INTRODUCTION
Glaucoma is a multifactorial eye disease worldwide leading to blindness characterized by retinal ganglion cells (RGCs) degeneration[1-3]. Visual formation involves pallium pillow of brain, eyeball and visual pathway and RGCs are the unique link to transport visual information between the two organs[4-7]. Furthermore, RGC axons play an important role in transporting nutrients within cycles through cell body and terminal[8]. As RGCs is axonal damage and steady loss,glaucomatous optic neuropathy progresses with gradual vision loss and visual field deficits[9-10].
Several risk factors of glaucoma are recognized including age, family history, elevated intraocular pressure (IOP)and myopia[11-12]. Among them abnormally elevated IOP is considered as a vital risk factor of visual function deficits[12-13].Clinical therapies focus on decreasing IOP to normal tension even the lower with eye drops or operations. Nevertheless these managements are not enough to prevent RGCs death[14]. In addition, eye drops have limitations such as poor compliance due to frequency and low bioavailability due to various barriers while outflow pathway blockade relapses several years after surgery[15]. Given this, gene therapy rises gradually in sorts of experiments.
Several nerve growth factors (NGFs) are confirmed to benefit to cultured RGCs such as brain derived neurotrophic factor (BDNF) and ciliary neurotrophic factor (CNTF) after injury[16-18]. However these peptides with short half-life have immediate effects[19]. If recombinant viral vector is conducted,survival rate of injured RGCs in vitro and in vivo increases in a considerable time owning to sustained NGFs especially CNTF[20-22]. Recent advances elucidate CNTF stimulates gene expression, cell proliferation and neural stem cell differentiation as well as provokes neuroprotection and axons regeneration[23-26]. And CNTF is reported to elicit biological effects for under several pathological conditions[25,27-28].
Mechanisms in relation to RGC destruction in glaucoma are currently deemed to incompetent axon transport of neurotrophic factors, reactive oxygen species (ROS), excitoxicity and chronic intermittent ischemia[29-30]. Among above factors, the interplay between ischemia and ROS for the formation of vicious circle induces RGCs death[29,31-32]. Besides ROS as a direct damage element of glaucomatous neurodegeneration, it also may participate in activating signal pathway to RGCs death as a second messager[33-34]. On the base of the pathology theory, we adopted hydrogen peroxide (H2O2) modeling on RGC-5 cell and focused on CNTF effect on injury model.
MATERIALS AND METHODS
Retinal Ganglion Cell-5 Identification by Immuno fluorescence RGC-5 cells were cultured by dulbecco’s modified eagle medium (DMEM) medium supplemented with 10 mL of 10% fetal bovine serum, 1 mL of 105 U/L penicillin and 1 mL of 100 mg/L streptomycin in each 100 mL medium. Cells were located in 5% CO2 incubator at 37℃ every 2d to change solution. The cell line RGC-5 at logarithmic phase was selected in the culture flask with 5% bovine serum albumin and 0.1% Triton X-100 in 0.01 mol/L phosphate buffered saline (BSAT) instead of medium at 4℃ over night. Then mixture of 5% BSAT solution and first antibody, Brn3a (Cell Signaling Technology. Inc., Boston, USA), with a proportion of 1:100 was added into flask without medium also at 4℃ over night. The following day poured away mixture, washed flask for five minutes by six times with 1×phosphate buffered saline and 0.1% Triton X-100 (PBST) and then added mixture of 5% BSAT solution and second antibody, Cy3 (Cell Signaling Technology. Inc., Boston, USA), with a proportion of 1:500 at indoor temperature for 2h.
Foundation of Lentiviral Expression Vector Carrying Ciliary Neurotrophic Factor Gene The target gene synthesis with upstream primer and downstream primer (Shanghai GeneChem Company. Ltd., Shanghai, China) was amplified for purified CNTF gene segments in polymerase chain reaction (PCR). Recombinant vector contained CNTF gene and plasmid GV287 (Shanghai GeneChem Company. Ltd.,Shanghai, China) (Figure 1). The forward primer sequences of CNTF was 5′-GAG GAT CCC CGG GTA CCG GTC GCC ACC ATG GCT TTC ACA GAG CAT TC-3′ and the reverse sequence was 5′-TCC TTG TAG TCC ATA CCC ATT TTC TTG TTG TTA GC-3′. The gene sequencing was conducted on the modified gene (pGC-FU-CNTF). After exact sequenced identification CNTF protein expression in 293T cells was assayed by Western blot. Correctly constructed recombinant plasmid of CNTF transfected bone mesenchymal stem cells(BMSCs) to detect virus titre by limited dilution. The viral titer of pGC-FU-CNTF was 1×108 TU/mL. Working solution with 10 μL of the virus suspension was to transfect BMSCs.
