INTRODUCTION
Diabetic retinopathy (DR) is the most common microvascular and a highly specific neurovascular complication of both, type 1 and type 2 diabetes mellitus (DM)[1]. Nearly 50%of the diabetic population will develop some degree of DR and 10% to 40% of these patients will have diabetic macular edema (DME)[2-3]. DME is the leading cause of visual loss among productive-age patients in developed countries[2,4-5]and its classified as center-involved (CI DME) or non-center involved (non-CI DME)[5-6]. Focal/grid laser photocoagulation has been the mainstay of treatment for clinically significant DME for nearly 30y[7]. In an era where the treatment of CI DME has shifted towards the intravitreal injection of antivascular endothelial growth factors (VEGF)[8-9], macular laser photocoagulation remains the suggested treatment for non-CI DME[10-12]. Existing and new treatment modalities should target not only for visual acuity (VA) stabilization, as has been the primary goal of the laser photocoagulation, but to recover structure and function in even earlier stages of DME, as could represent the non-CI DME.
The aim of the present study is to compare the efficacy(anatomical and visual results) of intravitreal bevacizumab(BV) and subthreshold macular laser photocoagulation (SMP),each as monotherapy, or combined for the treatment of non-CI DME.
SUBJECTS AND METHODS
This was a prospective, controlled, parallel-group randomized,single-blind clinical study. Institutional ethics committee review and approval was obtained. The study adhered to the tenets of the Declaration of Helsinki. All patients signed written informed consent. Consecutive recruitment of patients attending to the Retina and Vitreous Clinic at the Ophthalmology Department, University Hospital, The Autonomous University of Nuevo León was made. Inclusion criteria were patients older than 18y with type 2 DM, non-CI DME and a best-corrected visual acuity (BCVA) of 0.30 logarithm of the minimal angle of resolution (logMAR) or better. Exclusion criteria were media opacities that impeded laser treatment or proper evaluation, glaucoma, vitreous hemorrhage, intraocular surgery in the previous 12mo, laser treatment or intravitreal anti-VEGF injection in the previous 6mo.Patients were randomly assigned following simple randomization procedures (computerized random numbers) into 3 treatment groups. Group 1, monthly intravitreal BV; group 2, single application of SMP; group 3, single application of SMP and monthly intravitreal BV. If both eyes of a same patient were to be included, the second eye was assigned to the same treatment arm by cause of the systemic biodisponibility of BV and its possible effect on the uninjected eye[13-14]. Follow-up for 3mo was achieved.
All patients had a complete baseline ophthalmic evaluation including dilated fundoscopy, BCVA with Snellen chart and score conversion to the logMAR; macular optical coherence tomography (OCT) (macular thickness map and retinal map analysis report; Stratus OCT, Carl Zeiss Meditec, Dublin,CA, USA), and fluorescein angiography (FA) when needed(VISUCAM Lite, Carl Zeiss, Meditec, Dublin, CA, USA and a 5-mL intravenous injection of sodium fluorescein at 10%).Group 1 received intravitreal BV monthly until 3 doses were completed. Group 2 received SMP in one session[15]. Group 3 received intravitreal BV and prompt SMP. Patients were followed monthly with BCVA, complete ophthalmologic examination and IOP. After 3mo of follow up, a final evaluation was performed according to the monthly protocol of evaluation which included macular OCT and FA.
The SMP was performed with a diode-pumped solid-state laser(VISULAS 532s, Carl Zeiss Meditec AG, Jena, Germany)using the technique described by González-Cortés et al[15].Intravitreal injection of BV (1.25 mg/0.05 mL) was performed via pars plana in the superotemporal quadrant using a 1-mL syringe with a 31-gauge needle. All injections were performed in an operating room under sterile conditions with povidoneiodine, topical antibiotics and topical anesthesia. Immediately after the procedure, uncorrected VA was verified to be at least of count fingers. Prophylactic broad-spectrum antibiotic eye drops were prescribed 4 times a-day for 4d. All patients that received intravitreal injections underwent a security evaluation 48h after the procedure to look for adverse events such as ocular hypertension, iritis, cataract, vitritis, vitreous hemorrhage,macular exudates/hemorrhages, retinal detachment and endophthalmitis.
