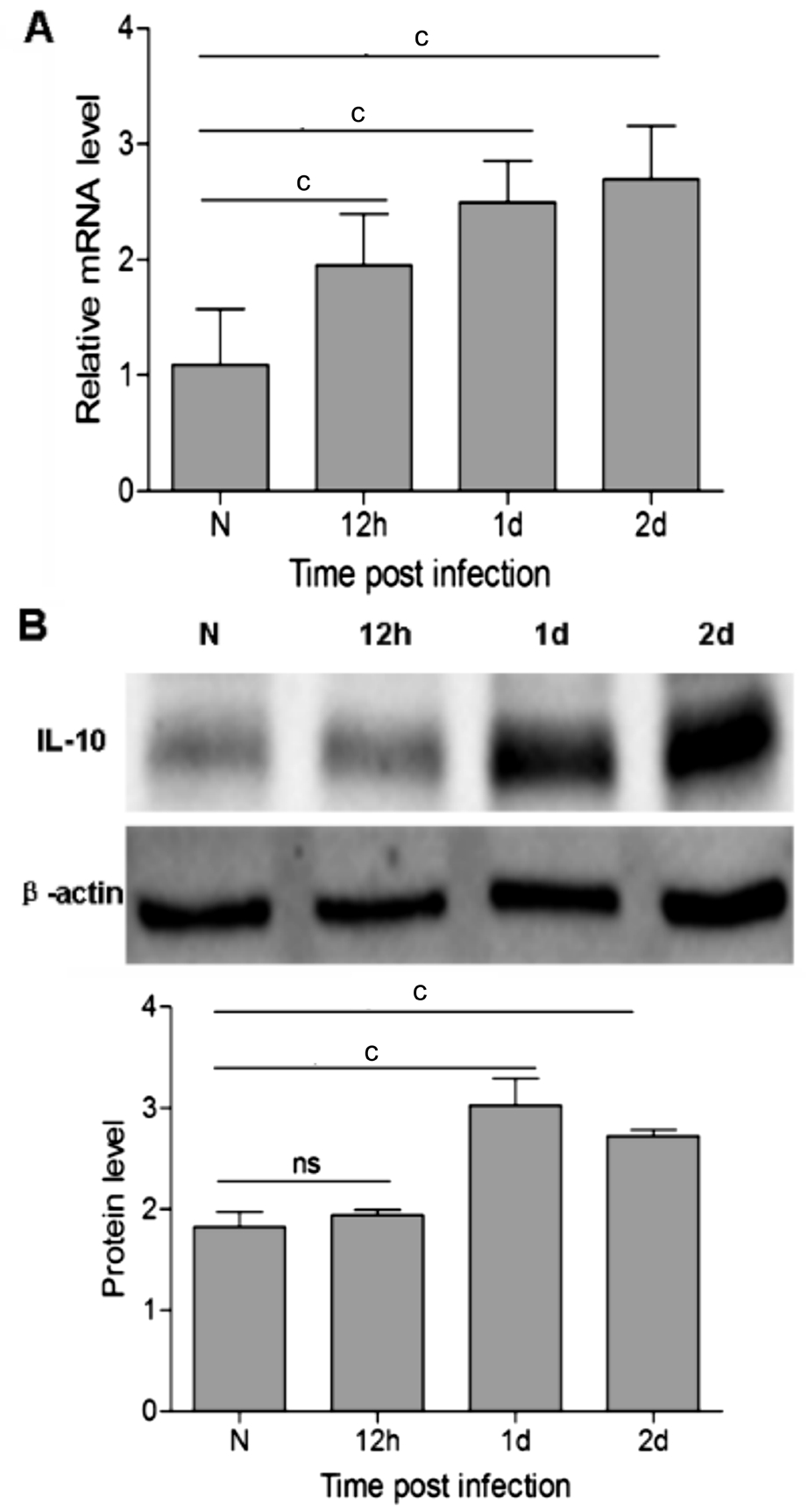
Figure 1 IL-10 expression was upregulated in mice with A.fumigatus keratitis A: IL-10 mRNA fold change in C57BL/6 mice corneas infected by A. fumigatus at 12h, 1d and 2d; B: IL-10 protein fold change in C57BL/6 mice corneas infected by A. fumigatus at 12h, 1d and 2d. cP<0.001.