Hydrogen Peroxide Injury Model of Retinal Ganglion Cell-5 RGC-5 cells were seeded on 60-well plates in 5% CO2 incubator at 37℃ for 24h to follow-on (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) experiment if inoculated cells were growth well. Experimental groups was set up by H2O2 concentration as follow: 0 (control group),50, 75, 100, 125 and 150 µmol/L. Cell proliferation was performed at four time points, respectively, 0.5, 1, 1.5 and 2h.An appropriate concentration was chosen for model on basis of MTT results.
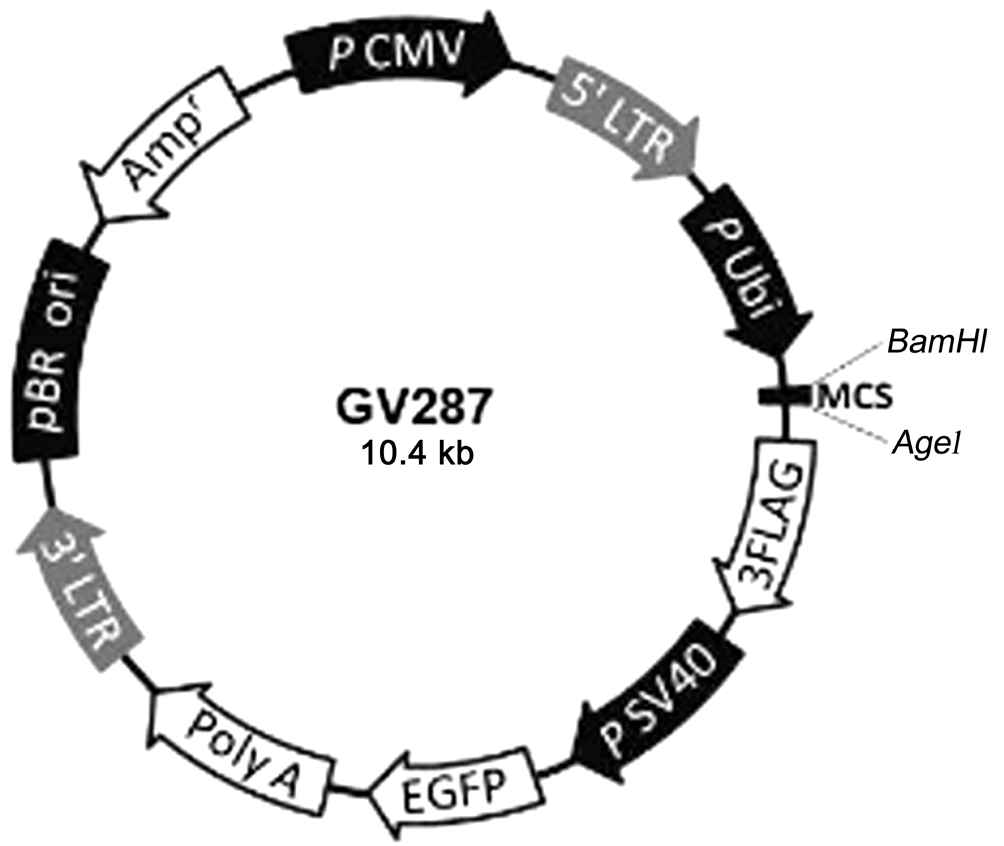
Figure 1 Plasmid GV287 diagram The circular plasmid containing AgeI cleavage site loaded with exogenous CNTF gene to construct recombinant plasmid.
Real-time Polymerase Chain Reaction While RGC-5 cells cocultured with BMSCs carrying recombinant plasmid, CNTF gene expression and neuroprotective agents such as glial cell line-derived neurotrophic factor (GDNF) and BDNF gene expression, measured by Real-time PCR at 3 and 7d versus control group without recombinant plasmid. Total RNA was extracted from cultured cells with TRIzol reagent (Shanghai Pufei Biotech Co., Ltd., Shanghai, China) according to the kit instructions. cDNA synthesis was implanted by reverse transcription by virtue of RNA PCR protocol (Promega Biotech Co., Ltd., Beijing, China). Glyceraldehyde phosphate dehydrogenase (GAPDH) was acted as a normalizing control.The several primers were as follows: CNTF: forward: 5′-CCA CCT ACA TCC CCA ATA CC-3′; reverse: 5′-GCT GAC ACT TAT GGA GAC CTT G-3′; BDNF: forward: 5′-GGT TAT TTC ATA CTT CGG TTG C-3′; reverse: 5′-CCC ATT CAC GCT CTC CAG-3′; GDNF: forward: 5′-CGA TAT TGT AGC GGT TCC TGT-3′; reverse: 5′-CCT TGT CAC TTG TTA GCC TTC T-3′; GAPDH: forward: 5′-TTC AAC GGC ACA GTC AAG G-3′; reverse: 5′-CTC AGC ACC AGC ATC ACC T-3′ (Genechem Co., Ltd., Shanghai, China). The PCR reaction system was performed in a volume of 25 μL using the SYBR Master Mix (Toyobo Co., Ltd., Shanghai, China) on the MX3000p real-time PCR instrument (Agilent Technologies Inc., California, USA). In SYBR Green PCRs the melting curve was in the 60℃-95℃ range to evaluate amplification products. Relative quantification of gene expression was performed using the 2-ΔΔCt method.