Evaluated parameters in OCT were: the central subfield thickness (CST), macular area of greatest thickness (MAGT)and total macular volume (TMV). Non-CI DME was defined as retinal thickness >250 μm in at least one zone of the ETDRS grid available in the macular OCT map and CST≤250 μm,or CST<320 μm with evidence of focal edema strictly of parafoveolar origin corroborated with FA. One experienced retinologist (Gonzalez-Cotres JH), blinded to the intervention that the patient received, evaluated all the imaging studies before and after treatment.
Statistical Analysis Statistical analysis was performed with IBM SPSS statistics for Windows (Version 22.0. Armonk, NY.IBM, Corp., USA). Quantitative variables were analyzed with ANOVA. A confidence interval of 95% was established, and P<0.05 was considered statistically significant.
RESULTS
A total of 32 eyes were included and randomized into the three treatment arms [group 1, n=10 (31.25%); group 2, n=12(37.5%); group 3, n=10 (31.25%)]. Demographics and the basal evaluation showed no statistically significant differences between groups (Table 1).
Outcomes at 3mo after treatment are summarized in Table 2.The three groups showed a tendency towards improvement in BCVA, but only the combination of BV plus SMP produced a statistically significant improvement after treatment, from 0.19±0.16 to 0.12±0.14 logMAR (P=0.041). Anatomically,BV as monotherapy produced a statistically significant change in the MAGT (from 325±26.26 to 298.20±44.85 μm, P=0.022)and TMV (from 7.79±0.57 to 7.50±0.56 mm3, P=0.047).BV with SMP showed significant reduction of TMV (from 7.90±0.57 mm3 to 7.65±0.73 mm3, P=0.025). Monotherapy with SMP failed to show significant changes after treatment in any of the parameters evaluated. No ocular or monocular adverse events were presented during the study, remarking the safety of the procedures.
DISCUSSION
This study on non-CI DME, demonstrates that the “loading phase” of 3 monthly intravitreal injections of BV as monotherapy or combined with SMP are superior in providing visual and anatomical improvement when compared to SMP as monotherapy. Functionally, significant improvement of BCVA was achieved only in the group of combined BV and SMP. Nonetheless, the groups