Regulation of LOX-1 on IL-10 in mouse A. fumigatus keratitis Since LOX-1 is involved in the defense of fungal infection, we set out to investigate the regulation of LOX-1 on IL-10 production in mice with A. fumigatus keratitis. The corneas of C57BL/6 mice were challenged with LOX-1 inhibitor Poly(I) before A. fumigatus infection. Compared with uninfected sterile water control, IL-10 mRNA expression in response to A. fumigatus infection was significantly increased (P<0.001; Figure 2A). However, compared with the group pretreated with sterile water before infection, Poly(I)pretreatment suppressed IL-10 mRNA expression significantly(P<0.001; Figure 2A).
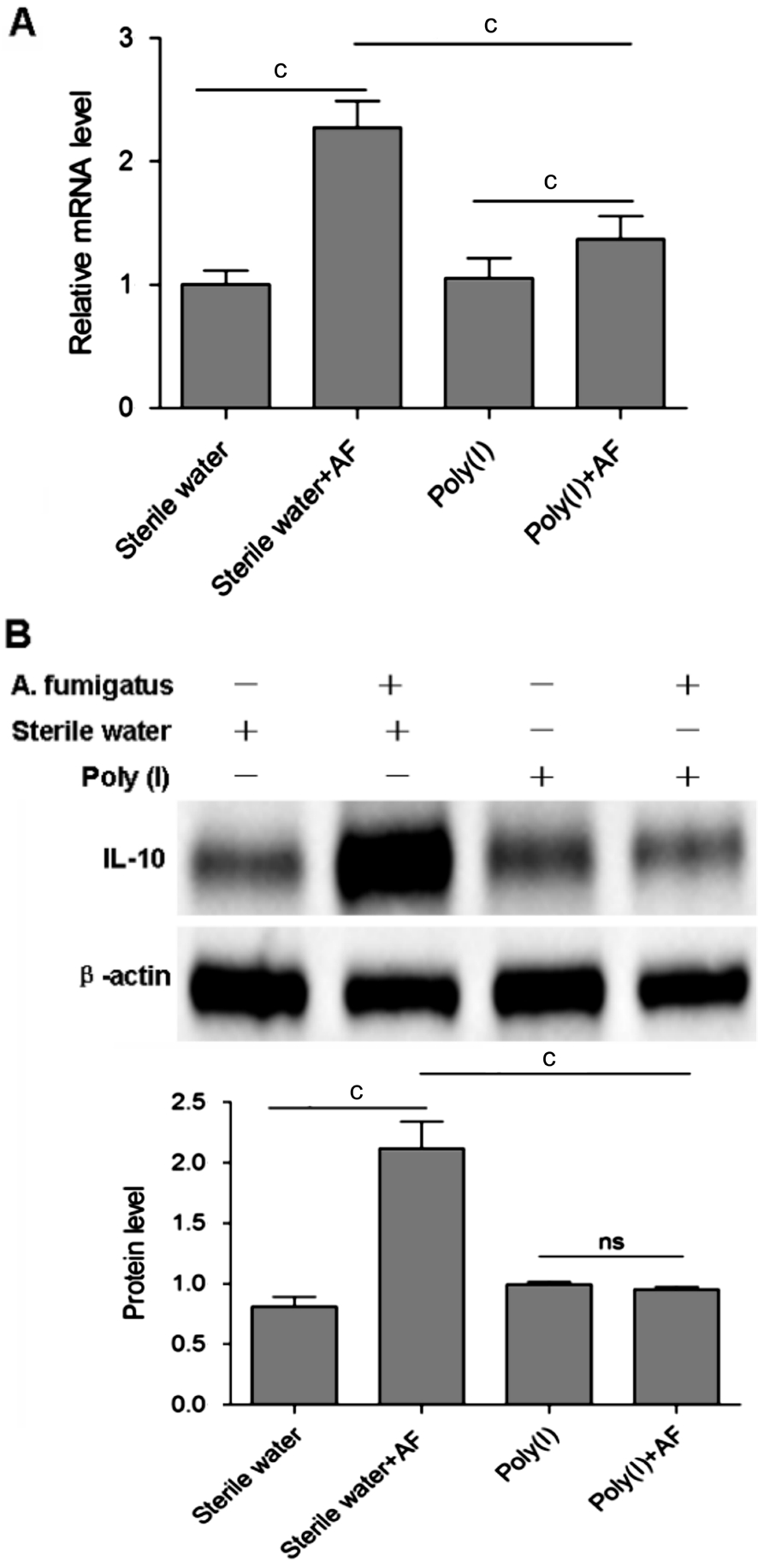
Figure 2 The change of IL-10 expression when C57BL/6 mice corneas were pretreated with LOX-1 inhibitor Poly(I) before A.fumigatus infection Compared with the group pretreated with sterile water before infection, Poly(I) pretreatment suppressed IL-10 mRNA(A) and protein (B) expression significantly. cP<0.001.
