INTRODUCTION
Cataract is a common reason for visual loss in both developed and developing countries. Current treatment for cataracts is performed by surgical removal of the crystalline lens, and replacement with an intraocular lens(IOL). Majority of patients can improve their visual acuity through this procedure. Since the living standards of people have been increased, patients with cataract nowadays have a higher demand on postoperative visual performance. Losing the accommodation is ineluctable with traditional monofocal intraocular lenses (MIOLs), reading glasses are required to focus desired objects. In order to improve visual outcomes of cataract surgeries, accommodating intraocular lenses (AIOLs)were designed[1]. AIOLs can provide good near vision with no compromise for distance vision by changing the refractive power of the eye through transmitting ciliary muscular contractions[2].Accommodation is achieved through the contraction of the ciliary body and the consequent relaxing zonular fibers,allowing the lens to turn into a more convex and dioptrically powerful form[3-4]. The decline in accommodation is inevitable due to age, but some researchers demonstrated that the even in old age, human ciliary muscle still maintains its contractile ability[5]. AIOLs are designed to induce accommodation by responding to the contraction of the ciliary body[6] to allow the postoperative patients obtain both good distance and near vision. Although AIOLs have its advantages, there are still some inevitable side effects, like limited amplitude of accommodation and high rate of posterior capsular opacification (PCO)[7]. In order to determine the postoperative outcomes, both subjective and objective measurements should be used in clinic[8].
The aim of this review is to describe different types of AIOLs and the measurements of postoperative performance, hoping to provide relative information on AIOL selection in clinic.
B R I E F I N T R O D U C T I O N O F VA R I O U S
ACCOMMODATING INTRAOCULAR LENSES
In the existing researches, AIOLs include single-optic, dualoptic and deformable surface accommodating IOLs.
Single-optic Accommodating Intraocular Lenses Singleoptic AIOL has flexible supporting elements that are consider to have the ability to transmit ciliary muscle contraction into a change of anterior displacement of the lens optic, resulting in increased dioptric power of the eye to improve near vision[9-11]. The working principle relies on the mechanism of natural accommodation which has various hypothetical assumptions. Nevertheless, the axial movement induced by accommodation is insufficient to generate desirable increase in refractive power[12-13]. Several single-optic AIOLs have been used in clinic such as the 1CU (HumanOptics AG, Erlangen,Germany), Tetra flex KH3500 (Lenstec, FL, USA), Crystalens AT-45 (Bausch and Lomb, NY, USA) and Crystalens HD(Bausch and Lomb, NY, USA)[14-18].
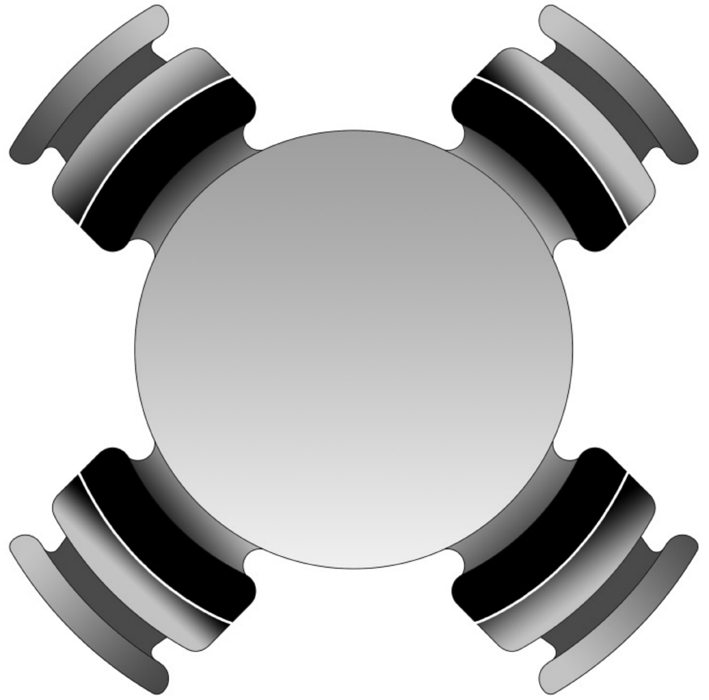
Figure 1 Schematic diagram of 1CU (HumanOptics AG, Erlangen,Germany): a biconvex optic with four wide-based haptics, and haptics are thin in the joint.