Detection of Cell Viability by Application of Ciliary Neurotrophic Factor by MTT Assay To explore CNTF efficacy to H2O2 injury model of RGC-5, each groups were measured by MTT assay. Experimental groups including: pGCFU-CNTF group, 50 ng/mL CNTF group, 100 ng/mL CNTF group, 150 ng/mL CNTF group, 200 ng/mL CNTF group and 250 ng/mL CNTF group acted on RGC-5 damage model while control group was without any treatment. Two effective approach to carry drugs, direct administration of CNTF and synthesis by pGC-FU-CNTF, had different administration times such as one day and seven days, respectively. The reason of different times was that pGC-FU-CNTF required for synthesis and secretion while the former one did not. After administration of CNTF, H2O2 intervened for same time then RGC-5 cell viability was measured by MTT assay.
Statistical Analysis Statistics were described by mean±standard deviation (SD). The differences between groups were analyzed by one-way ANOVA. P values less than 0.05 were considered statistically significant differences.
RESULTS
Culture and Identification of Retinal Ganglion Cell-5 RGC-5 cells showed logarithmic growth and the cell revealed long spindle in shape. Anti-Brn3a protein (1:100) for immunohistochemical identification of high-purity cultured cells fluoresced red under a inverted phase contrast microscope (Figure 2).
Hydrogen Peroxide Induced Retinal Ganglion Cell-5 Model Along with H2O2 concentration increasing from 50 to 150 µmol/L, RGC-5 cell viability showed a decreasing trend generally, and then had an obvious decrease of the cell viability at 150 µmol/L. In view of H2O2 was an acknowledged and effective modeling method and we done the curve with H2O2 in order to find a proper concentration and time point for our study. We ultimately decided to choose 125 µmol/L and 2h as the condition to establish model accounted for 23.20% cell death (Figure 3).
Efficacy of pGC-FU-CNTF Transfers into Bone Mesenchymal Stem Cells Immuno fluoresent assay was conducted to detect CNTF gene transferring efficacy in BMSCs. It was obvious that BMSCs with lentiviral vector carrying CNTF gene glow green fluorescent owing to green fluorescent protein junctional of recombinant plasmid (Figure 4A). RGC-5 cells were elongated spindle shape against the wall under a fluorescent microscope.
On the other hand, CNTF mRNA expression and production increased over time rather than control group with RGC-5 only. The mRNA expression levels of CNTF on day 7 (817.35±177.40)were higher than those in day 3 (8.30±2.64, P<0.05) and control group (1.01±0.18, P<0.05; Figure 4B).
Since BMSCs enable to secret various growth factors, as carriers they trigger upregulation of BDNF, GDNF expression and so on. Compared with control group, there was significant upregulation of BDNF gene expression in BMSCs additional groups at 7d (332.29±146.23, P<0.05) while GDNF gene expression was not significant in any experimental groups(Figure 4C, 4D). And elevated levels of BDNF expression was in accord with prolonged time.
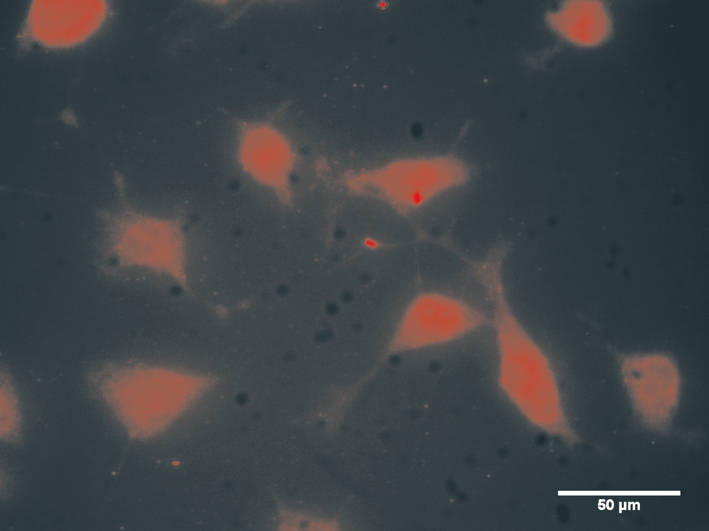
Figure 2 RGC-5 identification by immunofluorescence The detection of Brn3a showed strong red fluorescence staining revealing cell shape (×400).

