INTRODUCTION
Diabetic macular oedema (DMO) is a common complication of diabetic retinopathy (DR), and a major cause of vision loss[1]. The increasing prevalence of diabetes worldwide highlights the importance of DMO treatment[2]. Grid or focal laser photocoagulation of the macula has been demonstrated to lower the risk of vision loss in DMO, but vision is rarely improved[3]. Vascular endothelial growth factor (VEGF) is a key mediator of abnormal vascular permeability in DMO[4].Intravitreal anti-VEGF injections have been demonstrated to produce better outcomes compared to photocoagulation which had been the standard treatment for DMO prior[5-13].
The most used intravitreal anti-VEGF agents, aflibercept(Eylea, Regeneron-Bayer HealthCare), bevacizumab (Avastin,Genentech) and ranibizumab (Lucentis, Genentech), which have been demonstrated to be effective and relatively safe in treatment of DMO[5,14-17]. Aflibercept, a human recombinant fusion protein that binds VEGF-A and placental growth factor(PGF) to inhibit activation of VEGF receptors, has been investigated in trials on patients with DMO[11,15,18-20]. Aflibercept binds to VEGF-B and PGF, which are mediators involved in abnormal vascular permeability and retinal neovascularization,while ranibizumab and bevacizumab do not[21-22].
Intravitreal aflibercept has been approved for treatment of DMO in the United States, Europe, Japan, and Australia.The efficacy and safety of intravitreal aflibercept for DMO compared with photocoagulation has been demonstrated in the DA VINCI trial[11,20]. The subsequent studies, VISTA and VIVID, demonstrated that after nearly 3y of treatment,intravitreal aflibercept demonstrated superior visual and anatomic outcomes compared with photocoagulation. Ocular and systemic safety outcomes after nearly 3y of treatment were similar between aflibercept and photocoagulation[15]. The Diabetic Retinopathy Clinical Research Network (DRCRN)recently conducted a randomized clinical trial (RCT) assessing comparative efficacy and safety for intravitreal aflibercept,bevacizumab and ranibizumab for treatment of DMO[18-19].
As DMO may cause significant visual impairment, there is need for continuing evidence-based recommendations to be established regarding efficacy and safety of anti-VEGF agents as newer data become available. This Meta-analysis evaluates the relative efficacy and safety of aflibercept for treatment of DMO.
SUBJECTS AND METHODS
This meta-analysis was undertaken in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Search Strategy to Identify Eligible Studies A systematic literature review with searches of CENTRAL (The Cochrane Library 2017, Issue 4), Ovid MEDLINE, EMBASE, the Meta Register of Controlled Trials (mRCT), ClinicalTrials.gov,and the WHO International Clinical Trials Registry Platform(ICTRP). The final search was conducted on November 2017.
Inclusion and Exclusion Criteria Articles were considered for inclusion in the Meta-analysis if the study design was an RCT, the population was patients with DR and clinically significant macular oedema (CSMO)[3], and the intervention was aflibercept compared to another treatment. All trials had at least one year of follow up. Studies evaluating different doses of aflibercept compared with each other, with no control or comparator, and studies where aflibercept was used in combination with other treatments were excluded. Conference abstracts and full reports without raw data available, letters,reviews, duplicate publications and studies not available in English were excluded.
Types of Effect Estimates Efficacy was assessed as the proportion of patients with at least 15 Early Treatment Diabetic Retinopathy Study (ETDRS) letters (equivalent to 3 ETDRS lines or 0.3 logMAR) of gain or loss after one year or two years. Other measures of efficacy included mean change in best corrected visual acuity (BCVA), and mean reduction in central macular thickness (CMT) after one year or two years.Safety was assessed as the proportions of patients with death,thromboembolic events, and any systemic or ocular serious adverse event after one year or two years.
Selection of Studies Two review authors independently assessed the titles and abstracts found through the electronic searches (Nguyen CL, Wong E). Each record was classified as ‘not relevant’, ‘possibly relevant’, or ‘definitely relevant’.Full-text reports were obtained for all records classified as‘possibly relevant’ or ‘definitely relevant’ and then assessed to be included in the study (Nguyen CL, Wong E). Disagreement was settled with a third reviewer. Authors of studies were contacted for further details as needed to make adequate assessments.
Data Extraction Two reviewer authors independently extracted the data (Nguyen CL, Wong E). Discrepancies were settled with a third reviewer. The information extracted from each study included details on study methods, patients, interventions,the proportion of patients with at least 15 ETDRS letters of worsening and improvement, the mean change in BCVA and mean reduction in CMT measured after one year or two years,and the frequency of systemic and ocular adverse events.
