INTRODUCTION
Pathological myopia (PM) presents a variable spectrum of characteristic fundus lesions[1-2], including lacquer cracks,chorioretinal atrophy, myopic choroidal neovascularization(CNV), vitreomacular traction, and posterior staphyloma[3].PM is one of the most common causes of irreversible vision loss and blindness in the world, particularly in East Asia[4-6].Global estimation has speculated that the prevalence of myopia and high myopia will increase dramatically between 2000 and 2050[7], with high myopia possibly affecting up to 1 billion people. Thus, it is necessary to plan services to manage and prevent myopia, as well as its related complications.
Peripapillary atrophy (PPA) is one of the characteristic features of highly myopic eyes[8]. The mechanical stretching of the eye globe due to a longer axial length (AL) causes choroidal and retinal pigment epithelium (RPE) thinning,leading to degeneration of the retina, RPE, and choroid[9].This degeneration presents itself around the optic disc, and is often seen as a peripapillary crescent or halo, which can be seen ophthalmoscopically as a whitish discolored area of visible inner sclera with a distinguished border. The PPA region is divided into α, β and γ zones. One histological study[10] has shown that the PPA-β zone is the region with Bruch’s membrane, but without RPE, which is related to an almost absolute loss of photoreceptors and closure of the choriocapillaris. PPA-γ zone is the region without both Bruch’s membrane and RPE. Changes in the optic disc in children during the development of myopia have been tracked and shown to include disc tilting and an enlargement of the PPA-β zone. These changes are highly related to the development of myopia, the degree of refractive error[11], and glaucoma[12-13].PPA may be observed in early myopia, and is considered to be a possible risk factor or indicator of the development of pathological myopia later in life[14-15]. However, the relationships between the morphology of the optic disc and the other ocular parameters have not yet been determined in adult patients. It has been observed that when compared to the optic discs of emmetropic or hyperopic eyes, the optic discs of myopic eyes are usually larger in diameter, and the cupping tends to be more concentric and shallow[15]. However, the relationships between the optic disc morphology and the other ocular parameters have yet been thoroughly studied.
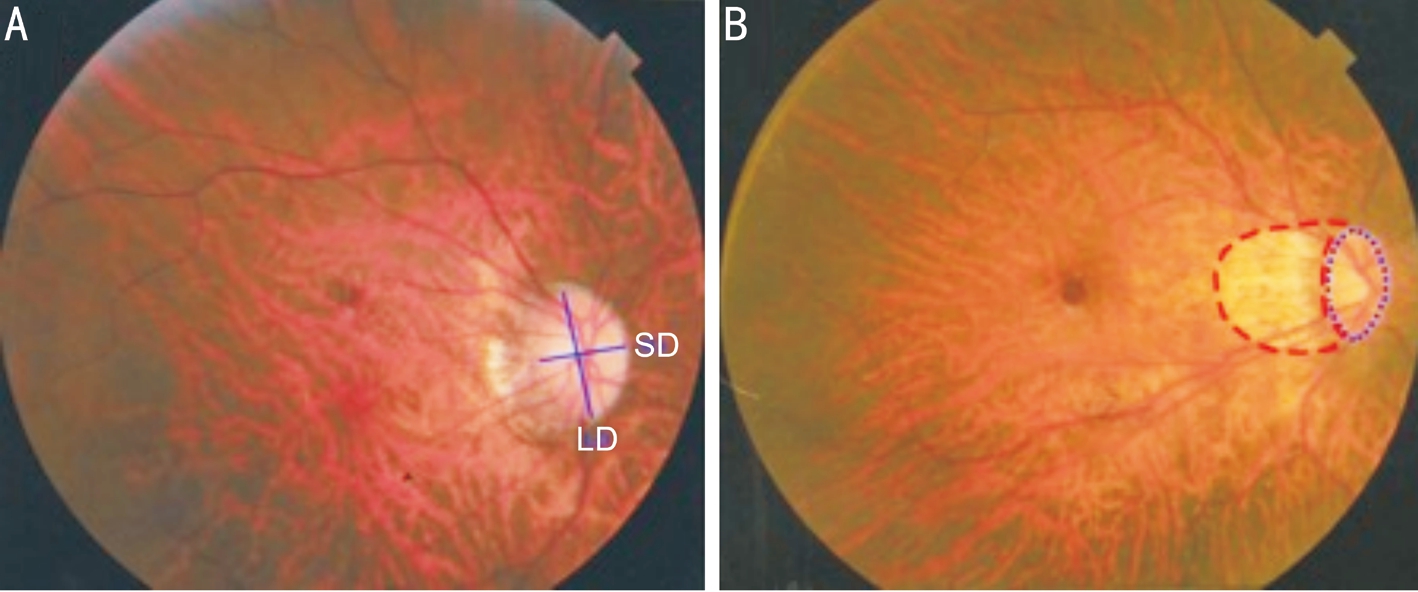
Figure 1 Identification of the ovality index and peripapillary atrophy area via Photoshop A: The ovality index was defined as the ratio between the shortest diameter (SD) and longest diameter (LD) of the optic disc; B: The peripapillary atrophy area was outlined manually, while the pixel area was calculated automatically using the software.
