INTRODUCTION
Familial exudative vitreoretinopathy (FEVR) was first discribed by Criswick and Schepens[1] in 1969 as a genetically related retinal vasuclar malformation, characterized by peripheral retinal vasucular abnormalities including retinal avascular zone, retinal neovascularization, exudate, macular traction, traction or rhegmatogenous retinal detachment(RD)[2-3]. The clinical features were confirmed by Canny and Oliver[4] in 1976 with fundus fluorescein angiography.
Although FEVR is known as familial, the presence among family members remains variant. Patients with FEVR can be asypomatic with peripheral avascular zone or vision loss as a result of retinal detachment if left untreated. Reports indicated that Wnt-receptor/β-catenin pathway was related with retinal formation and regulations of vascular endothelial growth factor (VEGF) expression. Mutation of protein that modulates the pathway might be involed in the pathogenesis of FEVR[5-7].Anti-VEGF therapy was tried for FEVR in a few reports and was found to be effective[8-10]. Lucentis (Ranubizumab, Novatis Company) was used in our study for inhibation of retinal neovascular activities as primary or combined therapy in FEVR of stage 2 or greater.
SUBJECTS AND METHODS
Retrospective, non-controlled clinical study. The study protocol was reviewed and approved by the Ethnic Committee of the First Affiliated Hospital of Chongqing Medical University and Beijing Tongren Hospital. Thirty patients (37 eyes) diagnosed with FEVR of stage 2 to 5 were enrolled in our study. Twenty patients (66.67%) were male and 10 patients (33.33%) were female. Age ranged from 0.4 to 35 years old (median 3y).Intravitreal ranubizumab (IVR) was used either as primary or as a combined therapy according to the retinal neovasuclar activities. The follow up ranged from 1 to 57mo with mean 16.73±15.73 (median 11)mo. The treatment effect of retinal neovasuclar activites were recorded as well as the ocular and systemic side effects. Patients were evaluated with ocular check-up including visual acuity (VA), intraocular pressure,indirect ophthalmoscopy, slit lamp examination, retinal photography and fundus fluorescein angiography. There were 16 patients old enough to cooperate with VA examination,the rest of patients were too young to VA taking. Their visions were discribed by parents according to their visual behaviors. Staging was done in accordence with the following classification (Pendergast and Trese, 1998):
Stage 1, avascular retina without extraretinal vessels; Stage 2, avascular retina with extraretinal vessels (A, no exudate;B, with exudate); Stage 3, partial RD-fovea spared (A, no exudate; B, with exudate); Stage 4, partial RD-fovea involved(A, no exudate; B, with exudate); Stage 5, total RD (A, no exudate; B, with exudate).
All patients diagnosed with FEVR of stage 2 or greater received intravitreal injection of ranubizumab. Consent form was signed prior to the treatment. Retinal photocoagulation was performed in cases with persistent or recurrent neovasuclar activities like hemorrahge and exudate. Buckle surgery was done when tractional RD detected.
Intravitreal injection of ranubizumab was performed 2-3 mm away from limbus. Dosage of injection was 0.5 mg (0.05 mL)ranubizumab for adult patient and 0.25 mg (0.025 mL) for children (age below 18 years old). All patients were examined on the following day and 1mo after injection, then followed monthly to determine if the vascular changes were stable or further treatments needed.
RESULTS
The enrolled patients’ data at baseline was listed in Table 1.Among 30 patients (37 eyes), 10 eyes received single IVR,1 eye received 2 injections. Three eyes were treated with IVR and simutanous laser photocoagulation. Laser indirect ophthalmoscopy (LIO) was applied in 5 eyes 1mo after the primary IVR. Seven eyes were treated surgically following the primary IVR due to persistent retinal neovasuclar activities and retinal traction either with vitrectomy or buckle surgery(Figures 1-3).
IVR was used as combined treatment with vitrectomy in 11 eyes. In eyes underwent vitrectomy, vitreous hemorrhage and traction were the main reasons for surgical intervention. IVR facilitied the retinal neovascular regression which minimize the chances of surgical complications such as bleeding and itraogenic retinal breaks.
Retinal neovascular regression such as diminished retinal hemorrhage and exudation was notified at 1mo following the primary IVR in all eyes, which was determined with indirectophthalmoscopy, fundus photogaphy and fundus fluorescein angiography. Vision in all patients improved or remained stable except in one patient with visions in both eyes decreased from 0.05 to light perception and no light perception due to persistent tractional RD. No IVR related ocular and systemic complications were recorded during follow up. The details of treatment and follow up were listed in Table 2.