1CU (Figure 1) is made of a hydrophilic acrylic material. The implant has an overall diameter of 9.8 mm and a biconvex optic diameter of 5.5 mm. It features four wide-based haptics,and haptics are thinner close to the optic[19]. The contraction or relaxation of ciliary muscle can transmit to the haptics and resulting in movement of the optic[20]. According to some studies, the accommodative amplitude for the patients after implanted the 1CU IOL was 0.63-2.00 diopters (D)[19,21-22], but it would decrease within 12-24mo, attributing to increased capsule fibrosis[22-23]. Besides, because of the thin junctions between optic and haptics, would weakening the shielding effect from the square edge, and because of the hydrophilic material, the rate of PCO has increased almost to 50%[24].
Tetraflex KH3500 (Figure 2) is made from hydrophilic hydroxyethyl methacrylate, and the diameter of square edge optic is 5.75 mm. The closed loop haptics feature a five-degree anterior angulation for the purpose of which facilitating anterior movement with the whole capsular bag during accommodation[25]. To decrease the incidence of PCO, the whole IOL has square edges to inhibit migration of lens epithelial cells[26]. The material of KH3500 is highly flexible and high water content, allowing the AIOL being able to get through a 2.5 mm corneal incision and facilitating forward movement of the implant[27]. Researchers reported the amplitude of accommodation could achieve 2.00 D, but the incidence of postoperative PCO is about 42.2%[25,28].
Crystalens AT-45 (Figure 3) is a three-piece silicone posterior chamber AIOL[29] with 4.5 mm diameter of biconvex optic and 11.5 mm overall length. The implant is comprising two plate haptics which are terminating in two T-shaped polyamide loops to remain stability within the capsular bag[26]. Meanwhile incorporates hinges across the plates and adjacent to the optic are designed for the lens to allow axial movement of optic through accommodative effort, and the flexing of the optic may contribute significantly to the accommodation by increasing ocular aberrations[26,29]. The accommodative ability of this AIOL was reported differently. Some scientists showed that it has the capacity to provide 1.00 D of accommodation[6],while others denoted the amplitude of accommodation is only 0.44 D[30], the disparity between the studies may be counted on different depth of field[31]. Contract sensitivity is a major factor which could influence the postoperative outcome. According to researches, the contract sensitivity in patients with this AIOL was no worse than MIOLs[29]. Additionally, because in the adjacent areas of optic contact to plates have no square edge, the incidence of PCO is higher than those lenses with a 360-degree square edge[29]. And the haptics are tenuous which may cause tilt of the optic when the capsule contract irregular,resulting in augment of astigmatism and spherical aberration[32].

Figure 2 Schematic diagram of Tetra flex KH3500 (Lenstec, FL,USA): the optic has square edge, and haptics feature a five-degree anterior angulation are closed loop.
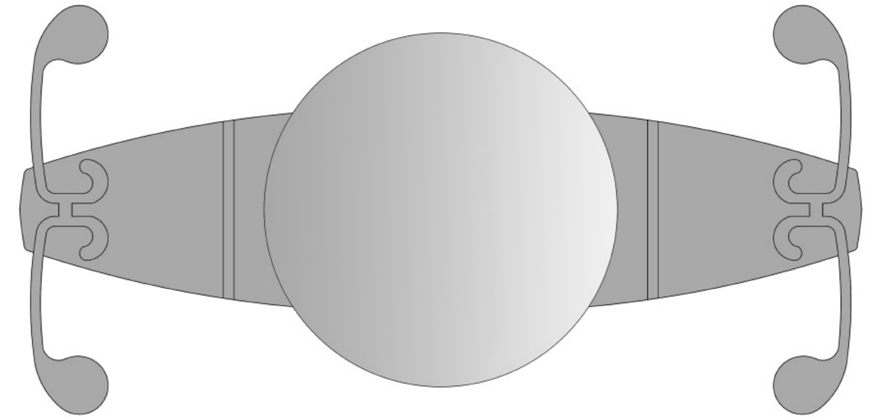
Figure 3 Schematic diagram of Crystalens AT-45 (Bausch and Lomb, NY, USA): the plate haptics are terminating in two T-shaped polyamide loop.