Therefore, we conducted this study in search of the morphological correlations of the optic disc, to measure the size of the PPA-β/γ area and ovality index, and to assess the relationships with the other ocular parameters, namely, the AL, refractive error, best corrected visual acuity (BCVA), choroidal thickness (CT) and age.
SUBJECTS AND METHODS
This observational case study was conducted in the Fundus Center of the Zhongshan Ophthalmic Center in Guangzhou,China between June 2013 and May 2016. This research was approved by the Institutional Review Board and Ethics Committee of the Zhongshan Ophthalmic Center. All of the investigations adhered to the principles of the Declaration of Helsinki.
Patients We retrospectively observed 667 patients with highly myopic eyes [spherical equivalent (SE) ≤-6.0 D, or AL≥26.5 mm]. Every patient had complete ophthalmological examination data, including refractometry, BCVA, fundus photography (CR-DGI; Canon Inc., Tokyo, Japan), A-mode ultrasonography (IOLMaster; Carl Zeiss Meditec AG, Jena,Germany), and enhanced spectral domain optical coherence tomography (SD-OCT; Spectralis HRA+OCT, Heidelberg Engineering, Heidelberg, Germany). Those eyes with advanced cataracts, a history of ocular surgery, and/or retinal detachment were excluded from this study.
Examinations The presenting BCVA was measured with correction using the logarithm of the minimal angle of resolution (logMAR) chart (Lighthouse International, NY,USA) at a distance of 4 m. If the patient could not read the largest number at 4 m, the chart was brought closer; then, the finger counting, hand motion, or light perception vision was assessed. The SE was calculated as the sum of the spherical power and one-half of the cylindrical power. The AL was obtained via the IOLMaster (Carl Zeiss Meditec AG, Jena,Germany) and OCT was used to measure the subfoveal CT and check for foveoschisis.
The fundus photographs of the macula and optic disc were obtained using a 45-degree digital retinal camera (Canon Inc.,Tokyo, Japan), after pupillary dilation with 1% tropicamide,following the Early Treatment for Diabetic Retinopathy Study standard photograph numbers 1 and 2. The photographs were viewed using Photoshop CS5 (Adobe Systems Inc., San Jose,California, USA). Two ophthalmologists were blinded to draw the boundaries and the minimal and maximal disc diameters.The optic disc ovality index was calculated by the ratio of the minimal to maximal optic disc diameters. “Round” was defined as an ovality index ≥0.8 and “oval” was defined as an ovality index <0.8[14]. The PPA-β/γ zone was characterized by a visible sclera and visible large choroidal vessels[16]. The PPA-β/γ area was plotted using a mouse-driven cursor to trace the disc and PPA-β/γ margins directly onto the image. The degree of PPA-β/γ zone was represented by the ratio of the PPA-β/γ area to the optic disc area (Figure 1).