The IL-10 protein expression was also detected. The change of IL-10 protein expression showed that IL-10 protein level was significantly increased after A. fumigatus infection compared with uninfected sterile water control (P<0.001; Figure 2B).But LOX-1 inhibitor Poly(I) suppressed the up-regulation significantly (P<0.001; Figure 2B). The results suggested that IL-10 was regulated by LOX-1 in mouse A. fumigatus keratitis.
Regulation of Dectin-1 on IL-10 in mouse A. fumigatus keratitis Upon easts and zymosan stumulation, Dectin-1 was involved in IL-10 production, to determine the role of Dectin-1 in A. fumigatus induced IL-10 production, special siRNA targeting Dectin-1 was used in this study. A higher level of IL-10 mRNA expression was detected in scrambled siRNA pretreated mice with A. fumigatus infection than uninfected scrambled siRNA control (P<0.001; Figure 3A). Dectin-1 silencing significantly reduced IL-10 mRNA expression in reponse to A. fumigatus infection when compared infected Dectin-1 siRNA pretreated group with scrambled siRNA control (P<0.001; Figure 3A).
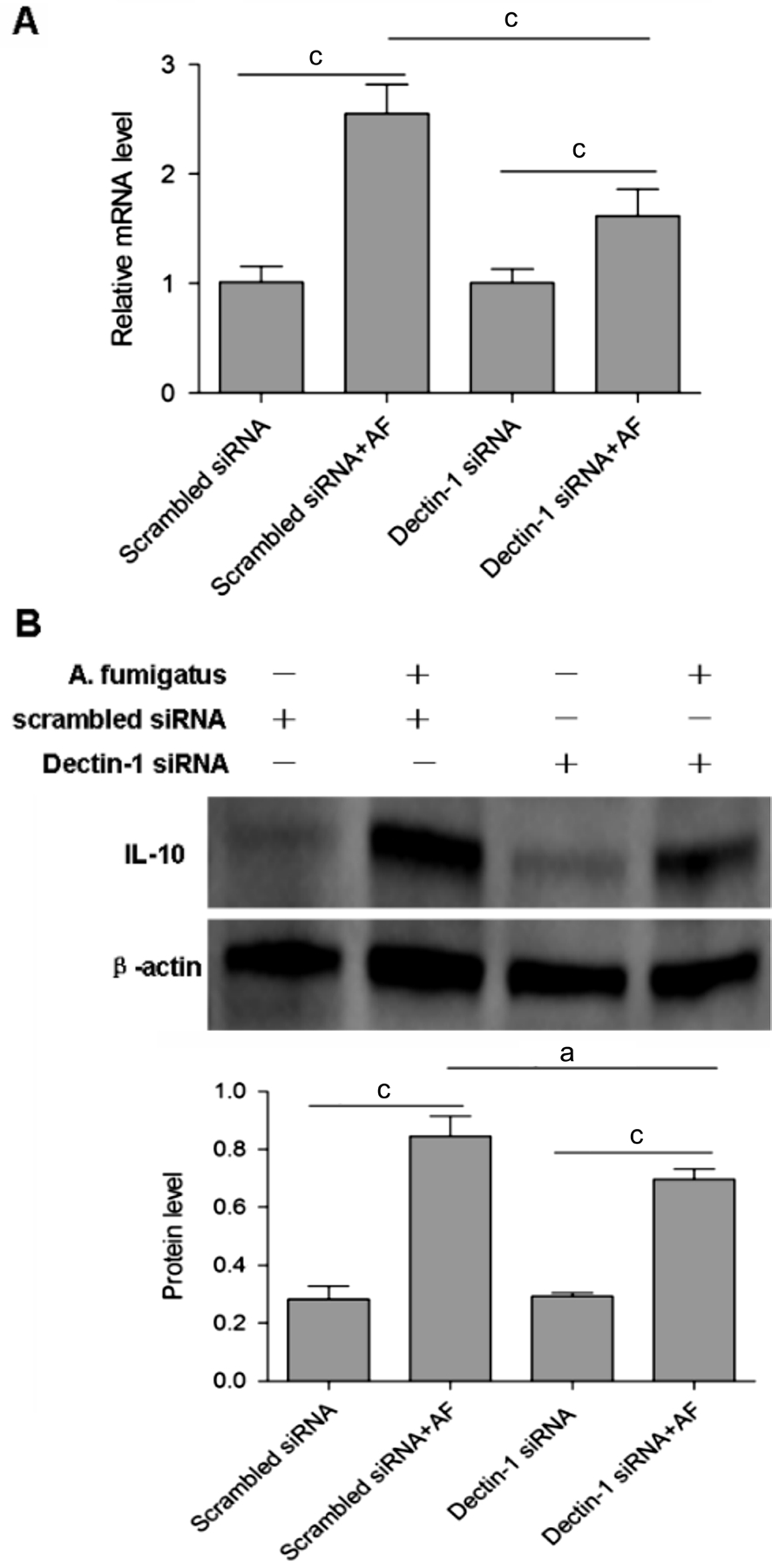
Figure 3 The change of IL-10 expression when C57BL/6 mice corneas were pretreated with Dectin-1 siRNA before A. fumigatus infection The group pretreated with Dectin-1 siRNA before A.fumigatus infection had a lower IL-10 mRNA (A) and protein (B)expression compared with the group pretreated with scrambled siRNA before A. fumigatus infection. aP<0.05, cP<0.001.
The result of Western Blot showed that the group pretreated with Dectin-1 siRNA before A. fumigatus infection had a lower IL-10 protein expression compared with the group pretreated with scrambled siRNA before A. fumigatus infection (P<0.05;Figure 3B). which is consistent with the fold change of mRNA.The results suggested that Dectin-1 induced the production of IL-10 in mouse A. fumigatus keratitis.