Qualitative Assessment Two review authors independently assessed the study quality. With the RCTs the authors used the risk of bias tool recommended by the Cochrane Collaboration as per methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Nguyen CL, Wong E)[23].Potential sources of bias in the following different domains were critically assessed: sequence generation, allocation concealment, blinding of patients, personnel and outcome assessors, incomplete outcome data, selective outcome reporting and other sources of bias. For each domain, the authors judged whether the risk of bias of that domain was high, low, or unclear. Authors of RCTs were contacted for information to adequately assess a study.
Statistical Analysis Quantitative data were recorded using Review Manager (RevMan, version 5.3). For continuous variables, the weighted mean difference was measured. For dichotomous variables, the risk ratios (RR) were measured.These were reported with a 95% confidence interval (CI),and P<0.05 was statistically significant on the test for overall effect. The I2-statistic was used to assess heterogeneity between studies (P<0.05 was statistically significant heterogeneity)[24].A random-effects model was utilized if there was heterogeneity between studies. Otherwise, a fixed effects model was used.
RESULTS
Overall Characteristics of Included Trials and Quality Assessment A total of 1745 articles were initially identified and of these 1695 were rejected. The 50 remaining articles with full texts were assessed for eligibility[11,15,18-20,25-26]. A total of 4 studies were included. One study compared aflibercept with bevacizumab and ranibizumab for centre-involved DMO[18-19].As this was the only study comparing aflibercept with other anti-VEGF agents and there was no photocoagulation control arm, it was excluded from the Meta-analysis. Three trials compared aflibercept with control with up to 148wk of follow up. Meta-analysis was conducted on the trials at follow up of 1 and 2y[26]. In total, there were four RCTs included in this study.Three of these trials were included in the Meta-analysis, and comprised a total of 661 patients: 331 patients in the aflibercept group and 330 patients in the photocoagulation group (Figure 1).The characteristics of the studies included and risk of bias assessment are summarized in Table 1 and Figure 2.
The DA VINCI study compared photocoagulation with monthly, bimonthly and as needed or pro re nata (PRN)intravitreal aflibercept regimens. The PRN regimen was chosen for data extraction as this is the present practice with other anti-VEGF agents. The VISTA and VIVID studies compared photocoagulation with 4-weekly intravitreal aflibercept (2q4),and a regimen of five initial 4-weekly intravitreal aflibercept followed by 8-weekly injections (2q8). The 2q8 regimen was selected for data extraction as the complete number of injections in the first year was smaller, and this is comparable to PRN regimens of other studies. The DRCRN trial randomlyassigned 660 patients with DMO to be treated with intravitreal aflibercept (2.0 mg dose, 224 patients), bevacizumab (1.25 mg,218), or ranibizumab (0.3 mg, 218). The injections were carried out as often as every 4wk according to an algorithm.
Table 1 Characteristics of randomized controlled trials of aflibercept included in this review
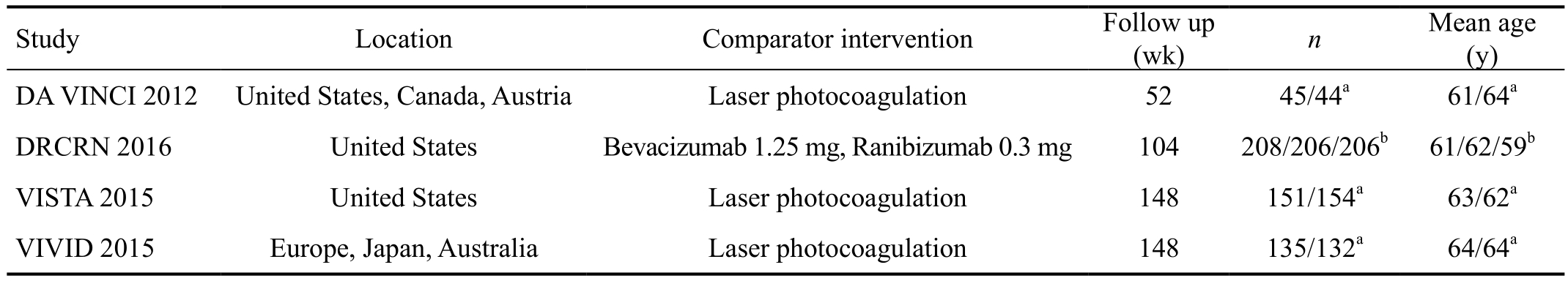
aAflibercept group/laser photocoagulation group; bAflibercept group/bevacizumab group/ranibizumab group.