Statistical Analyses One eye per subject was chosen based on the highest myopic refractive error. We examined the associations between the pathological myopia findings and the BCVA, age, SE, CT, and AL. The linear measurements were showed as the mean±SD. Differences between the two groups were analyzed statistically using the Mann-Whitney U test.Spearman’s rank correlation coefficient was used to describe the relationships between two groups. A probability value of P<0.05 was considered to be statistically significant for all of the analyses. The statistical analyses were performed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
A total of 667 highly myopic eyes from 667 patients (252 males and 415 females, ages ranging from 18 to 88 years old)were enrolled in this study. The patient characteristics, means,and range values for the measurements are summarized in Table 1. Correlations among the measurements with regard to the patient age were shown in Table 2. The biometric parameters were correlated with one another. The patient age was significantly negatively correlated with the ovality index(r=-0.262, P<0.01) and CT (r=-0.352, P<0.01), positively correlated with PPA (r=0.478, P<0.01) and BCVA (r=0.358,P<0.01), but not with the AL (r=0.09, P=0.124) and refractive error (r=-0.021, P=0.743).
In total, PPA-β/γ zone was found in 86.5% (577/667) eyes.PPA-β/γ area was significantly positively correlated with the AL (r=0.899, P<0.001) and BCVA (r=0.426, P<0.001),negatively correlated with ovality index (r=-0.232, P<0.001),refractive error (r=-0.312, P<0.001) and CT (r=-0.215,P<0.001). PPA-β/γ zone was subgrouped into peripapillary halos and peripapillary crescents. These two groups were significantly different with regard to the age (51.42±15.20 vs 41.56±14.57, P<0.001), AL (30.65±2.33 vs 28.91±2.09),BCVA (0.62±0.55 vs 0.45±0.53, P=0.011), refractive error(-17.58±6.09 vs -13.32±4.66, P<0.001), PPA (6.00±5.77 vs 2.22±1.80 P<0.001), and CT (61.93±56.08 vs 102.43±79.34,P<0.001). However, the difference was not significant with regard to the ovality index (0.70±0.11 vs 0.71±0.11, P=0.285)(Table 3).
The ovality index was significantly negatively correlated with the AL (r=-0.328, P<0.001), BCVA (r=-0.248, P<0.001) and age (r=-0.262, P<0.001), positively correlated with refractive error (r=0.306, P<0.001) and CT (r=0.294, P<0.001). Table 4 shows the characteristic differences between the highly myopic eyes with round discs and those with oval discs. The groups differed significantly with regard to the age (39.94±15.51 vs 46.57±15.25, P=0.002), AL (28.51±2.06 vs 29.83±2.32,P<0.001), refractive error (-11.98±4.85 vs -15.28±5.41,P<0.001), BCVA (0.34±0.45 vs 0.56±0.56, P=0.003), PPA(2.07±2.13 vs 4.01±2.29, P=0.001), and CT (113.32±83.24 vs 73.53±58.54, P=0.002).