DISCUSSION
Fungal recognition and uptake by the cornea induces the production of inflammatory response, which can remove fungi on the one hand and, cause corneal tissue damage on the other hand. How to limit tissue damage caused by inflammatory response while removing the fungus?Our study found that IL-10 expression was elevated in A.fumigatus keratitis. IL-10 was initially classified as a Th2-type cytokine, it functions to mediate anti-inflammatory response and limits bystander damage[6]. For example, it can inhibit the synthesis and release of inflammatory cytokines TNF-α, IL-1, IL-6, IL-12 and so on[17], suppress antigen presenting cell function[18-19], and inhibit Th1 type immune response[6]. However, on the other hand, IL-10 mediated anti-inflammatory response can be exploited by pathogens,which contributes to establish pathogenic persistence and chronic infection status[6,20-22]. For example, many chronic infection of bacteria and viruses were associated with elevated IL-10 expression[23]; DCs and macrophages induced IL-10 production can serve as an immune evasion mechanism for many pathogens; IL-10 and IL-10 induced Treg cells impairs the control and clearance of Candida albicans[20],Mycobacterium tuberculosis[24], and Schistosoma mansoni[25]in these infection models. Given that the role of IL-10 in the host inflammatory response may not show redundancy.It’s important for us to investigate the regulation of IL-10 production in mouse with A. fumigatus keratitis. Our study showed that IL-10 was regulated by LOX-1 and Dectin-1 in A. fumigatus keratitis.