Table 1 Patients’ data at baseline n (%)
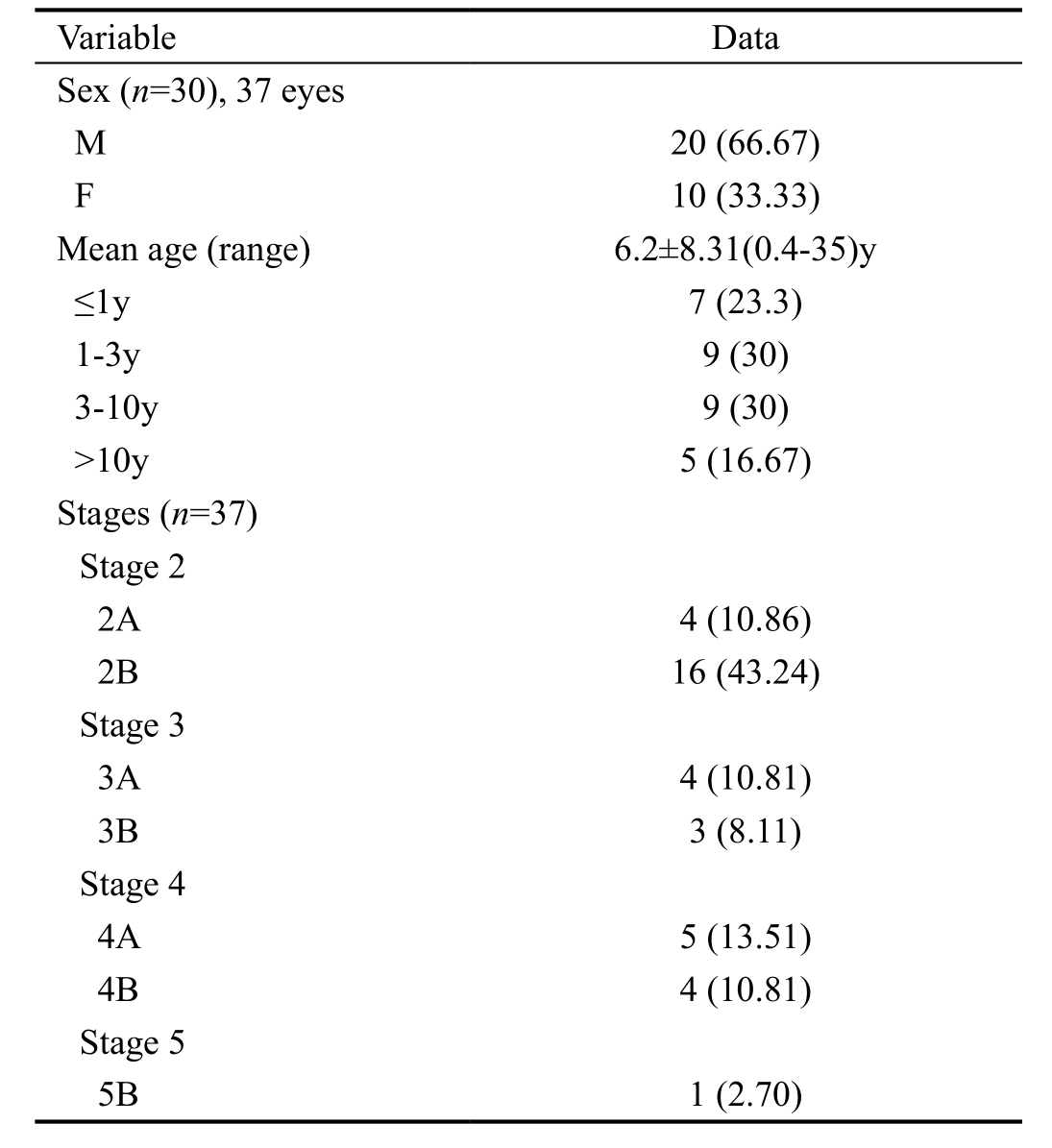

Figure 1 Fundus image of patient No.4 before IVR, showing retinal hemorrhage, exudate and minimal peripheral traction retinal detachment.
DISCUSSION
For patients with FEVR, the main causes of vision loss are retinal neovascularization and the associated complications,such as traction RD and macular edema etc. The treatment target of FEVR of stage 2 or greater is to minimize the retinal neovascular activities by all possible means. Conventional therapies include laser photocoagulation, cryotherapy, or buckle and vitrectomy in cases with RD.