DISCUSSION
The PPA area is characterized by a visible, almost bare sclera exposed by an atrophic choroid with distinct large choroidal vessels. As myopia progresses, the myopic disc tilt deviates from the temporal to the nasal region[17]. The results of our study showed that the PPA area in highly myopic eyes was significantly associated with a longer AL, higher myopic refractive error, worse BCVA, and older age. The factor most closely associated with the PPA area was the AL, suggesting that the mechanical tension acting on the retina and choroid due to the elongation of the globe was one of the principle causes of PPA in high myopia. Moreover, the peripapillary region of highly myopic eyes consists of a significantly thinscleral flange and retinal nerve fiber tissue, while lacking Bruch’s membrane and choroidal tissue[18]. This may explain why the PPA was significantly larger with the highly myopic biometrics. In comparison with other relevant studies[19-20],our results showed similar PPA relationships with the AL and refractive error. However, it was previously observed that in PPA eyes with optic disc crescents, the size of the crescent was not associated with a longer AL[21]. This discrepancy is probably due to the fact that our PPA data was measured by the ratio of the PPA to the optic disc area, which corrected the retinal magnification induced by the different ALs, and that most of the patients in our study were very highly myopic(mean AL: 29.53±2.33 mm). When comparing those eyes with peripapillary halos to those with peripapillary crescents,we also found an older age, longer AL, worse BCVA, higher refractive error, greater PPA, and thinner CT in those with peripapillary halos. This finding suggests that different mechanisms underlie the pathogenesis of the PPA enlargement associated with aging and axial elongation.
Table 1 Characteristics of the 667 highly myopic eyes of the 667 patients mean±SD (range)
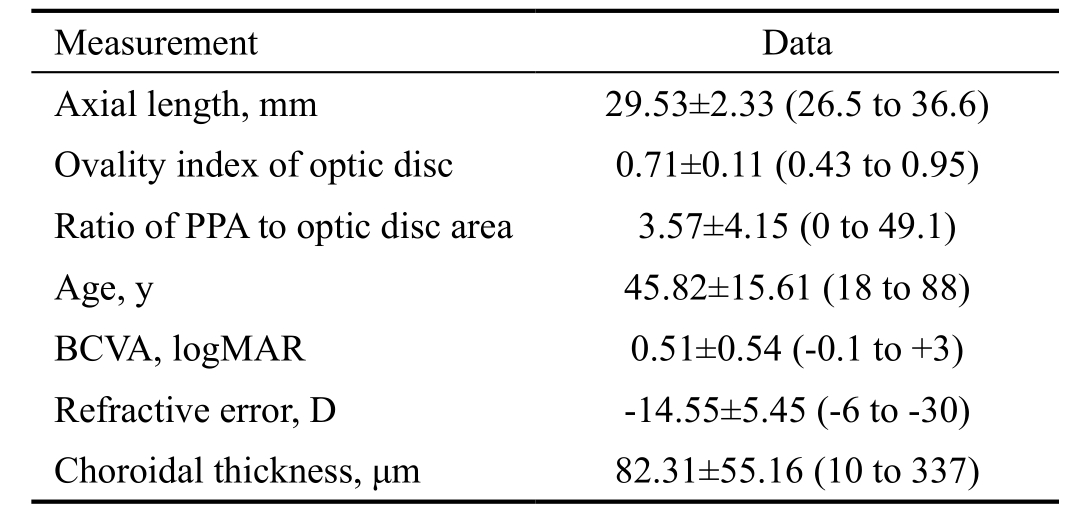
SD: Standard deviation; PPA: Peripapillary atrophy; BCVA: Best corrected visual acuity; D: Diopters.
We also compared the data between the PPA eyes with round discs and those with oval discs and found that an oval disc was correlated with an older age, longer AL, higher refractive error,worse BCVA, greater PPA, and thinner CT. The changes in the optic nerve head, including its marked enlargement, first begin at an AL of approximately 26.5 mm, or at a refractive error of approximately -8.0 D. With the progression of myopia, there is a reduction in the ovality index of the optic disc, along with a greater width in the PPA area[22-23]. In other words, we observed that the optic nerve tilt was a result of posterior protrusion,which is contrary to the study performed by How et al[24].These differences may be explained by the different definition of a tilted optic disc (e.g. index of tilt <0.75 in How et al[24]).In our study, only highly myopic patients were recruited, but in the How et al’s study[24], the population included a wider range of refractive errors.