PRRs detect PAMPs of the pathogens in the first line of host immunity and, then trigger an immune response[26-28]. TLRs and CLRs expressed on DCs and other immune cells are a good example of PRRs[29-31]. DCs, as an immune cell, were initially considered to be important in starting immune response, but more recent evidence suggests that they play a key role in regulating quality of the immune response and in inhibiting the immune response[26-27]. PRRs are essential for immune cells to perform their functions. LOX-1 and Dectin-1 belong to CLRs.LOX-1, one of the major receptors of oxidized low density lipoprotein (oxLDL), plays a role of promoting atherosclerosis through activating NF-κB to promote gene expression of pro-inflammatory cytokines[32]. However, it shows an atheroprotective aspect by receptor-mediated oxLDL uptake in the presence of anti-inflammatory cytokines such as IL-10[33].IL-10 increases the expression of LOX-1, and IL-10-induced LOX-1 contributed to scavenging of oxLDL without affecting the pro-inflammatory signaling[34]. LOX-1 induces increased expression of pro-inflammatory cytokines in mice with fungal keratitis[3,15-16], while can it also induce the expression of IL-10?Our study showed that LOX-1 enhanced the expression of IL-10 in mice with A. fumigatus keratitis.
Dectin-1 recognizes the β-glucans on the surface of fungal cell walls and is involved in anti-fungal immunity[35-37]. A variety of cellular functions can be mediated by Dectin-1, including identification and combination of fungi, uptake and killing,inducing a protective Th1 cells response and the production of cytokines and chemokines[9,38-39], these all contribute to antifungal immunity. Studies have shown that Dectin-1 is also involved in the production of IL-10. Recognition of yeasts and zymosan by Dectin-1 induced IL-10 production, which could suppress inflammatory cytokines production and contribute to the Treg cells development, thereby limiting inflammation as well as contributing to fungal tolerance and long-term infection[38,40-43]. Our study showed that Dectin-1 induced the expression of IL-10 in mice with A. fumigatus keratitis, which may play a role in rebalancing the inflammatory response and contributing to fungal tolerance.
Taken together, IL-10 was upregulated in mice with A. fumigatus keratitis, and it was regulated by LOX-1 and Dectin-1. This suggests that inflammation evoke by LOX-1 and Dectin-1 contributes to fungal clearance, at the same time LOX-1 and Dectin-1 can induce the production of IL-10, which may play a role in rebalancing inflammation and limiting tissue damage,even in immune evasion.
ACKNOWLEDGEMENTS
Foundations: Supported by the National Natural Science Foundation of China (No.81470609; No.81300730;No.81500695); China Postdoctoral Science Foundation(No.2018M630482); the National Natural Science Foundation of Shandong (No.ZR2017BH025); Key Research Project of Shandong (No.2018GSF118193); Applied Basic Research Project of Qingdao (No.16-5-1-65-jch).
Conflicts of Interest: Che CY, None; Yuan KL, None; Zhao GQ, None; Li C, None; Lin J, None; Zhu GQ, None; Liu M,None.
REFERENCES
1 Leal SM, Pearlman E. The role of cytokines and pathogen recognition molecules in fungal keratitis-insights from human disease and animal models. Cytokine 2012;58(1):107-111.
2 Florcruz NV, Peczon I Jr. Medical interventions for fungal keratitis.Cochrane Database Syst Rev 2008;(1):CD004241.
3 Gao X, Zhao G, Li C, Lin J, Jiang N, Wang Q, Hu L, Xu Q, Peng X, He K,Zhu G. LOX-1 and TLR4 affect each other and regulate the generation of ROS in A. fumigatus keratitis. Int Immunopharmacol 2016;40:392-399.