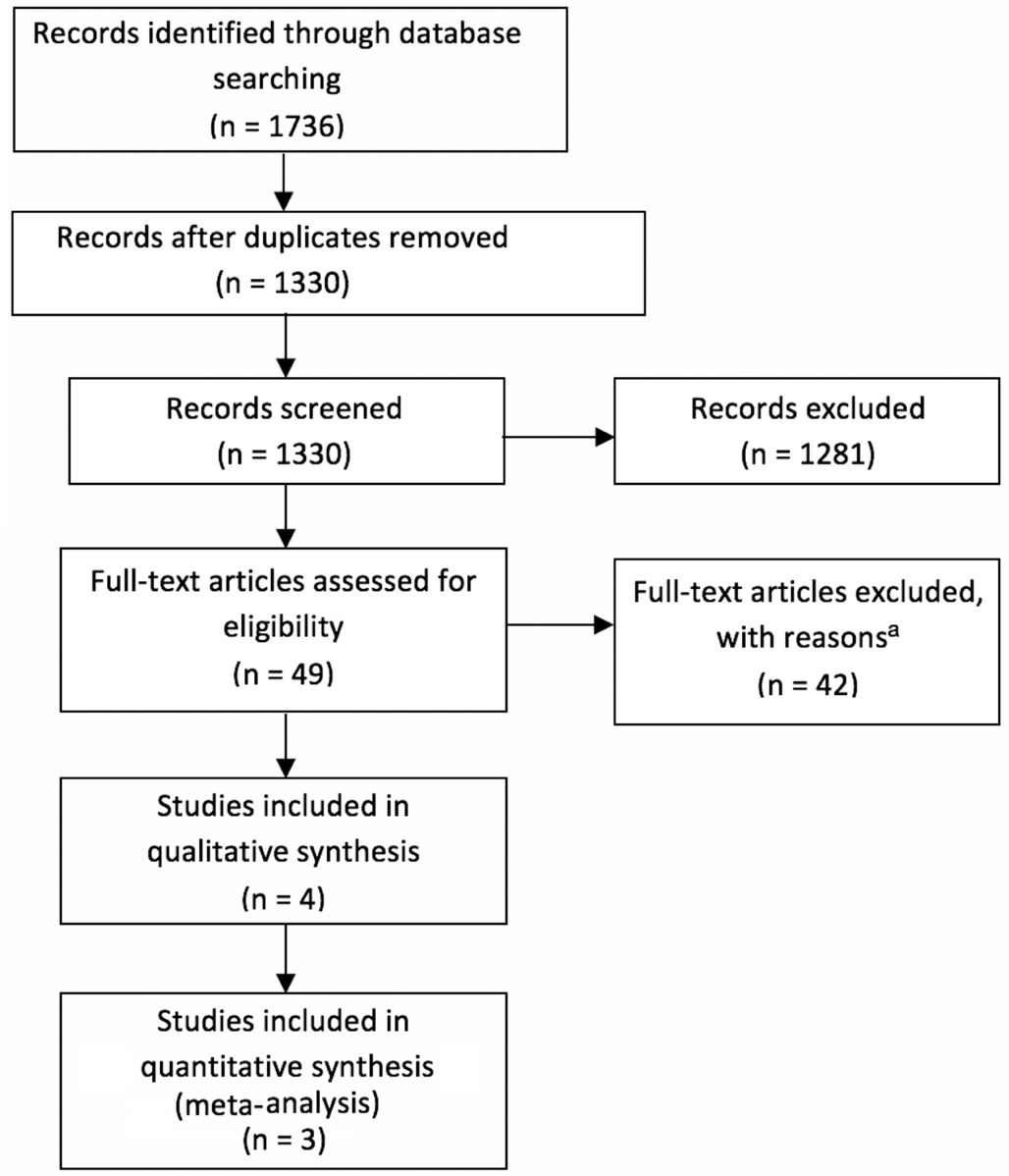
Figure 1 Flow diagram of studies included in this Meta-analysis aStudies had less than one year of follow up; trials of different doses of aflibercept compared to each other, with no control or comparator;studies used aflibercept in combination with other treatments; reviews.
Efficacy Analysis Patients receiving intravitreal aflibercept were more likely to gain 3 or more lines of visual acuity (VA)over 1y or 2y compared to patients treated with photocoagulation(RR=3.81, 95%CI 2.61 to 5.56, 661 patients, 3 studies,P<0.00001, I2=0; RR=2.56, 95%CI 1.80 to 3.62, 572 patients,2 studies, P<0.00001, I2=0, respectively; Figure 3).
Those receiving aflibercept were also less likely to lose 3 or more lines of VA over 1 or 2y (RR=0.06, 95%CI 0.01 to 0.23, 661 patients, 3 studies, P<0.0001, I2=0; RR=0.09,95%CI 0.03 to 0.30, 572 patients, 2 studies, P<0.0001, I2=0,respectively; Figure 4).

Figure 2 Risk of bias of included studies.
Two studies involving 572 patients compared aflibercept with photocoagulation in regards to mean change in BCVA at 1y or 2y from baseline. The combined results demonstrated that aflibercept produced significantly greater improvement in BCVA at 1y or 2y (weighted mean difference =10.01,95%CI 8.32 to 11.69, P<0.00001, I2=0; weighted mean difference =9.42, 95%CI 7.49 to 11.35, P<0.00001, I2=0,respectively; Figure 5).