The region without Bruch’s membrane and without RPE has been called as parapapillary γ zone[20,25-26]. This γ zone is significantly correlated with high axial myopia, while the βzone is associated with the presence of glaucoma[20,26]. In our study, the PPA-β/γ zone was employed for analysis because we focused on the data obtained from the fundus photographs,which is easily available in all clinical settings. Since the patients in our study had a higher refractive error and were older, we found that the PPA-β/γ zone was also correlated with high myopia.
Table 2 Correlations of the biometric parameters in the highly myopic eyes
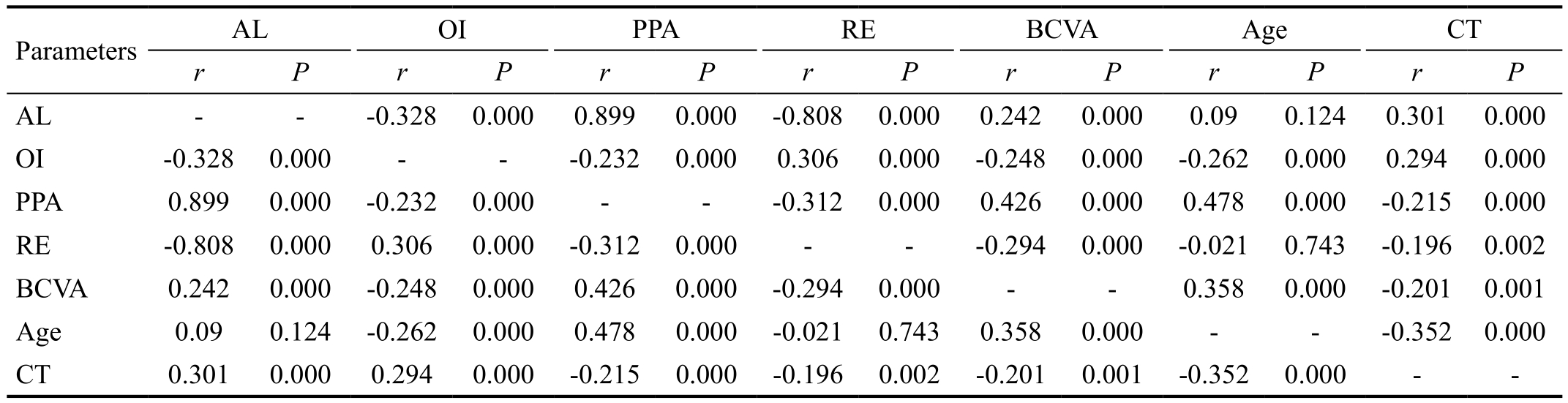
AL: Axial length; OI: Ovality index; PPA: Peripapillary atrophy; RE: Refractive error; BCVA: Best corrected visual acuity; CT: Choroidal thickness.
Table 3 Characteristics of eyes with peripapillary halos versus peripapillary crescents

BCVA: Best corrected visual acuity; PPA: Peripapillary atrophy; CT: Choroidal thickness.