treated with monotherapy of BV and SMP showed a tendency towards improvement. A possible reason for a lack of significant gain in BCVA might be that one of our inclusion criteria was a BCVA of 0.30 logMAR or better. Thus, the niche for significant visual improvement in our sample was small, and these results should not be generalized to patients with worse baseline VA. Anatomically,the group of BV as monotherapy showed significant improvement in TMV and MAGT. Whereas the group of combined BV and SMP demonstrated significant reduction of the TMV only. Interestingly, monotherapy with SMP failed to
achieve significant improvement of the parameters evaluated.The greater improvement in the groups treated with BV in our study might because it is after the “loading phase” of an anti-VEGF when results are more remarkable[16] and our follow-up consisted of 3mo.
Table 1 Demographic and baseline measurements
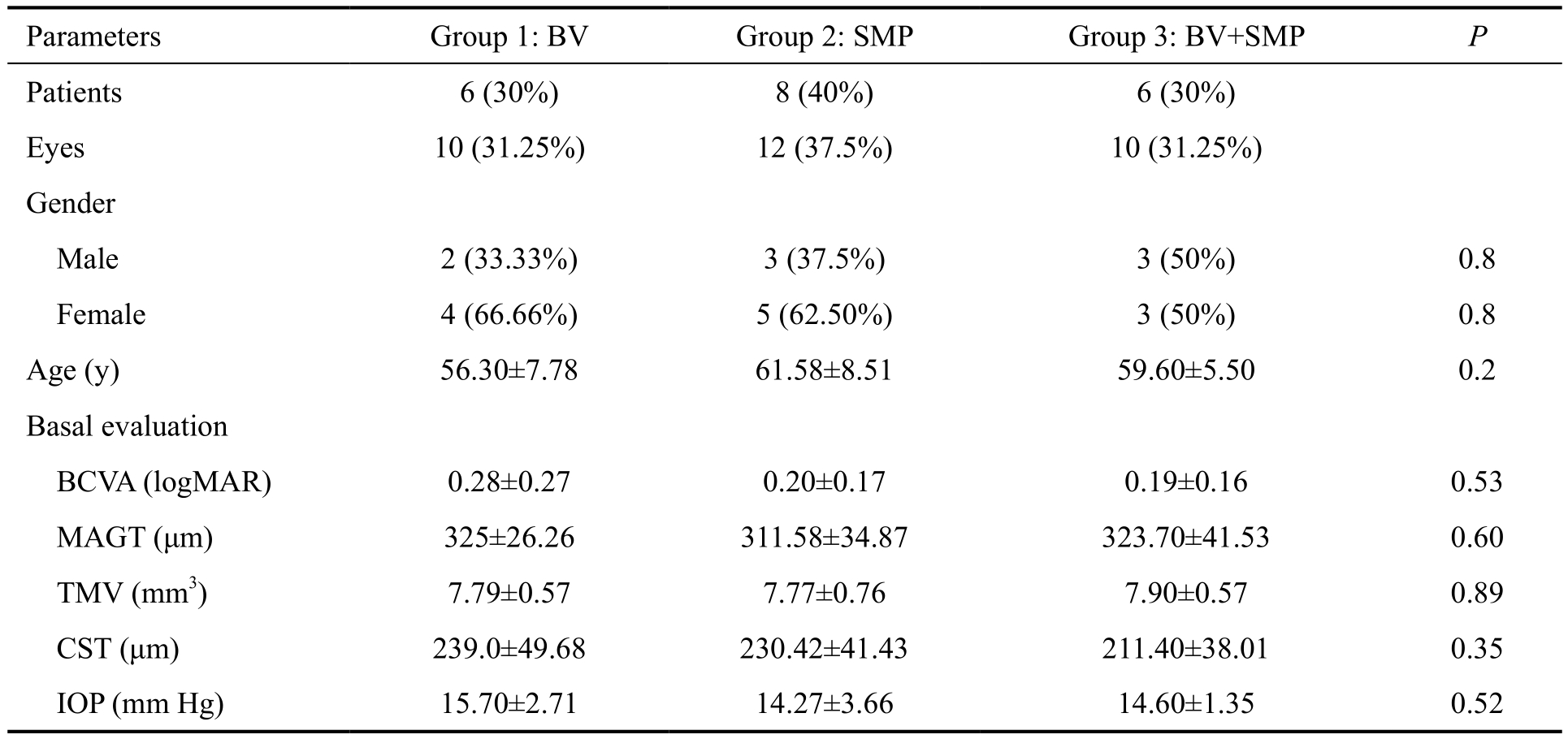
BV: Bevacizumab; SMP: Subthreshold laser macular photocoagulation; BCVA: Best-corrected visual acuity; MAGT: Macular area of greater thickness; TMV: Total macular volume; CST: Central sub field thickness; IOP: Intraocular pressure.
Table 2 Treatment response by group before and 3mo after treatment mean±SD
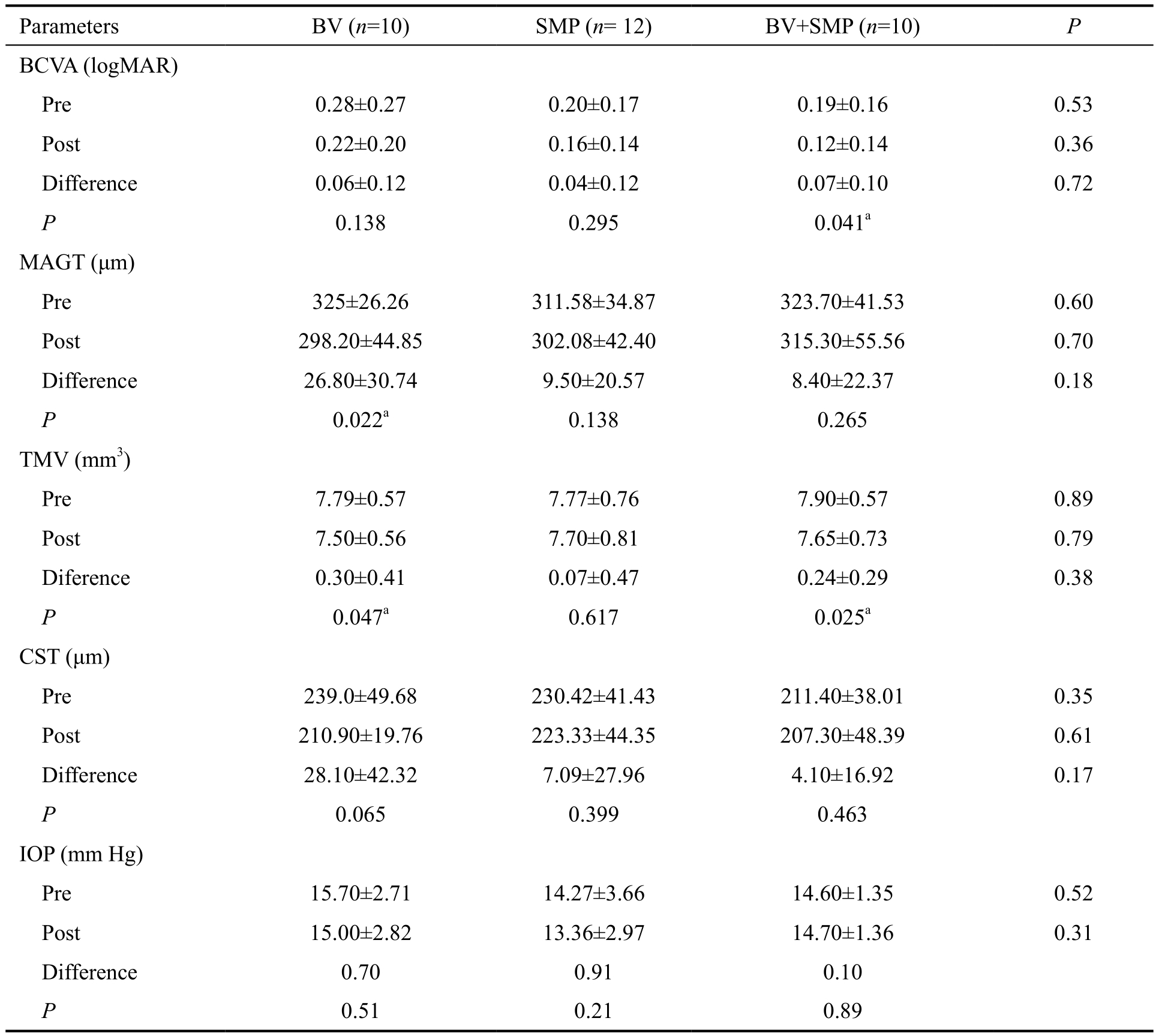
BV: Bevacizumab; SMP: Subthreshold laser macular photocoagulation; BCVA: Best-corrected visual acuity; MAGT: Macular area of greater thickness; TMV: Total macular volume; CST: Central sub field thickness; IOP: Intraocular pressure. aStatistically significant values.
Our results show that BV alone or combined with SMP is/are better than SMP as monotherapy. Similarly, the intravitreal injection of any anti-VEGF as monotherapy or combined with laser treatment has shown long-term superior outcomes when compared to monotherapy with laser in randomized clinical trials[7,17-25]. The DRCR.net Protocol I, analyzed the effect of intravitreal ranibizumab (0.5 mg) combined with prompt or deferred (24wk) focal/grid laser treatment for CI DME[17,26].The 5-year report[17], found no significant changes in BCVA and CST by OCT between the 2 laser groups. These results suggest that prompt laser treatment is not better than deferring laser in eyes with