The combined results also demonstrated that aflibercept was more efficacious in reducing CMT at 1y or 2y (weighted mean difference =119.02, 95%CI 95.47 to 142.57, 661 patients, 3 studies, P<0.00001, I2=0; weighted mean difference=108.80,95%CI 83.20 to 134.40, 572 patients, 2 studies, P<0.00001,I2=0, respectively; Figure 6).
Eyes treated with intravitreal aflibercept in the VISTA and VIVID trials demonstrated significantly greater improvements in visual and anatomic outcomes compared with eyes treated with photocoagulation at 1 and 2y. The improvements observed at 2y in mean baseline BCVA and CMT, and proportion that gained 3 or more lines of VA, were maintained over 148wk of treatment.
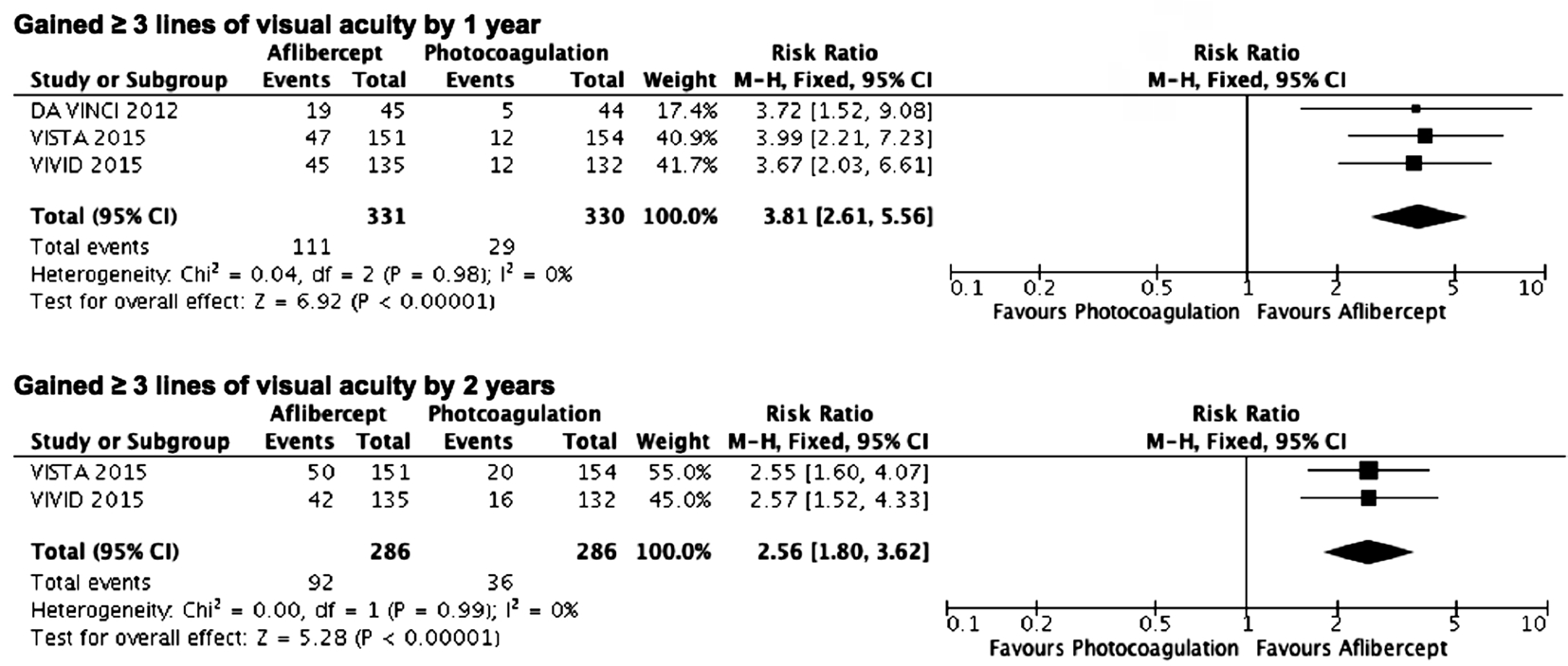
Figure 3 Estimated risk ratios of gains of 3 or more lines of visual acuity from baseline as measured on a logMAR chart M-H: Mantel-Haenszel statistics.

Figure 4 Estimated risk ratios of loss of 3 or more lines of visual acuity from baseline as measured on a logMAR chart M-H: Mantel-Haenszel statistics.