Figure 3 A cell viability assay of RGC-5 cells subject to different concentrations of H2O2 in several times by MTT Cell survival rate was in inverse proportion of H2O2 concentrations and intervened time.Longitudinal changes in H2O2-induced RGC-5 cell death at 0.5, 1, 1.5 and 2h increased along with concentrations.
Ciliary Neurotrophic Factor Effect of Two Ways Against Hydrogen Peroxide Induced Retinal Ganglion Cell-5 Model Two ways of CNTF as follow one was addition of CNTF in the culture medium directly and the other one was recombinant plasmid carrying CNTF gene while they were adopted with its own advantages such as certain dosage and continuous pharmic infusion. As shown in Figure 3, 125 µmol/L H2O2 alone was toxic to the cells causing at most 30.24% cell death while the effects of CNTF differentiated to some extent on resistance to oxidative damage. In the first day the survival rate of RGC-5 cells with additional CNTF had no significant difference among every CNTF concentration groups. When RGC-5 cells cultured in second day, the low level of CNTF groups (50 and 100 ng/mL) had statistic difference with high concentration groups (150, 200 and 250 ng/mL) in cell viability.However there were no difference in every high concentration groups. And in third day the results were same as the previous day. Only 50 ng/mL group had difference with the groups of the other concentrations in fourth day. While analyses from all groups revealed no difference five days later (Figure 5).Therefore we chose 150 ng/mL to intervene for three days and this method of administration would be compared with the approach by recombinant lentivirus.
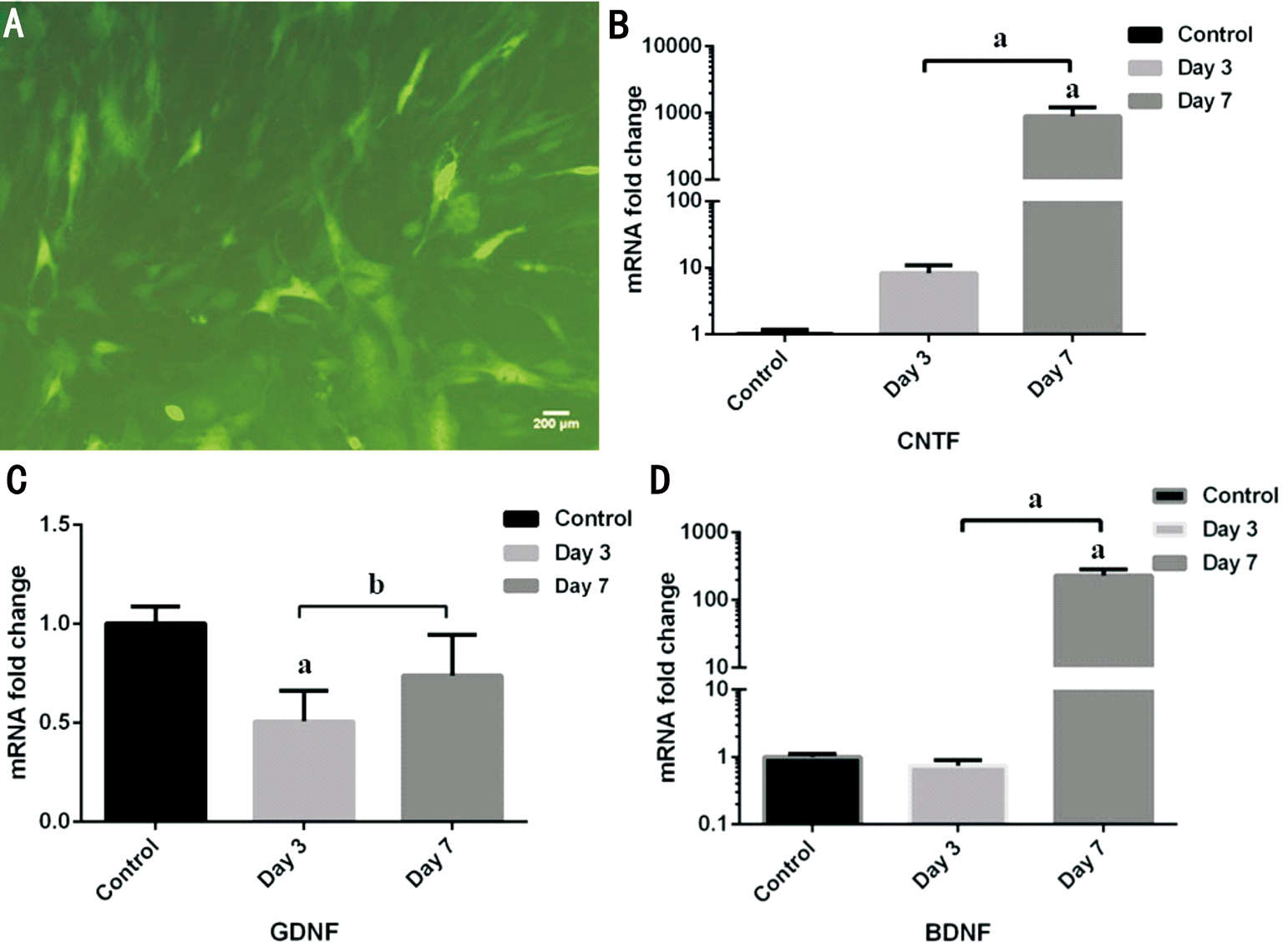
Figure 4 Transfection efficiency of recombined lentivirus in BMSCs A: Representative confocal image of BMSCs immuolabelled with green fluorescence protein for its gene was transferred into BMSCs by lentivirus vector (×100). B-D: Real-time PCR confirmed mRNA expression levels of three neurotrophic factors in day 3, day 7 groups after recombined lentivirus transfection and control group. B: CNTF gene expression in day 7 group had statistically