CI DME. Nevertheless, the number of intravitreal injections was higher in the group of deferred laser than in the group of prompt laser (17 and 13 injections,respectively). It is worth noting that the previous mentioned studies didn’t make a distinction between CI or non-CI DME.Fewer studies exclusively on non-CI DME are available and our work is one of them. The report of the DRCR.net[27] and its subsequent analysis by Scott et al[6] are two recent studies that evaluate the therapeutic effect of modified ETDRS (focal/grid)and mild macular grid laser photocoagulation in patients with non-CI DME. The results suggest stability in VA with a median decrease in VA letter score of 1 letter. Most of the eyes (50%)having a ±4 letter improvement and a median retinal thickness reduction measured with OCT of 4 to 12 μm after a 12-month follow-up[6,27]. This demonstrates the relative stability in VA and retinal thickness with laser photocoagulation monotherapy as has been known since the first ETDRS report[7].
Despite that the current guidelines recommend laser photocoagulation as the treatment of choice for non-CI DME[10-12,28], the clinical judgement has always a role of paramount importance in the treatment of patients with DME and the clinical question of whether to start intravitreal anti-VEGF for selected non-CI DME cases can be raised. Our results provide useful information about the superiority of combined therapy with BV and SMP or BV alone. In addition,we highlight the safety profile of the procedures at a short-term follow-up, since all the parameters evaluated improved with a tendency towards significance in each group, including the group treated with SMP as monotherapy.
Our study has several limitations requiring careful interpretation.Simple randomization and a small eye sample influence on the results and limit the power of our study. In addition, we report the results and the effect that the “loading phase” of this treatment modalities have on non-CI DME after a short follow-up. The metabolic control status of the patients was not included in our analysis, nevertheless, all patients were managed by an internist or endocrinologist. Long-term results need to be evaluated and reported in oncoming projects.