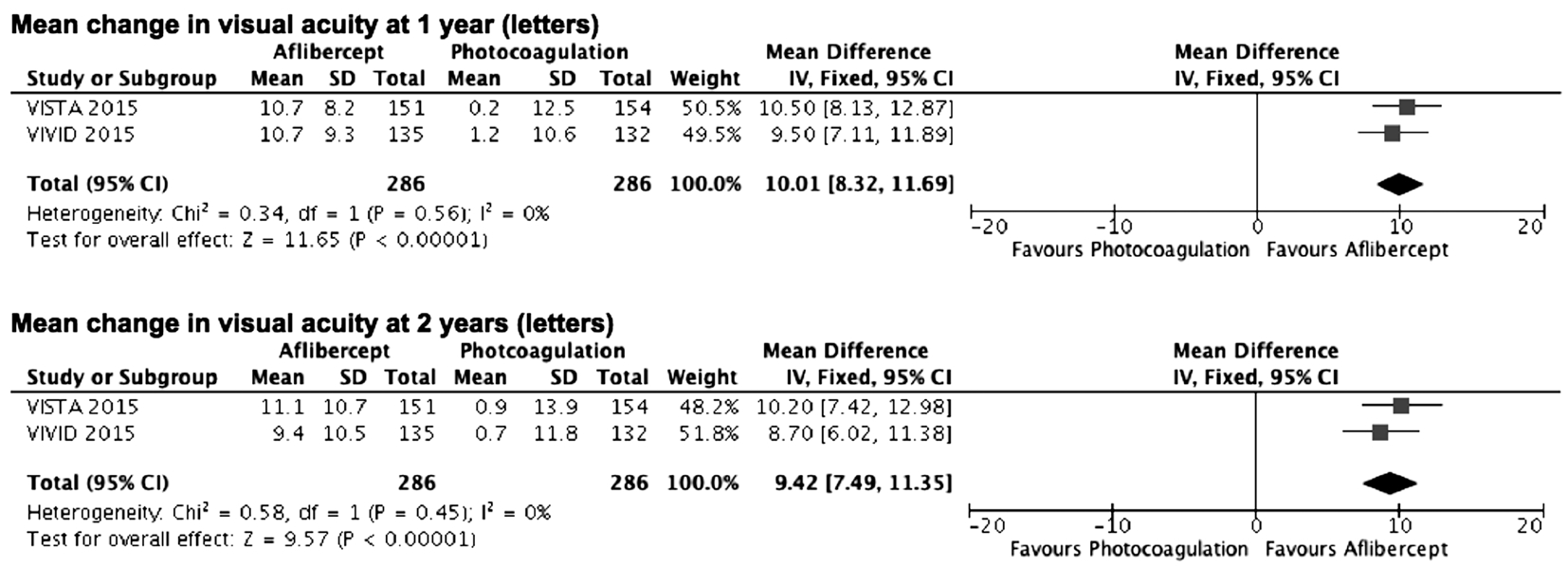
Figure 5 Estimated mean difference in changes from baseline to follow up best corrected visual acuity (in letters) IV: Inverse variance;SD: Standard deviation.
In patients with worse baseline VA letter score (VA letter score of less than 69, Snellen equivalent 20/50 or worse) in the DRCRN trial, aflibercept was superior to bevacizumab and ranibizumab at improving 15 or more letters at one-year follow up (P<0.001 and P=0.008, respectively). However,in patients with better baseline VA (VA letter score 78 to 69,Snellen equivalent 20/32 to 20/40), there were no significant differences in the gain or loss of 15 or more letters between the anti-VEGF agents at one-year follow up. There were no statistically significant differences between the anti-VEGF agents for patients with at least 15-letter changes at the twoyear follow up.