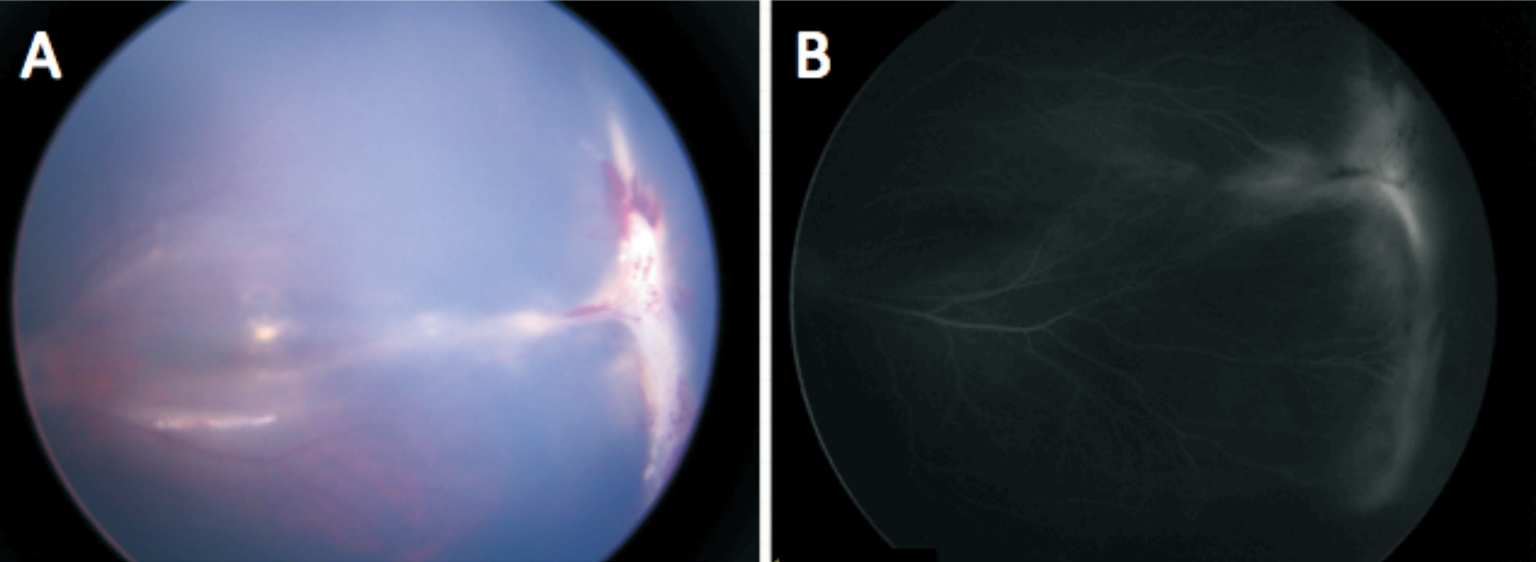
Figure 2 Fundus photographs of patient 1mo after IVR Color image (A) and fluorescence fundus angiography (B) of patient No.4 showing reduced retinal hemorrhage, exudate and leakage.
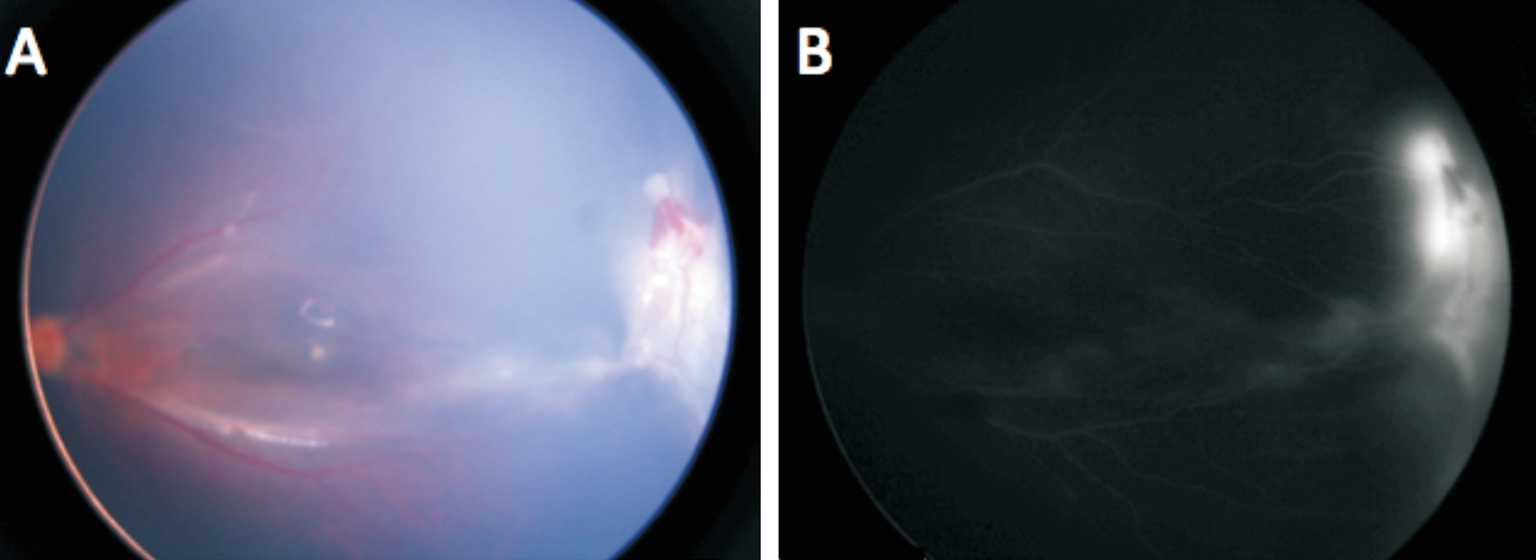
Figure 3 Fundus photographs of patient 4mo after IVR Images of fundus (A) and fluorescence fundus angiography (B) of patient No.4 showing retinal hemorrhage and exudate remained minimal, traction retinal detachment increased. The patient received buckle surgery afterwards and remained stable.
Anti-VEGF therapy has become the primary treatment of retinal neovascular problems such as wet age-related macular degeneration (AMD), retinal vein occlusion and diabetic macular edema. It has been found to be able to reduce retinal vascular permeability and reduction of exudate. There have been a number of reports of anti-VEGF for pediatric retinal diseases like Coat’s disease and retinopathy of prematurity[11-20].Reports indicated that Wnt-receptor/β-catenin pathway was related with retinal formation and regulations of VEGF expression. Mutation of protein that modulates the pathway might be involed in the pathogenesis of FEVR[5-7]. Anti-VEGF therapy was tried for FEVR in a few reports and was found to be effective[8-10].
In our study, the IVR was used as a primary or combined treatment of FEVR in case of stage 2 and greater, in which retinal neovascular activities were present. Conventional laser photocoagulation reacts poorly in severe retinal hemorrhage and exudate while cryotherapy causes prominent intraocular inflammation. The primary anti-VEGF therapy helped to reduce the retinal hemorrhage and exudate, which greatly facilitated the photocoagulation effect with minimal intensity.In our study, retinal neovascularization