significant differences versus the other two groups; C: GDNF gene expression in day 3 group was below control level with significant differences but no significant differences was found between intervened groups; D: The increased BDNF gene expression in day 7 group had statistically significant differences versus the other two groups. aP<0.05, bP>0.05 (one-way ANOVA followed by post hoc comparisons).
Both 150 ng/mL CNTF and recombinant plasmid made a difference on resistance to oxidative damage and on protection of RGCs. As comparison with the optimal concentration group of 24.57% cell death, recombinant plasmid group reduced cell death to 19.23% with no statistically significant differences(P>0.05; Figure 6).
DISCUSSION
RGCs, as a precious medium to visual formation, reduce in glaucoma and there is no available remission as far. In many researches, NGF are confirmed to protective factors for injured RGCs as well as our study demonstrated that CNTF had a positive effect on RGC-5 cell death induced by H2O2.Taken together, the acknowledged mechanisms of RGCs death are as follows: 1) neurotrophic factors deficiency due to invalid axonal transport; 2) glutamic toxicity; 3) free radical concentration; 4) nitric oxide toxicity; 5) apoptosis[35]. H2O2,one member of ROS as same as free radical, was used to make model in the study to cause RGC-5 loss by oxidative stress.The negative correlation between H2O2 concentration and RGC-5 survival lay in the irreversible cell injure model. H2O2 is danger to RGC-5 and is pathogenic microenvironment to disease. Complicated and various etiological factors always are unlikely indicated in detail, what we can do is to improve microenvironment as far as possible. So then, the aid of protective agents can yet be regarded as a novel option. For RGC-5 involved with vision, it is critical to make full use of CNTF.
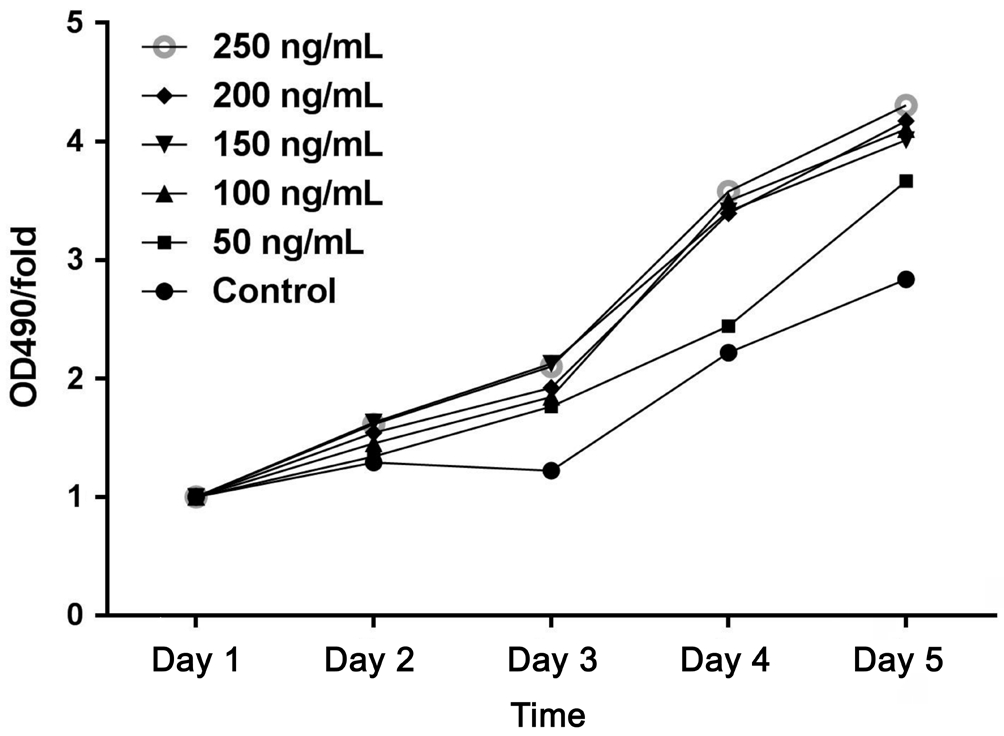
Figure 5 Dose- and time-dependent changes in the numbers of RGCs represented by OD490 value in the other groups versus day 1 Whether with CNTF addition or not OD490/fold value increased linearly in all groups with cultivation time.