4 Thaiss CA, Zmora N, Levy M, Elinav E. The microbiome and innate immunity. Nature 2016;535(7610):65-74.
5 Medzhitov R. Origin and physiological roles of inflammation. Nature 2008;454(7203):428-435.
6 Hazlett LD, Jiang X, McClellan SA. IL-10 function, regulation, and in bacterial keratitis. J Ocul Pharmacol Ther 2014;30(5):373-380.
7 Hutchins AP, Diez D, Miranda-Saavedra D. The IL-10/STAT3-mediated anti-inflammatory response: recent developments and future challenges.Brief Funct Genomics 2013;12(6):489-498.
8 Paul J, Karmakar S, De T. TLR-mediated distinct IFN-γ/IL-10 pattern induces protective immunity against murine visceral leishmaniasis. Eur J Immunol 2012;42(8):2087-2099.
9 Teixeira-Coelho M, Guedes J, Ferreirinha P, Howes A, Pedrosa J,Rodrigues F, Lai WS, Blackshear PJ, O'Garra A, Castro AG, Saraiva M.Differential post-transcriptional regulation of IL-10 by TLR2 and TLR4-activated macrophages. Eur J Immunol 2014;44(3):856-866.
10 Liu BS, Cao Y, Huizinga TW, Hafler DA, Toes RE. TLR-mediated STAT3 and ERK activation controls IL-10 secretion by human B cells.Eur J Immunol 2014;44(7):2121-2129.
11 Mion F, Tonon S, Toffoletto B, Cesselli D, Pucillo CE, Vitale G. IL-10 production by B cells is differentially regulated by immune-mediated and infectious stimuli and requires p38 activation. Mol Immunol 2014;62(2):266-276.
12 Ng SS, Li A, Pavlakis GN, Ozato K, Kino T. Viral infection increases glucocorticoid-induced interleukin-10 production through ERK-mediated phosphorylation of the glucocorticoid receptor in dendritic cells: potential clinicalimplications. PLoS One 2013;8(5):e63587.
13 Saijo S, Iwakura Y. Dectin-1 and Dectin-2 in innate immunity against fungi. Int Immunol 2011;23(8):467-472.
14 Yuan K, Zhao G, Che C, Li C, Lin J, Zhu G, He K. Dectin-1 is essential for IL-1β production through JNK activation and apoptosis in Aspergillus fumigatus keratitis. Int Immunopharmacol 2017;52:168-175.15 Li C, Zhao G, Che C, Lin J, Li N, Hu L, Jiang N, Liu Y. The Role of LOX-1 in innate immunity to Aspergillus fumigatus in corneal epithelial cells. Invest Ophthalmol Vis Sci 2015;56(6):3593-3603.
16 He K, Yue LH, Zhao GQ, Li C, Lin J, Jiang N, Wang Q, Xu Q, Peng XD, Hu LT, Zhang J. The role of LOX-1 on innate immunity against Aspergillus keratitis in mice. Int J Ophthalmol 2016;9(9):1245-1250.
17 de Waal Malefyt R. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 1991;174(5):1209-1220.
18 Mittal SK, Roche PA. Suppression of antigen presentation by IL-10.Curr Opin Immunol 2015;34:22-27.
19 Mannino MH, Zhu Z, Xiao H, Bai Q, Wake field MR, Fang Y. The paradoxical role of IL-10 in immunity and cancer. Cancer Lett 2015;367(2):103-107.
20 Netea MG, Sutmuller R, Hermann C, Van der Graaf CA, Van der Meer JW, van Krieken JH, Hartung T, Adema G, Kullberg BJ. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J Immunol 2004;172(6):3712-3718.
21 Montagnoli C, Bacci A, Bozza S, Gaziano R, Mosci P, Sharpe AH,Romani L. B7/CD28-dependent CD4+CD25+ regulatory T cells are essential components of the memory-protective immunity to Candida albicans. J Immunol 2002;169(11):6298-6308.