Table 4 Characteristics of eyes with an ovality index ≥0.8 versus <0.8
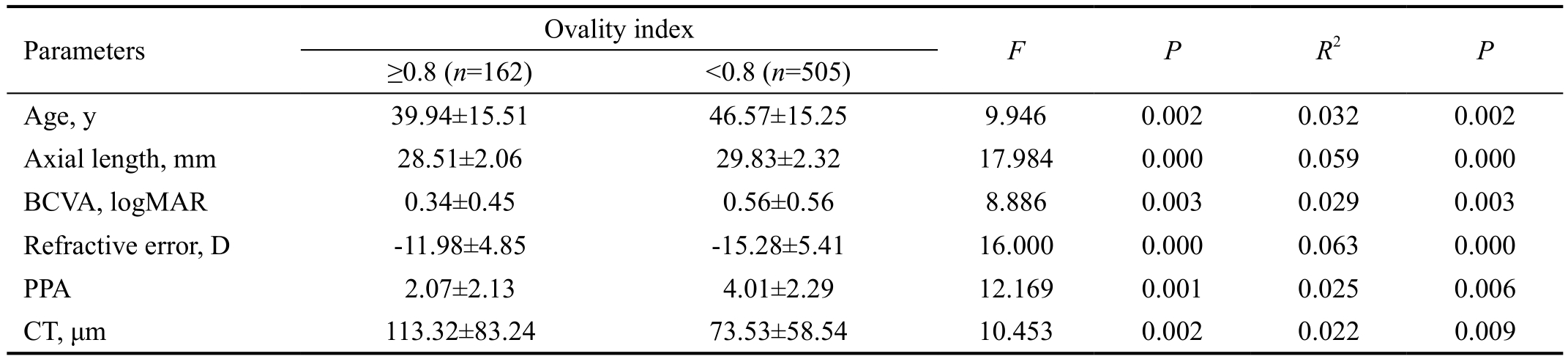
BCVA: Best corrected visual acuity; PPA: Peripapillary atrophy; CT: Choroidal thickness.
Our study showed relationships among the PPA-β/γ area,ovality index, age, AL, refractive error, BCVA, peripapillary halo versus peripapillary crescent, round versus oval disc shape. However, this study did have several limitations. For example, we did not perform magnetic resonance imaging(MRI) to determine the posterior staphyloma location,which has a tremendous effect on both the biometric indices and retinal anatomy. In addition, our study subjects were recruited from a referral clinic-based practice and not via a population-based screening. Future prospective studies, using larger sample sizes, are needed to definitively determine the abovementioned associations.
We believe that these measurements and associations will enhance the overall understanding of PPA-β/γ and the ovality index. In addition, the variables determining the size of the PPA-β/γ area and ovality index may be valuable for evaluating the degree of myopia, such as the AL, CT, and BCVA.
ACKNOWLEDGEMENTS
Foundations: Supported by the National Natural Science Foundation of China (No.81570862); the Guangdong Provincial Science and Technology Grant (No.2016A020215096); Guangzhou Science and Technology Project (No.3030901006039).
Conflicts of Interest: Zhao XJ, None; Jiang HY, None; Li YH, None; Liu BQ, None; Xu HX, None; Zhou J, None;Chen XH, None; Lyu CC, None; Ma W, None; Ma J, None;Liang XL, None; Jin CJ, None; Ding XY, None; Lu L,None.
REFERENCES
1 Wong TY, Ferreira A, Hughes R, Carter G, Mitchell P. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol 2014;157(1):9-25.e12.
2 Soubrane G. Choroidal neovascularization in pathologic myopia: recent developments in diagnosis and treatment. Surv Ophthalmol 2008;53(2):121-138.
3 Grossniklaus HE, Green WR. Green, Pathologic findings in pathologic myopia. Retina 1992;12(2):127-133.
4 Klaver CC, Wolfs RC, Vingerling JR, Hofman A, de Jong PT. Agespecific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Arch Ophthalmol 1998;116(5):653-658.
5 Xu L, Wang Y, Li Y, Wang Y, Cui T, Li J, Jonas JB. Causes of blindness and visual impairment in urban and rural areas in Beijing: the Beijing Eye Study. Ophthalmology 2006;113(7):1134.e1-11.
6 Iwase A, Araie M, Tomidokoro A, Yamamoto T, Shimizu H, Kitazawa Y; Tajimi Study Group. Prevalence and causes of low vision and blindness in a Japanese adult population: the Tajimi Study. Ophthalmology 2006;113(8):1354-1362.
7 Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ, Resnikoff S. Global Prevalence of myopia and high myopia and temporal trends from 2000 through 2050.Ophthalmology 2016;123(5):1036-1042.
8 Fantes FE, Anderson DR. Clinical histologic correlation of human peripapillary anatomy. Ophthalmology 1989;96(1):20-25.