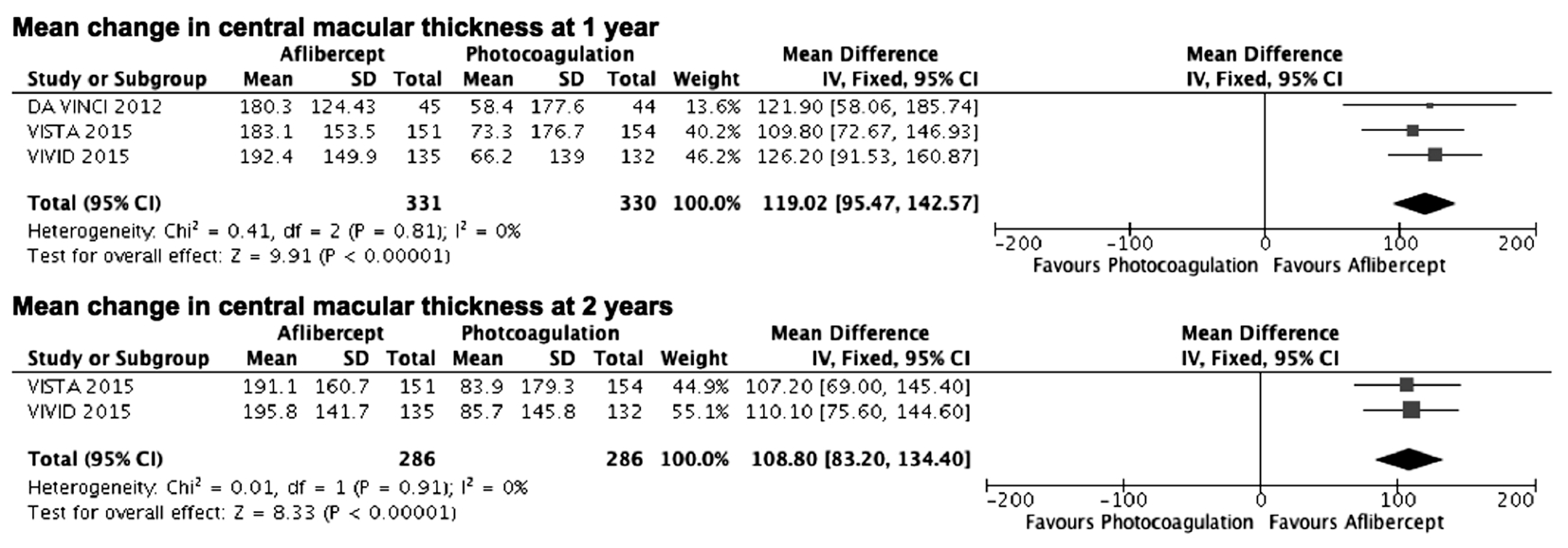
Figure 6 Estimated mean difference in changes from baseline to follow up central macular thickness IV: Inverse variance; SD: Standard deviation.
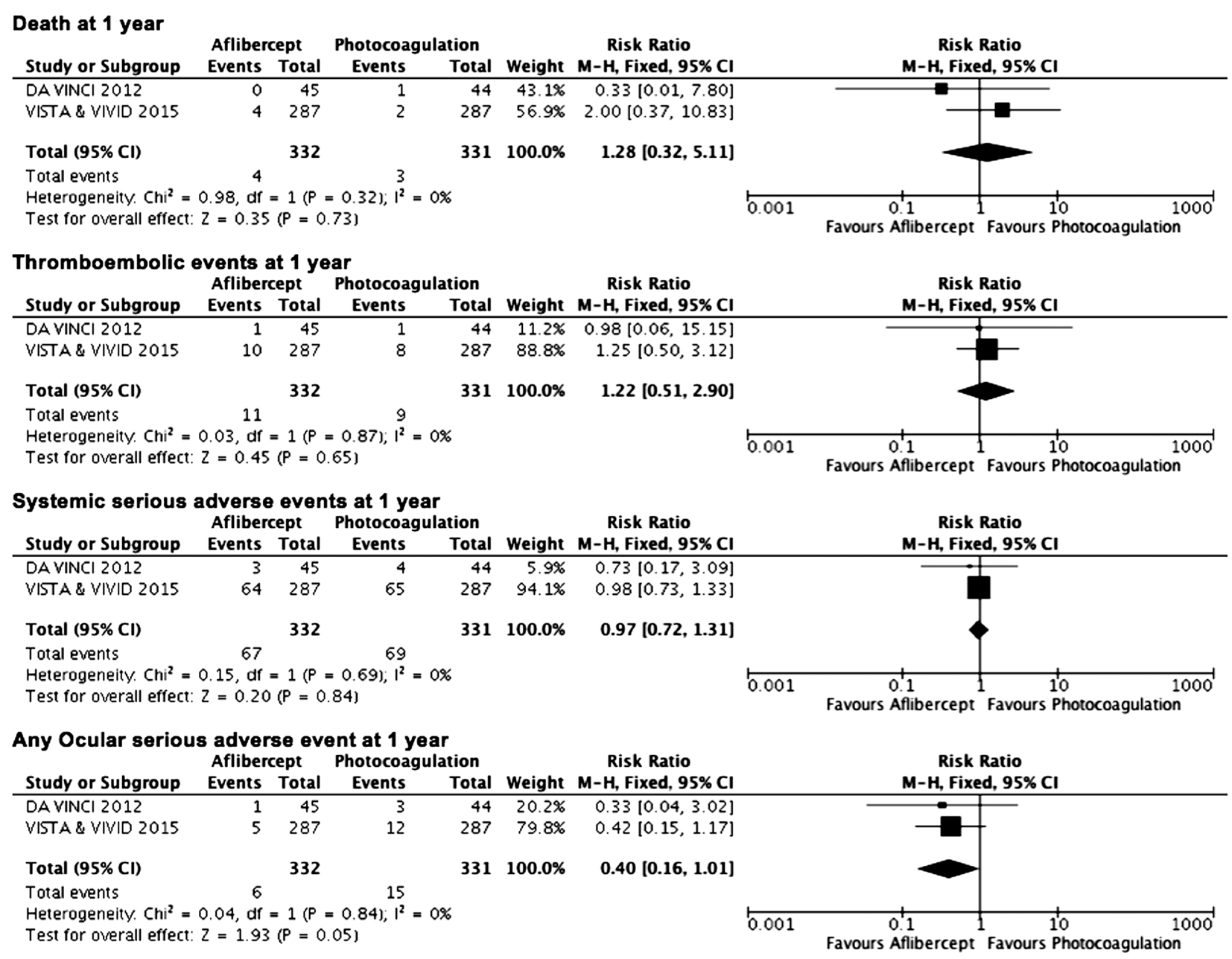
Figure 7 Estimated relative risks of serious adverse events M-H: Mantel-Haenszel statistics.