We recommend the use of BV for patients with non-CI DME in which macular anatomy is most affected, since this group showed the most significant anatomical recovery. The addition of SMP to BV might be reserved for patients with worse baseline BCVA, because the group with combined therapy was the only one that had a significant improvement in BCVA.Despite its limitations, our work provides valuable information in the short-term management of non-CI DME. Further work with longer follow-up, greater samples and higher resolution imaging is needed to stablish the best therapeutic modality for these cases. In conclusion, our study suggests that BV as monotherapy or its combination with SMP, are superior for VA and anatomic recovery in eyes with non-CI DME, when compared to SMP alone.
ACKNOWLEDGEMENTS
Research was conducted using resources from the division of Ophthalmology of our Institution.
Conflicts of Interest: Cuervo-Lozano E, None; González-Cortés JH, None; Olvera-Barrios A, None: Treviño-Cavazos E, None; Rodríguez-Pedraza J, None; Mohamed-Noriega K, None; Mohamed-Hamsho J, None.
REFERENCES
1 Solomon SD, Chew E, Duh EJ, Sobrin L, Sun JK, VanderBeek BL,Wykoff CC, Gardner TW. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care 2017;40(3):412-418.
2 Chun DW, Heier JS, Topping TM, Duker JS, Bankert JM. A pilot study of multiple intravitreal injections of ranibizumab in patients with centerinvolving clinically significant diabetic macular edema. Ophthalmology 2006;113(10):1706-1712.
3 Williams R, Airey M, Baxter H, Forrester J, Kennedy-Martin T,Girach A. Epidemiology of diabetic retinopathy and macular oedema: a systematic review. Eye (Lond) 2004;18(10):963-983.
4 Massin P, Paques M, Pournaras JA. Diabetic Macular Edema, in Ocular disease: mechanisms and management. 2010, Saunders/Elsevier:Philadelphia. p.519-526.
5 González-Cortés JH. Treatment of diabetic macular edema (DME):shifting paradigms. Med Univer 2015;17(69):243-247.
6 Scott IU, Danis RP, Bressler SB, Bressler NM, Browning DJ, Qin H;Diabetic Retinopathy Clinical Research Network. Effect of focal/grid photocoagulation on visual acuity and retinal thickening in eyes with noncenter-involved diabetic macular edema. Retina 2009;29(5):613-617.
7 Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol 1985;103(12):1796-1806.
8 Wykoff CC, Hariprasad SM. DRCR protocol-T: reconciling 1- and 2-year data for managing diabetic macular edema. Ophthalmic Surg Lasers Imaging Retina 2016;47(4):308-312.
9 Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, Mitchell P, Sharp D, Wolf-Schnurrbusch UE, Gekkieva M,Weichselberger A, Wolf S. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized,controlled, double-masked, multicenter phase II study. Diabetes Care 2010;33(11):2399-2405.
10 Hooper P, Boucher MC, Colleaux K, Cruess A, Greve M, Lam WC,Shortt S, Tourville E. Contemporary management of diabetic retinopathy in Canada: from guidelines to algorithm guidance. Ophthalmologica 2014;231(1):2-15.
11 Bandello F, Cunha-Vaz J, Chong NV, Lang GE, Massin P, Mitchell P,Porta M, Prünte C, Schlingemann R, Schmidt-Erfurth U. New approaches for the treatment of diabetic macular oedema: recommendations by an expert panel. Eye (Lond) 2012;26(4):485-493.
12 Mitchell P, Wong TY; Diabetic Macular Edema Treatment Guideline Working Group. Management paradigms for diabetic macular edema. Am J Ophthalmol 2014;157(3):505-513.
13 Bakri SJ, Snyder MR, Reid JM, Pulido JS, Singh RJ. Pharmacokinetics of intravitreal bevacizumab (Avastin). Ophthalmology 2007;114(5):855-859.
14 Nomoto H, Shiraga F, Kuno N, Kimura E, Fujii S, Shinomiya K,Nugent AK, Hirooka K, Baba T. Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits. Invest Ophthalmol Vis Sci 2009;50(10):4807-4813.
15 González CJH, Treviño CEE, Cuervo LEE, Martínez LPA, Mohamed HJ. Tratamiento del edema macular diabético clínicamente significativo con láser Nd:Yag subumbral de doble frecuencia. Med Univer 2008;10(41):190-199.