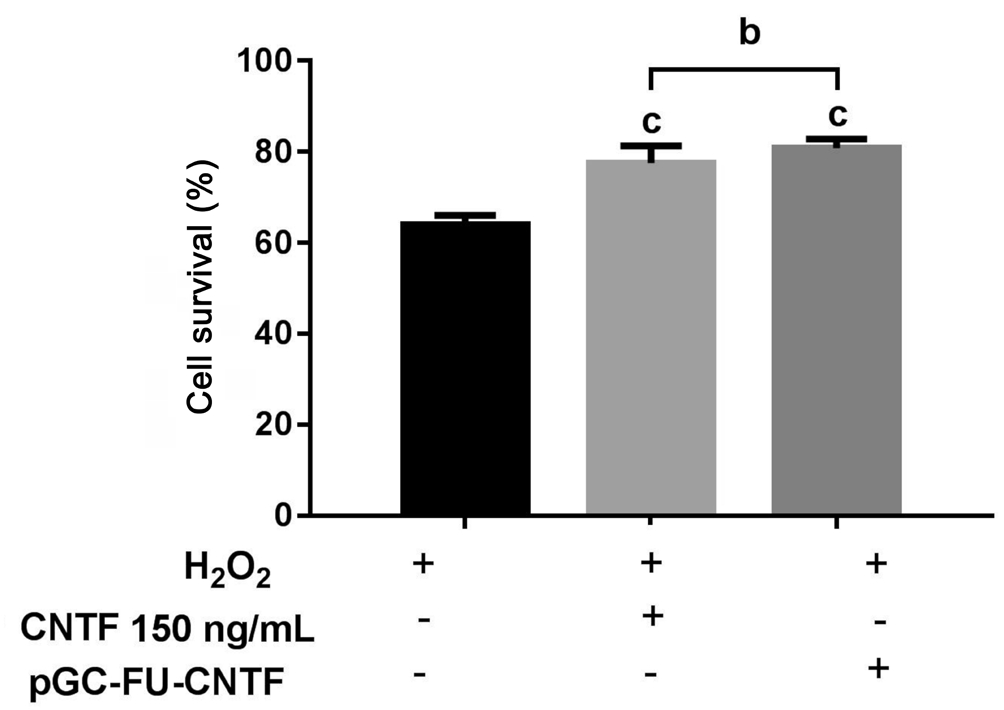
Figure 6 RGC-5 cell survival rate in different treatments for H2O2 injure H2O2 injure only had significant differences with two experiment groups-CNTF 150 ng/mL given in the culture media directly and by recombinant plasmid. However there was no significant differences between the experiment groups. bP>0.05(one-way ANOVA followed by post hoc comparisons); cP<0.001 vs control.
In the course of exploring appropriate H2O2 concentration to intervene, gradually increasing concentration brought about a rise in the number of dead cells but the correlation applied in the range of 50 to 150 µmol/L. Once H2O2 concentration is above 150 µmol/L, the mortality rate of RGC-5 obviously increased with rare survival cells. Even if the relative survival rate was about 33.36% in 150 µmol/L group at each time,maybe the surrounding microenvironment abounding in ROS was too terrible to exist. At the beginning, RGC-5 was in outset of cell degeneration and overmuch ROS finally induced irreversible cell damage. Hence early detection and diagnosis of RGC related opthalmopathy (e.g. optic neuropathy) are worth preventing hypopsia and blindness. Eventually we chose 125 µmol/L H2O2 for 2h to establish model. Because there are very low cell survival rate when the concentration of H2O2 reaches 150 µmol/L and it is available to assure cellular activity in the follow-up study.