In the DRCRN trial at one-year follow up, in patients with worse baseline VA, the mean difference in BCVA was 6.5(95%CI 2.9 to 10.1, P<0.001), and 4.7 letters (95%CI 1.4 to 8.0, P=0.003) for aflibercept versus bevacizumab, and aflibercept versus ranibizumab respectively. At two-years follow up, in patients with worse baseline VA, there remained a significant difference between aflibercept versus bevacizumab,with aflibercept providing superior mean change in BCVA(mean difference =4.7, 95%CI 0.5 to 8.8, P=0.02).
After two-years of treatment, aflibercept provided superior reduction in CMT overall compared to bevacizumab(mean difference =48.5, 95%CI 27 to 70, P<0.001) and ranibizumab (mean difference =15.5, 95% CI 2.0 to 33.0,P=0.08).
Safety Analysis There were no significant differences between aflibercept and photocoagulation with respect to death,thromboembolic events, systemic serious adverse events or any ocular serious adverse events at 1y. There was no statistical heterogeneity detected between the studies (Figure 7).Similarly, at 2y, combined data from two trials did not demonstrate significant differences between aflibercept and photocoagulation. No new safety concerns were demonstrated in the VISTA and VIVID trials with a longer treatment period of 148wk.
In the DRCRN trial, there were no significant differences with aflibercept when compared to bevacizumab or ranibizumab in rates of death, any systemic serious adverse event, or any ocular serious adverse event. At two-year follow up, the higher rate of Antiplatelet Trialists’ Collaboration (APTC)thromboembolic events for ranibizumab included more nonfatal strokes (11 for ranibizumab, 6 for bevacizumab, and 2 for aflibercept) and vascular deaths (9 for ranibizumab, 8 for bevacizumab, and 3 for aflibercept). This was significant when aflibercept was compared to ranibizumab (P=0.047).
DISCUSSION
Four RCTs were reviewed, with 3 RCTs (331 patients in the aflibercept group and 330 patients in the photocoagulation group) included in the Meta-analysis. In terms of efficacy,patients receiving aflibercept were more likely to gain 3 or more lines of VA, and less likely to lose 3 or more lines of VA over one year or two years. In addition, intravitreal aflibercept demonstrated greater efficacy in terms of effect on improvement in mean BCVA and reduction in mean CMT when compared to photocoagulation at 1 or 2y. In terms of safety, rates of death, thromboembolic events, any systemic serious adverse event or any ocular serious adverse event at 1 or 2y were similar with aflibercept and photocoagulation.Intravitreal aflibercept treatment provided visual and anatomic improvements as well as comparable safety through to week 148 of treatment[26].
There was high quality evidence that aflibercept provides superior benefit compared to laser photocoagulation in combined clinical trial populations at one or two years. Nevertheless,the conditions in clinical trials are highly controlled and the patients may not be representative of the real-world population with DMO. Studies assessing the real-world effectiveness of aflibercept for DMO would be of benefit to determine whether the results of clinical trials translate to real-world conditions.Treatment of DMO with anti-VEGF agents is now established.As well as the need to investigate real-world effects, future research should compare different anti-VEGF agents and treatment regimens. Differences among anti-VEGF agents were evaluated in only one high quality trial comparing aflibercept to bevacizumab and ranibizumab. The DRCRN trial compared treatment with intravitreal aflibercept, bevacizumab,or ranibizumab in eyes with centre-involving DMO and found some differences between the anti-VEGF agents in terms of efficacy and safety[18-19]. At one-year follow up aflibercept demonstrated greater improvement in VA compared to ranibizumab and bevacizumab amongst patients with poorer levels of baseline VA. Although the superiority of aflibercept over bevacizumab for VA improvement for these patients persisted through 2y, the difference between aflibercept and ranibizumab seen at year one did not persist.