Crystalens HD is made of silicon, having 5 mm biconvex optic with square edge, and it has a 1.5 mm central bispheric modification to increase the depth of focus, intending to provide better near and intermediate focus[33]. Two sizes of the implant are available depending on the required power,12.0 mm for 10.00 D to 16.50 D and 11.5 mm for 17.00 D to 33.00 D[34]. Accommodation of this AIOL depends on changes in axial position and shape of optic[26,34]. Studies showed the anterior shift of the optic is about 1.4 mm, which can provide better near vision compares to MIOLs[35], as well as the intermedia vision[36]. Because this AIOL only has 240-degree square edge[33,37], the incidence of PCO was high, being reported to reach 40.7%[33,37].
Both single-optic AIOLs and traditional MIOLs can bring patients with good correct distance visual acuity, but compared to traditional MIOLs single-optic AIOLs can also improve near and intermedia visual acuity at same time[38]. The amplitude of accommodation and near vision acuity through distance correction was better than traditional MIOLs even at the 6 and 12mo postoperatively[20,39-41]. Some research also showed better reading ability in single-optic AIOLs[2], and Tetra flex KH3500 was better than Crystalens[27]. However, the study of reading ability is not sufficient yet. Harman et al[20] reported that the spectacle independence was higher in single-optic AIOLs group than in traditional MIOLs group, but this study was not a double-blind test and had a poor reliability. In general,for those patients with high demand of postoperative visual performance, single-optic AIOL is a considerable choice.
The main disadvantage of single-optic AIOLs is the limited amplitude of accommodation[2]. In theory, 1 mm of anterior displacement of the IOL attributes an average of 1.50 D-1.90 D of accommodative amplitude[42], but research reported heretofore that the maximum forward shift of IOL was about 0.4 mm[43-44].According to Pérez-Merino’s study[45], accommodative amplitude of single-optic AIOLs were below 0.40 D, and the ability of accommodation would decline following the capsule fibrosis formation[46], because the stiffness of capsular bag limits axial movement of the lens and thus decrease the accommodative capacity[7]. So the existent publications can only demonstrate that accommodative amplitude of single-optic AIOLs is limited, and unable to achieve normal physiological state of accommodation. Some investigators compared the clinical outcomes between 1CU and AT-45,and revealed that the accommodative amplitude and distancecorrected near visual acuity of 1CU was superior to AT-45 one year after operation, however, other results had no significant differences[41]. Secondly, glare can be a detrimental factor for clinical outcome after operation. Studies showed that it appeared in patients with 1CU, and part of the patients still experienced moderate to severe glare even at 18mo postoperatively, in the contrary, incidence of glare was lower in patients with MIOLs[20]. Nevertheless, because the sample size was small, this consequence may be uncertain. Also,lots of researchers illustrated the occurrence of PCO in AIOL group was higher than MIOL group[39-40]. This phenomenon might be caused by the design of IOL, especially the material and the shape[47]. It has been showed that incidence of PCO was lower in eyes with acrylic hydrophobic IOLs than those with acrylic hydrophilic IOLs[48]; the design of square edge and polishing the anterior capsule during the surgery could avoid proliferation of cortex by inhibiting transplantation of lens epithelia cells from anterior capsule to posterior capsule[49].Furthermore, neodymium: YAG capsulotomies was necessary for patients with PCO to improve visual acuity, and this method may not affect accommodative ability[50].
Figure 4 Schematic diagram of Synchrony (Visiogen, Irvine,California, USA): there are two optics connected, the anterior optic is smaller than posterior optic.
Dual-optic Accommodating Intraocular Lenses Dual-optic AIOLs consist of two separate optics including a high powered plus anterior optic of fixed dioptric power and a minus posterior optic, coupled by a spring haptics[10,51-52]. The lens were designed to occupy the capsular bag completely, thus the capsular tension could change the distance between the anterior and posterior optic. When the ciliary muscular contract,the capsule relax, forward displacement of the anterior lens induced an increase in dioptric power, then the focus turn to near objects[26,53]. The Synchrony (Visiogen, Irvine, California,USA) and Sarfarazi (Bausch and Lomb, NY, USA) dual-optic AIOLs have been developed more recently.