was significantly diminished following IVR with the reduction of hemorrhage and exudate in all patients. Secondary laser photocoagulation was applied in 5 eyes because of persistent precence of neovascular activities. Two eyes was finally treated with buckle surgery because of increased traction. IVR was used as combined treatment with vitrectomy in 11 eyes. In eyes underwent vitrectomy, vitreous hemorrhage and traction were the main reasons for surgical intervention. IVR facilitied the retinal neovascular regression which minimize the chances of surgical complications such as bleeding and itraogenic retinal breaks.
Vision in the most of the eyes with IVR had remained stable or improved in our study. Similar effect of anti-VEGF for FEVR was reported by Quiram et al[8].
Complications of IVR may include vitreous hemorrhage,retinal breaks and detachment, endothphalmitis and uncertain systemic side effects. In our study, neither ocular nor systemic side effects were recorded, implying that IVR for FEVR may be a safe treatment as recongnized in wet AMD treatment.
In general, our study has shown that IVR may be an effective modality in the treatment of FEVR either as the primary or as an ajunct to the conventional therapies. The long term effect and safty of IVR still need further research.
Table 2 Eyes underwent IVR for FEVR of more than stage 2

a3 IVR. VH: Vitreous hemorrhage; LSV: Lens-sparing vitrectomy; L&V: Lensectomy and vitrectomy; CF: Counting fingers; SB: Scleral buckle;PC: Photocoagulation; VA: Visual acuity; NLP: No light perception; LP: Light perception; IVR: Intravitreal ranubizumab; CRO: Cryotherapy;SO: Silicone oil; LIO: Laser indirect ophthalmoscopy.
ACKNOWLEDGEMENTS
Conflicts of Interest: Lu YZ, None; Deng GD, None; Liu JH, None; Yan H, None.