22 Vazquez-Torres A, Jones-Carson J, Wagne RD, Warner T, Balish E. Early resistance of interleukin-10 knockout mice to acute systemic candidiasis. Infect Immun 1999;67(2):670-674.
23 Moore K, Vieira P, Fiorentino D, Trounstine M, Khan T, Mosmann T.Homology of cytokine synthesis inhibitoryfactor (IL-10) to the Epstein-Barr virus gene BCRFI. Science 1990;248(4960):1230-1234.
24 Jang S, Uematsu S, Akira S, Salgame P. IL-6 and IL-10 induction from dendritic cells in response to Mycobacterium tuberculosis is predominantly dependent on TLR2-mediated recognition. J Immunol 2004;173(5):3392-3397.
25 van der Kleij D, Latz E, Brouwers JF, Kruize YC, Schmitz M, Kurt-Jones EA, Espevik T, de Jong EC, Kapsenberg ML, Golenbock DT,Tielens AG, Yazdanbakhsh M. A novel host-parasite lipid cross-talk.Schistosomal lyso-phosphatidylserine activates toll-like receptor 2 and affects immune polarization. J Biol Chem 2002;277(50):48122-48129.
26 Janeway CA Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 1989;54 Pt 1:1-13.
27 Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol 2002;2(3):151-161.
28 Pulendran B. Modulating vaccine responses with dendritic cells and Toll-like receptors. Immunol Rev 2004;199(1):227-250.
29 Pasare C, Medzhitov R. Toll-like receptors and acquired immunity.Semin Immunol 2004;16(1):23-26.
30 Cambi A, Figdor CG. Dual function of C-type lectin-like receptors in the immune system. Curr Opin Cell Biol 2003;15(5):539-546.
31 Gordon S. Pattern recognition receptors: doubling up for the innate immune response. Cell 2002;111(7):927-930.
32 Xu S, Ogura S, Chen J, Little PJ, Moss J, Liu P. LOX-1 in atherosclerosis:biological functions and pharmacological modifiers. Cell Mol Life Sci 2013;70(16):2859-2872.
33 Zeya B, Arjuman A, Chandra NC. Lectin-like oxidized low-density lipoprotein (LDL) receptor (LOX-1): a chameleon receptor for oxidized LDL. Biochemistry 2016;55(32):4437-4444.
34 Arjuman A, Chandra NC. Effect of IL-10 on LOX-1 expression,signalling and functional activity: an atheroprotective response. Diab Vasc Dis Res 2013;10(5):442-451.
35 Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H,Haynes K, Steele C, Botto M, Gordon S, Brown GD. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol 2007;8(1):31-38.
36 Brown GD, Gordon S. Immune recognition: a new receptor for β-glucans. Nature 2001;413(6851):36-37.
37 Underhill DM, Pearlman E. Immune interactions with pathogenic and commensal fungi: a two-way street. Immunity 2015;43(5):845-858.
38 Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol 2006;6(1):33-43.
39 Gantner BN, Simmons RM, Underhill DM. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments.EMBO J 2005;24(6):1277-1286.
40 Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, Williams DL, Gordon S, Tybulewicz VL, Brown GD, Reis e Sousa C.Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 2005;22(4):507-517.
41 Elcombe SE, Naqvi S, Van Den Bosch MW, MacKenzie KF,Cianfanelli F, Brown GD, Arthur JS. Dectin-1 regulates IL-10 production via a MSK1/2 and CREB dependent pathway and promotes the induction of regulatory macrophage markers. PLoS One 2013;8(3):e60086.
42 Dillon S, Agrawal S, Banerjee K, Letterio J, Denning TL, Oswald-Richter K, Kasprowicz DJ, Kellar K, Pare J, van Dyke T, Ziegler S,Unutmaz D, Pulendran B. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest 2006;116(4):916-928.
43 Meyer-Wentrup F, Cambi A, Adema GJ, Figdor CG. "Sweet talk":closing in on C type lectin signaling. Immunity 2005;22(4):399-400.