Synchrony AIOL (Figure 4) is a silicone-made, singlepiece IOL, total length is 9.5 mm and width is 9.8 mm[52,54].Its anterior optic has a fixed dioptric of +32.00 D, and the diameter is 5.5 mm, which designed to minimize the contact area with the anterior capsule, thus facilitating the flow of aqueous humor[47,55]. The diameter of the posterior optic is 6 mm which is longer than anterior optic, for maintaining the stability within the capsular bag[55]. The power of the posterior optic can be varied depending on the dioptric power of different patient[56]. When the accommodation occurred,constriction of ciliary body would lead to release the tension of capsular bag, then the two optics been separated, resulting in augment of the dioptric power of the eye[47]. The haptics were designed to allow a displacement of 1.5 mm of the anterior optic, and 1.0 mm forward movement roughly equal to 2.60 D of accommodation theoretically[52]. All the components of the implant were designed to control the distance of two optics while the ciliary body relaxed and capsule contracted[52].Furthermore, the Synchrony AIOL can be preloaded in a cartridge, and allow the IOL through a small incision ranging between 3.8 mm and 4.00 mm[10,33,56]. According to a study, the amplitude of accommodation in eyes with Synchrony AIOL is about 3.00 D[10].
Sarfarazi (Figure 5) is made of silicone, consisted by 2 optic lenses of 5.0 mm in diameter. The lens connected by 3 haptics[26]. And the elliptical optic designed for conforming to natural morphology of the capsule[16]. Method of changing diopter was as same as the Synchrony which through the displacement of anterior optic[16,26]. The Sarfarazi has been tested using sophisticated models, indicating that the amplitude of accommodation could reach 4.00 D in humans. But it depends on a 1.9 mm movement of the optic was achieved[57].However, there is not adequate evidence to confirm the accommodating ability with this AIOL.
There are some researchers reported that dual-optic AIOLs could offer better reflective power and accommodative ability to patients[26,57], which reflecting in wider range of defocus curve[33]. Besides, some studies further showed the performance of accommodation was related to the axial length of eye.The amplitude of accommodation was wider in short axial length (<23 cm) with single-optic AIOLs[51], however, others indicated that accommodative amplitude and axial length was irrelevant[20]. About the degeneration of accommodative ability, studies showed those patients with dual-optic AIOLs had no reduction in accommodative ability with the time[56],but year-long follow-up study was inadequate. There was also research proved indiscrimination of near and intermediate vision between single-optic and dual-optic AIOLs[33], although contract sensitivity was better in dual-optic AIOLs group[33].
The shortages of dual-optic AIOLs are also existed, primary one was the limitation of accommodative amplitude. Alió et al[33] proved the improvement of near vision was limited,and the result of accommodation would diminish obviously with age[45]. Therefore, it is a major challenge to maintain the elasticity of capsule depending on the accommodative theory, because the fibrosis of capsule must influence the accommodative ability after surgery. Besides, in order to acquire satisfying visual performance, patients may need some visual training[56]. And accommodative ability varies for different operative procedure and postoperative recovery,so that patients may have different visual performance with the same IOL after operation[4]. To those patients with high myopia, the anterior chamber is deeper and zonular fibers are weaker, because of the big and slack capsule[58].These patients may hardly to get expected results, but the performance of visual in high myopia patients demands more observation. The secondary disadvantage is magnification of the image, this effects been considered as a vital factor that influences the postoperative outcomes of the patients with dual-optic AIOLs[53]. It is because of the distance between the image space nodal point and retina is increased in dualoptic AIOLs during the movement of the anterior lens of dualoptic AIOLs in accommodation, thus the occurrence rate was higher[59]. Studies showed magnification of the image might have positive correlation with the axial length[60], researcher also found that implanting a single-optic AIOL in one eye and a dual-optic AIOL in the other, this phenomenon may be more obvious[61]. Implanting dual-optic AIOLs bilaterally may be a solution[47,54,59], but this method may cause different accommodative response due to the differences of the surgery itself and recovery responses after surgery[4]. Anisoaccommodation of about 1.00 D would induce a retinal image size disparity, resulting in compromise of binocular vision,even this may not cause ambiopia in patients[59]. Furthermore,magnification of the image could influence the accuracy of near vison test which may be overestimated because of bigger image. At last, about the incidence of PCO, researcher verified that the incidence of PCO was lower in patients with dualoptic AIOLs than those with single-optic AIOLs[33].