The advantage of direct intervention was known dose while recombinant lentiviral plasmid provided continuous CNTF. If in vitro and in vivo experiments selected a proper CNTF, an implantable drug delivery system in eyes can conduct in the above dose. A positive correlation was confirmed between a rise of CNTF concentration and RGC-5 survival rate. CNTF function in neuron via heterotrimeric complex that is ciliary neurotrophic factor receptor-α (CNTFRα) the only one part anchored at surface membrane, gp130 and leukemia inhibitory factor-β (LIFRβ)[36]. CNTF binds CNTFRα at the first step then does the heterodimer of gp130 and LIFRβ[37-39]. Whereas RGCs are unable to secrete proteins, the choice of BMSCs as medium was because not only it had the stable biological characteristics but also could secrete CNTF itself. Based on the influence of CNTF on cell model we could select the optimal concentration which was a hint for in vivo experiments by intravitreal injection or implanted intraocular secretion apparatus.
In general, CNTF is an effective neurotrophin for RGCs through sorts of signal pathways acting on recipient cells.Special process about how CNTF plays a pivotal role is still an open question.
ACKNOWLEDGEMENTS
Foundation: Supported by Ph.D. Programs Foundation of Ministry of Education of China (No.20130141120052).
Conflicts of Interest: Wang WJ, None; Jin W, None; Yang AH, None; Chen Z, None; Xing YQ, None.
REFERENCES
1 Agudo-Barriuso M, Villegas-Pérez MP, de Imperial JM, Vidal-Sanz M.Anatomical and functional damage in experimental glaucoma. Curr Opin Pharmacol 2013;13(1):5-11.
2 Sippl C, Bosserhoff AK, Fischer D, Tamm ER. Depletion of optineurin in RGC-5 cells derived from retinal neurons causes apoptosis and reduces the secretion of neurotrophins. Exp Eye Res 2011;93(5):669-680.
3 Sluch VM, Zack DJ. Stem cells, retinal ganglion cells, and glaucoma.Dev Ophthalmol 2014;53:111-121.
4 Wang H, Wang R, Thrimawithana T, Little PJ, Xu J, Feng ZP, Zheng W.The nerve growth factor signaling and its potential as therapeutic target for glaucoma. Biomed Res Int 2014;2014:759473.
5 Crish SD, Calkins DJ. Central visual pathways in glaucoma: evidence for distal mechanisms of neuronal self-repair. J Neuroophthalmol 2015;35(Suppl 1):S29-S37.
6 Lin B, Peng EB. Retinal ganglion cells are resistant to photoreceptor loss in retinal degeneration. PLoS One 2013;8(6):e68084.
7 O’Brien EE, Greferath U, Fletcher EL. The effect of photoreceptor degeneration on ganglion cell morphology. J Comp Neurol 2014;522(5):1155-1170.
8 Nuschke AC, Farrell SR, Levesque JM, Chauhan BC. Assessment of retinal ganglion cell damage in glaucomatous optic neuropathy: axon transport, injury and soma loss. Exp Eye Res 2015;141:111-124.
9 Tian K, Shibata-Germanos S, Pahlitzsch M, Cordeiro MF. Current perspective of neuroprotection and glaucoma. Clin Ophthalmol 2015;9:2109-2118.
10 Wei T, Kang Q, Ma B, Gao S, Li X, Liu Y. Activation of autophagy and paraptosis in retinal ganglion cells after retinal ischemia and reperfusion injury in rats. Exp Ther Med 2015;9(2):476-482.
11 Cholkar K, Trinh HM, Pal D, Mitra AK. Discovery of novel inhibitors for the treatment of glaucoma. Expert Opin Drug Discov 2015;10(3):293-313.
12 Howell GR, Soto I, Libby RT, John SW. Intrinsic axonal degeneration pathways are critical for glaucomatous damage. Exp Neurol 2013;246:54-61.
13 Nickells RW, Howell GR, Soto I, John SW. Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu Rev Neurosci 2012;35:153-179.
14 Wang Y, Huang C, Zhang H, Wu R. Autophagy in glaucoma: crosstalk with apoptosis and its implications. Brain Res Bull 2015;117:1-9.
15 Pita-Thomas DW, Goldberg JL. Nanotechnology and glaucoma: little particles for a big disease. Curr Opin Ophthalmol 2013;24(2):130-135.
16 Uchida S, Suzuki Y, Araie M, Kashiwagi K, Otori Y, Sakuragawa N.Factors secreted by human amniotic epithelial cells promote the survival of rat retinal ganglion cells. Neurosci Lett 2003;341(1):1-4.
17 Sarup V, Patil K, Sharma SC. Ciliary neurotrophic factor and its receptors are differentially expressed in the optic nerve transected adult rat retina. Brain Res 2004;1013(2):152-158.
18 Roberti G, Mantelli F, Macchi I, Massaro-Giordano M, Centofanti M.Nerve growth factor modulation of retinal ganglion cell physiology. J Cell Physiol 2014;229(9):1130-1133.