The lack of other comparative effectiveness trials between aflibercept and other anti-VEGF agents did not allow for this Meta-analysis. Further RCTs comparing aflibercept to other anti-VEGF agents will be of benefit to ophthalmologists and patients when deciding between treatment options.Comparison of aflibercept with bevacizumab and ranibizumab in regards to safety profile will also be important as higher APTC thromboembolic events were seen with ranibizumab[19].This review has limitations in that publication bias could not be fully eliminated. Nevertheless, the findings indicate that aflibercept offers greater benefits in terms of improving and maintaining vision when compared to photocoagulation. This Meta-analysis also demonstrates the safety of aflibercept to treat DMO; there were no differences between aflibercept and photocoagulation in terms of rates of death, thromboembolic events, any serious systemic adverse event, or any serious ocular adverse event at 1 or 2y.
This Meta-analysis confirms the comparable safety and superior efficacy of aflibercept over photocoagulation for patients with DMO. It also highlights the need for further comparative trials of anti-VEGF agents and investigations assessing treatment effects in the real world.
ACKNOWLEDGEMENTS
Foundation: Supported by the University of Sydney, Sydney,Australia.
Conflicts of Interest: Nguyen CL, None; Lindsay A, None;Wong E, None; Chilov M, None.
REFERENCES
1 Varma R, Bressler NM, Doan QV, Gleeson M, Danese M, Bower JK, Selvin E, Dolan C, Fine J, Colman S, Turpcu A. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol 2014;132(11):1334-1340.
2 Diabetes: facts and figures. Brussels: international diabetes federation,2015. Accessed August 2016. Available at: https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/13-diabetes-atlas-seventh-edition.html.
3 Early Treatment Diabetic Retinopathy Study Research Group.Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Archives of Ophthalmology 1985;103(12):1796-1806.
4 Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW.Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem 1999;274(33):23463-23467.
5 Edwards P. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Acta Ophthalmology 2010;88:0.
6 Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012;119(4):789-801.
7 Nguyen QD, Shah SM, Khwaja AA, et al. Two-year outcomes of the ranibizumab for edema of the macula in diabetes (READ-2) study.Ophthalmology 2010;117(11):2146-2151.
8 Scott IU, Edwards AR, Beck RW, et al. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema.Ophthalmology 2007;114(10):1860.
9 Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615-625.
10 Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data:report 2. Ophthalmology 2010;117(6):1078-1086.e2.
11 Do DV, Nguyen QD, Boyer D, et al. One-year outcomes of the da Vinci Study of VEGF Trap-Eye in eyes with diabetic macular edema.Ophthalmology 2012;119(8):1658-1665.
12 Arevalo JF, Sanchez JG, Fromow-Guerra J, et al. Comparison of two doses of primary intravitreal bevacizumab (Avastin) for diffuse diabetic macular edema: results from the Pan-American Collaborative Retina Study Group (PACORES) at 12-month follow-up. Graefes Arch Clin Exp Ophthalmol 2009;247(6):735-743.
13 Virgili G, Parravano M, Menchini F, Evans JR. Anti-vascular endothelial growth factor for diabetic macular oedema. Cochrane Database Syst Rev 2014(10):CD007419.
14 Arevalo JF, Lasave AF, Wu L, Diaz-Llopis M, Gallego-Pinazo R,Alezzandrini AA, Berrocal MH. Intravitreal bevacizumab plus grid laser photocoagulation or intravitreal bevacizumab or grid laser photocoagulation for diffuse diabetic macular edema: results of the Pan-American Collaborative Retina Study Group at 24 months. Retina 2013;33(2):403-413.
15 Hashmonay R, Parikh S. Re: Korobelnik, et al. Intravitreal aflibercept for diabetic macular edema (Ophthalmology 2014;121:2247-54).Ophthalmology 2015;122(6):e37-e38.
16 Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology 2013;120(10):2013-2022.
17 Rajendram R, Fraser-Bell S, Kaines A, Michaelides M, Hamilton RD,Esposti SD, Peto T, Egan C, Bunce C, Leslie RD, Hykin PG. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema:24-month data: report 3. Arch Ophthalmol 2012;130(8):972-979.
18 Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab,or ranibizumab for diabetic macular edema. N Engl J Med 2015;372(13):1193-1203.
19 Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab,or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology 2016;123(6):1351-1359.
20 Do DV, Schmidt-Erfurth U, Gonzalez VH, et al. The DA VINCI Study:phase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edema. Ophthalmology 2011;118(9):1819-1826.
21 Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012;15(2):171-185.
22 Rakic JM, Lambert V, Devy L, Luttun A, Carmeliet P, Claes C,Nguyen L, Foidart JM, Noël A, Munaut C. Placental growth factor, a member of the VEGF family, contributes to the development of choroidal neovascularization. Invest Opthalmol Vis Sci 2003;44(7):3186-3193.
23 Higgins JPT, Altman DG, Sterne JACe. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 510. The Cochrane Collaboration, 2011 Available at: http://training.cochrane.org/handbook. Access on 2011.
24 Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-560.
25 Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology 2015;122(10):2044-2052.
26 Heier JS, Korobelnik JF, Brown DM, et al. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology 2016;123(11):2376-2385.