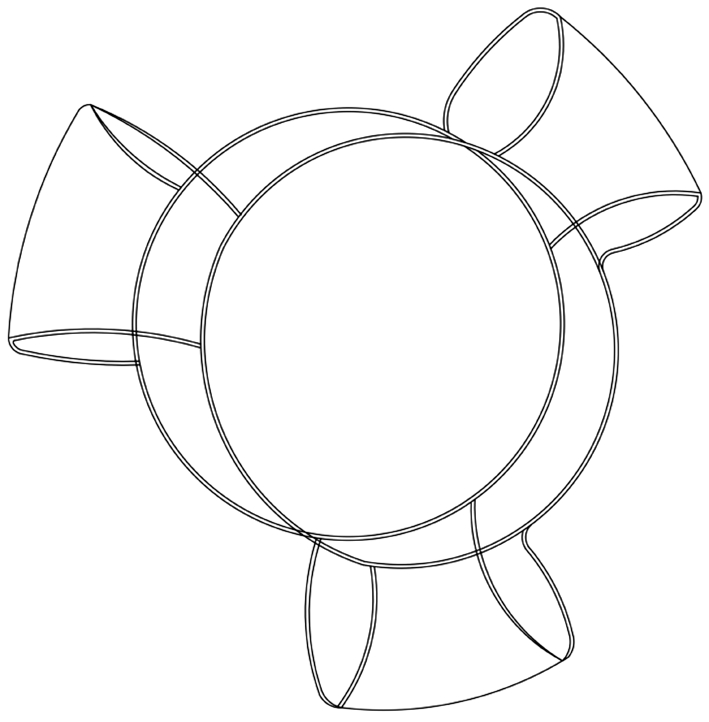
Figure 5 Schematic diagram of Sarfarazi (Bausch and Lomb, NY,USA): two elliptical optics have the same diameter, and connected by 3 haptics.
Deformable Surface Accommodating Intraocular Lenses Deformable surface AIOLs (Figure 6) have been designed in many different models. Changing the shape of lens’s surface during the accommodation is the principle of transforming refractive power[62]. This kind of AIOL was in the stage of study, and has not yet been put into use in clinic. The NuLens is a new concept in deformable surface AIOLs, consists of a flexible gel contained in a small chamber. The implant is made of polymethyl methacrylate, and has an anterior reference plane with a round hole in the center and a piston in the posterior[62-63]. The ciliary muscles provide kinetic energy and capsular acts as a diaphragm. When the ciliary muscles contracted or relaxed, the capsule diaphragm can make displacement of the gel component, to diminishes or bulges to a planar surface through the round hole, then the refract power would be changed[62,64]. The magnitude of the bulging correlates with the forces generated by the ciliary muscles and transferred to the piston[62]. Only one research have used NuLens in people, the amplitude of accommodation could reach 10.00 D after surgery[62]. There needs more research on the feasibility and security of deformable surface AIOLs.