REFERENCES
1 Criswick VG, Schepens CL. Familial exudative vitreoretinopathy. Am J Ophthalmol 1969;68(4):578-594.
2 Kashani AH, Brown KT, Chang E, Drenser KA, Capone A, Trese MT.Diversity of retinal vascular anomalies in patients with familial exudative vitreoretinopathy. Ophthalmology 2014;121(11):2220-2227.
3 Shastry BS, Trese MT. Cosegregation of two unlinked mutant alleles in some cases of autosomal dominant familial exudative vitreoretinopathy.Eur J Hum Genet 2004;12(1):79-82.
4 Canny CL, Oliver GL. Fluorescein angiographic findings in familial exudative vitreoretinopathy. Arch Ophthalmology 1976;94(7):1114-1120.
5 Moon RT. Wnt/beta-catenin pathway. Sci STKE 2005;2005(271):cm1.
6 Wu CH, Nusse R. Ligand receptor interactions in the Wnt signaling pathway in Drosophila. J Biol Chem 2002;277(44):41762-41769.
7 Van Raay TJ, Vetter ML. Wnt/frizzled signaling during vertebrate retinal development. Dev Neurosci 2004;26(5-6):352-358.
8 Quiram PA, Drenser KA, Lai MM, Capone A Jr, Trese MT. Treatment of vascularly active familial exudative vitreoretinopathy with pegaptanib sodium (Macugen). Retina 2008;28(3 Suppl):S8-S12.
9 Tagami M, Kusuhara S, Honda S, Tsukahara Y, Negi A. Rapid regression of retinal hemorrhage and neovascularization in a case of familial exudative vitreoretinopathy treated with intravitreal bevacizumab.Graefes Arch Clin Exp Ophthalmol 2008;246(12):1787-1789.
10 Henry CR, Sisk RA, Tzu JH, Albini TA, Davis JL, Murray TG,Berrocal AM. Long-term follow-up of intravitreal bevacizumab for the treatment of pediatric retinal and choroidal diseases. J AAPOS 2015;19(6):541-548.
11 Ells AL, Wesolosky JD, Ingram AD, Mitchell PC, Platt AS. Lowdose ranibizumab as primary treatment of posterior type I retinopathy of prematurity. Can J Ophthalmol 2017;52(5):468-474.
12 Wallace DK, Kraker RT, Freedman SF, et al. Assessment of lower doses of intravitreous bevacizumab for retinopathy of prematurity: a phase 1 dosing study. JAMA Ophthalmol 2017;135(6):654-656.
13 Huang Q, Zhang Q, Fei P, Xu Y, Lyu J, Ji X, Peng J, Li YA, Zhao P.Ranibizumab injection as primary treatment in patients with retinopathy of prematurity: anatomic outcomes and in fluencing factors. Ophthalmology 2017;124(8):1156-1164.
14 VanderVeen DK, Melia M, Yang MB, Hutchinson AK, Wilson LB,Lambert SR. Anti-vascular endothelial growth factor therapy for primary treatment of type 1 retinopathy of prematurity: a report by the American academy of ophthalmology. Ophthalmology 2017;124(5):619-633.
15 Wallace DK. Retinopathy of prematurity: anti-VEGF treatment for ROP: which drug and what dose? J AAPOS 2016;20(6):476-478.
16 Sun Y, Jain A, Moshfeghi DM. Elevated vascular endothelial growth factor levels in Coats’ disease: rapid response to pegaptanib sodium.Graefes Arch Clin Exp Ophthalmol 2007;245(9):1387-1388.
17 Jun JH, Kim YC, Kim KS. Resolution of severe macular edema in adult Coats’ disease with intravitreal triamcinolone and bevacizumab injection. Korean J Ophthalmol 2008;22(3):190.
18 Park S, Cho HJ, Lee DW, Kim CG, Kim JW. Intravitreal bevacizumab injections combined with laser photocoagulation for adult-onset Coats'disease. Graefes Arch Clin Exp Ophthalmol 2016;254(8):1511-1517.
19 Li S, Deng G, Liu J, Ma Y, Lu H. The effects of a treatment combination of anti-VEGF injections, laser coagulation and cryotherapy on patients with type 3 Coat's disease. BMC Ophthalmol 2017;17(1):76.
20 Ramasubramanian A, Shields CL. Bevacizumab for Coats' disease with exudative retinal detachment and risk of vitreoretinal traction. Br J Ophthalmol 2012;96(3):356-359.