19 Leaver SG, Cui Q, Plant GW, Arulpragasam A, Hisheh S, Verhaagen J, Harvey AR. AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene Ther 2006;13(18):1328-1341.
20 Hu Y, Cui Q, Harvey AR. Interactive effects of C3, cyclic AMP and ciliary neurotrophic factor on adult retinal ganglion cell survival and axonal regeneration. Mol Cell Neurosci 2007;34(1):88-98.
21 Pease ME, Zack DJ, Berlinicke C, Bloom K, Cone F, Wang Y, Klein RL, Hauswirth WW, Quigley HA. Effect of CNTF on retinal ganglion cell survival in experimental glaucoma. Invest Ophthalmol Vis Sci 2009;50(5):2194-2200.
22 Almasieh M, Wilson AM, Morquette B, Cueva Vargas JL, Di Polo A.The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res 2012;31(2):152-181.
23 Pernet V, Joly S, Dalkara D, Jordi N, Schwarz O, Christ F, Schaffer DV, Flannery JG, Schwab ME. Long-distance axonal regeneration induced by CNTF gene transfer is impaired by axonal misguidance in the injured adult optic nerve. Neurobiol Dis 2013;51:202-213.
24 Cen LP, Luo JM, Zhang CW, Fan YM, Song Y, So KF, van Rooijen N, Pang CP, Lam DSC, Cui Q. Chemotactic effect of ciliary neurotrophic factor on macrophages in retinal ganglion cell survival and axonal regeneration. Invest Ophthalmol Vis Sci 2007;48(9):4257-4266.
25 Cui Q, Harvey AR. CNTF promotes the regrowth of retinal ganglion cell axons into murine peripheral nerve grafts. Neuroreport 2000;11(18):3999-4002.
26 Harvey AR, Hellström M, Rodger J. Gene therapy and transplantation in the retinofugal pathway. Prog Brain Res 2009;175:151-161.
27 Hellström M, Pollett MA, Harvey AR. Post-injury delivery of rAAV2-CNTF combined with short-term pharmacotherapy is neuroprotective and promotes extensive axonal regeneration after optic nerve trauma. J Neurotrauma 2011;28(12):2475-2483.
28 Mathews MK, Guo Y, Langenberg P, Bernstein SL. Ciliary neurotrophic factor (CNTF)-mediated ganglion cell survival in a rodent model of non-arteritic anterior ischaemic optic neuropathy (NAION). Br J Ophthalmol 2015;99(1):133-137.
29 Chang EE, Goldberg JL. Glaucoma 2.0: neuroprotection, neuroregeneration,neuroenhancement. Ophthalmology 2012;119(5):979-986.
30 Aihara M, Chen YN, Uchida S, Nakayama M, Araie M. Hyperbaric pressure and increased susceptibility to glutamate toxicity in retinal ganglion cells in vitro. Mol Vis 2014;20:606-615.
31 Wang Y, Xu K, Zhang H, Zhao J, Zhu X, Wang Y, Wu R. Retinal ganglion cell death is triggered by paraptosis via reactive oxygen species production: a brief literature review presenting a novel hypothesis in glaucoma pathology. Mol Med Rep 2014;10(3):1179-1183.
32.Iizuka Y, Hong S, Kim CY, Yang WI, Lee JE, Seong GJ. Protective mechanism of agmatine pretreatment on RGC-5 cells injured by oxidative stress. Braz J Med Biol Res 2010;43(4):356-358.
33 Tezel G. Oxidative stress in glaucomatous neurodegeneration:mechanisms and consequences. Prog Retin Eye Res 2006;25(5):490-513.
34 Wang Z, Pan X, Wang D, Sun H, Han F, Lv C, Zhang X. Protective effects of protocatechuic acid on retinal ganglion cells from oxidative damage induced by H2O2. Neurol Res 2015;37(2):159-166.
35 Kaushik S, Pandav SS, Ram J. Neuroprotection in glaucoma. J Postgrad Med 2003;49(1):90-95.
36 Wen R, Tao W, Li Y, Sieving PA. CNTF and retina. Prog Retin Eye Res 2012;31(2):136-151.
37 Sleeman MW, Anderson KD, Lambert PD, Yancopoulos GD, Wiegand SJ. The ciliary neurotrophic factor and its receptor, CNTFR alpha. Pharm Acta Helv 2000;74(2-3):265-272.
38 Vergara C, Ramirez B. CNTF, a pleiotropic cytokine: emphasis on its myotrophic role. Brain Res Rev 2004;47(1):161-173.
39 Allen TL, Matthews VB, Febbraio MA. Overcoming insulin resistance with ciliary neurotrophic factor. Handb Exp Pharmacol 2011;(203):179-199.