9 Fujiwara T, Imamura Y, Margolis R, Slakter JS, Spaide RF. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. Am J Ophthalmol 2009;148(3):445-450.
10 Jonas JB, Holbach L, Panda-Jonas S. Peripapillary ring: histology and correlations. Acta Ophthalmol 2014;92(4):e273-e279.
11 Kim TW, Kim M, Weinreb RN, Woo SJ, Park KH, Hwang JM.Optic disc change with incipient myopia of childhood. Ophthalmology 2012;119(1):21-26.e1-3.
12 Jonas JB, Fernández MC, Naumann GO. Glaucomatous parapapillary atrophy. Occurrence and correlations. Arch Ophthalmol 1992;110(2):214-222.
13 Healey PR, Mitchell P, Gilbert CE, Lee AJ, Ge D, Snieder H, Spector TD, Hammond CJ. The inheritance of peripapillary atrophy. Invest Ophthalmol Vis Sci 2007;48(6):2529-2534.
14 Samarawickrama C, Mitchell P, Tong L, Gazzard G, Lim L, Wong TY, Saw SM. Myopia-related optic disc and retinal changes in adolescent children from Singapore. Ophthalmology 2011;118(10):2050-2057.
15 Hayashi K, Ohno-Matsui K, Shimada N, Moriyama M, Kojima A,Hayashi W, Yasuzumi K, Nagaoka N, Saka N, Yoshida T, Tokoro T,Mochizuki M. Long-term pattern of progression of myopic maculopathy:a natural history study. Ophthalmology 2010;117(8):1595-1611, 1611.e1-4.
16 Jonas JB, Nguyen XN, Gusek GC, Naumann GO. Parapapillary chorioretinal atrophy in normal and glaucoma eyes. I. Morphometric data.Invest Ophthalmol Vis Sci 1989;30(5):908-918.
17 Apple DJ, Rabb MF, Walsh PM. Congenital anomalies of the optic disc. Surv Ophthalmol 1982;27(1):3-41.
18 Jonas JB, Jonas SB, Jonas RA, Holbach L, Panda-Jonas S.Histology of the parapapillary region in high myopia. Am J Ophthalmol 2011;152(6):1021-1029.
19 Jonas JB, Dichtl A. Optic disc morphology in myopic primary openangle glaucoma. Graefes Arch Clin Exp Ophthalmol 1997;235(10):627-633.20 Jonas JB, Gusek GC, Naumann GO. Optic disk morphometry in high myopia. Graefes Arch Clin Exp Ophthalmol 1988;226(6):587-590.
21 Fulk GW, Goss DA, Christensen MT, Cline KB, Herrin-Lawson GA.Optic nerve crescents and refractive error. Optom Vis Sci 1992;69(3):208-213.
22 Toda R, Mochizuki H, Sone T, Kiuchi Y. Changes in optic disc shape and size in two patients with suspected glaucoma during a two- and threeyear follow-up period. Jpn J Ophthalmol 2010;54(1):94-96.
23 Nakazawa M, Kurotaki J, Ruike H. Longterm findings in peripapillary crescent formation in eyes with mild or moderate myopia. Acta Ophthalmol 2008;86(6):626-629.
24 How AC, Tan GS, Chan YH, Wong TT, Seah SK, Foster PJ, Aung T. Population prevalence of tilted and torted optic discs among an adult Chinese population in Singapore: the Tanjong Pagar Study. Arch Ophthalmol 2009;127(7):894-899.
25 Reis AS, Sharpe GP, Yang H, Nicolela MT, Burgoyne CF, Chauhan BC. Optic disc margin anatomy in patients with glaucoma and normal controls with spectral domain optical coherence tomography.Ophthalmology 2012;119(4):738-747.
26 Dai Y, Jonas JB, Huang H, Wang M, Sun X. Microstructure of parapapillary atrophy: beta zone and gamma zone. Invest Ophthalmol Vis Sci 2013;54(3):2013-2018.