16 Nicholson L, Patrao NV, Ramu J, Vazquez-Alfageme C, Muwas M,Rajendram R, Hykin PG, Sivaprasad S. Influence of baseline diabetic retinopathy status on initial anatomical response of intravitreal ranibizumab therapy for diabetic macular oedema. Eye (Lond) 2017;31(9):1358-1364.
17 Elman MJ, Ayala A, Bressler NM, Browning D, Flaxel CJ, Glassman AR, Jampol LM, Stone TW; Diabetic Retinopathy Clinical Research Network. Intravitreal Ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results.Ophthalmology 2015;122(2):375-381.
18 Ishibashi T, Li X, Koh A, Lai TY, Lee FL, Lee WK, Ma Z, Ohji M,Tan N, Cha SB, Shamsazar J, Yau CL; REVEAL Study Group. The REVEAL Study: ranibizumab monotherapy or combined with laser versus laser monotherapy in asian patients with diabetic macular edema.Ophthalmology 2015;122(7):1402-1415.
19 Photocoagulation for diabetic macular edema: Early Treatment Diabetic Retinopathy Study Report no. 4. The Early Treatment Diabetic Retinopathy Study Research Group. Int Ophthalmol Clin 1987;27(4):265-272.
20 Focal photocoagulation treatment of diabetic macular edema.Relationship of treatment effect to fluorescein angiographic and other retinal characteristics at baseline: ETDRS report no. 19. Early Treatment Diabetic Retinopathy Study Research Group. Arch Ophthalmol 1995;113(9):1144-1155.
21 Arevalo JF, Sanchez JG, Fromow-Guerra J, Wu L, Berrocal MH,Farah ME, Cardillo J, Rodríguez FJ; Pan-American Collaborative Retina Study Group (PACORES). Comparison of two doses of primary intravitreal bevacizumab (Avastin) for diffuse diabetic macular edema:results from the Pan-American Collaborative Retina Study Group(PACORES) at 12-month follow-up. Graefes Arch Clin Exp Ophthalmol 2009;247(6):735-743.
22 Heier JS, Korobelnik JF, Brown DM, Schmidt-Erfurth U, Do DV,Midena E, Boyer DS, Terasaki H, Kaiser PK, Marcus DM, Nguyen QD,Jaffe GJ, Slakter JS, Simader C, Soo Y, Schmelter T, Vitti R, Berliner AJ, Zeitz O, Metzig C, Holz FG. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID Studies.Ophthalmology 2016;123(11):2376-2385.
23 Schmidt-Erfurth U, Lang GE, Holz FG, Schlingemann RO, Lanzetta P, Massin P, Gerstner O, Bouazza AS, ShenH, Osborne A, Mitchell P; RESTORE Extension Study Group. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology 2014;121(5):1045-1053.
24 Rajendram R, Fraser-Bell S, Kaines A, Michaelides M, Hamilton RD,Esposti SD, Peto T, Egan C, Bunce C, Leslie RD, Hykin PG. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema:24-month data: report 3. Arch Ophthalmol 2012;130(8):972-979.
25 Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV,Boyer D, Heier JS, Abraham P, Thach AB, Lit ES, Foster BS, Kruger E, Dugel P, Chang T, Das A, Ciulla TA, Pollack JS, Lim JI, Eliott D,Eliot D, CampochiaroPA; READ-2 Study Group. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study.Ophthalmology 2010;117(11):2146-2151.
26 Diabetic Retinopathy Clinical Research Network, Elman MJ, Qin H,Aiello LP, Beck RW, Bressler NM, Ferris FL 3rd, Glassman AR, Maturi RK, Melia M. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology 2012;119(11):2312-2318.
27 Writing Committee for the Diabetic Retinopathy Clinical Research Network, Fong DS, Strauber SF, Aiello LP, Beck RW, Callanan DG, Danis RP, Davis MD, Feman SS, Ferris F, Friedman SM, Garcia CA, Glassman AR, Han DP, Le D, Kollman C, Lauer AK, Recchia FM, Solomon SD.Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol 2007;125(4):469-480.
28 Das T, Aurora A, Chhablani J, Giridhar A, Kumar A, Raman R,Nagpal M, Narayanan R, Natarajan S, Ramasamay K, Tyagi M, Verma L. Evidence-based review of diabetic macular edema management:Consensus statement on Indian treatment guidelines. Indian J Ophthalmol 2016;64(1):14-25.