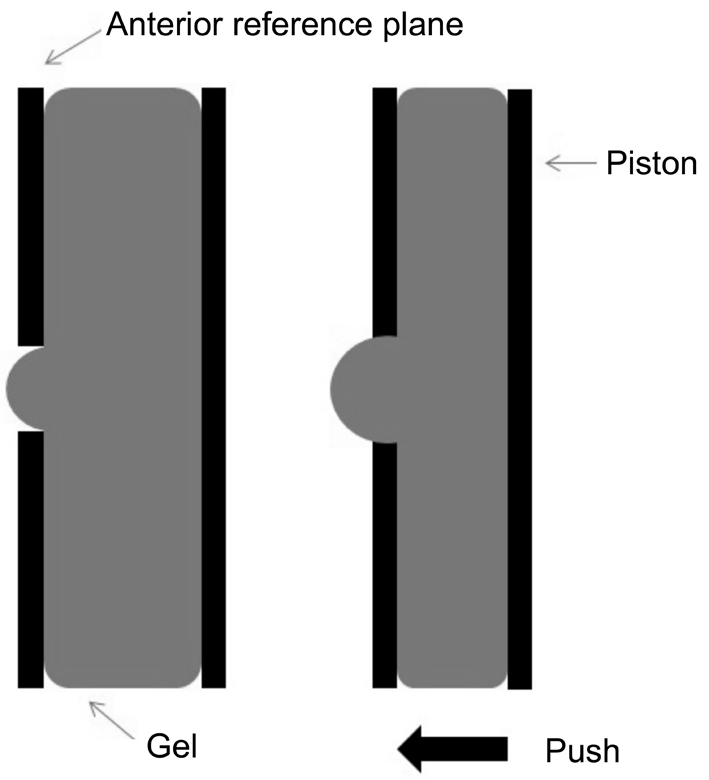
Figure 6 Schematic diagram of deformable surface AIOLs:accommodation occurred during the changing of the lens’s surface.
Other Types of Accommodating Intraocular Lenses To provide higher accommodative amplitude and overcome some of the deficiency of existing AIOLs, a series of further design concepts is in the early stages of development. These AIOLs including Magnet-driven active shift IOL, Fluid Vision IOL (Power Vision Inc., California, USA) and Smart IOL(Medennium Inc., California, USA).
Magnet-driven active shift IOLs have pairs of magnets which are repellent, the lens and capsular bag can be driven move forwards through repulsive forces during the accommodation[9].The outer magnets are implanted under the superior and inferior rectus muscle[9,26]. Because the concept of this kind of implant is based on synchronous movement of both lens and capsular bag, the effects of postoperative capsular fibrosis and PCO were expected to be lower[26].
Fluid Vision IOL has hollow haptics and optic to facilitate fluid displacement within the IOLs during ciliary muscle contraction or relaxation, resulting in changing surface curvature then vary the refractive power[65]. Early clinical trials of the Fluid Vision IOL are under study.
Smart IOL is made by a thermoplastic hydrophilic acrylic material, regarding to IOL power the specifications are able to determine. At room temperature, it is a rod shape of 2 mm by 30 mm, after implantation into capsular it recovers to its original shape because of the body temperature[66]. During the accommodation, AIOL with predicted increases in axial thickness and surface curvatures like physiological change of crystalline lens[9,16,26]. The full-sized design may reduce incidence of PCO and minimize edge glare[26].
All these kind of novel AIOLs demand further research to be improved, and need to be experimented on the stability and effectiveness.
INFLUENCE FACTORS OF POSTOPERATIVE OUTCOMES IN PATIENTS WITH ACCOMMODATING INTRAOCULAR LENSES
Dhital et al[67] confirmed that accommodative effect was not only related to the displacement of optic, but was at least due to depth of focus, which could be changed by myosis[68-69],low myopia[70], irregular astigmatism[71] and high-order aberration, particularly spherical aberration and coma[72].Other researchers[73-74] deduced that the forward movement of AIOLs has been attributed partly to vitreous pressure acting on the lens, so surgery should be performed more carefully to ensure no loss of vitreous[75]. A long-term experiment showed that anterior capsular opacification (ACO) and PCO is a factor in reduction of accommodative amplitude[76], this made thorough cortical wash and vacuum polishing of the capsule became important for postoperative accommodation.The previous study proved that the human ciliary muscles maintain its contractile ability well into old age, even in pseudophakic subjects[77]. But the use of ciliary muscles declined in presbyopia, and the function of ciliary muscles restricted, through appropriate training may augment the accommodative ability[78]. There is no standardized objective technique in common usage to assessing near visual function,so it is difficult to compare within and between studies. And visual acuity is not the only crucial aspect of visual function assessment, so avoiding side effects is also important.
Contrast sensitivity can be influenced by multiple factors, like illumination conditions, pupillary diameter, refraction values of cornea and lens[79], so more particular research is demanded.The choice of AIOL depends on three sides, patients’ selfconditions (including physical and economic aspect), surgeon’s technology and surgical instruments. Some of the studies use corneal astigmatism as an exclusion criterion when it is greater than 2.00 D[10,47], or greater than 1.50 D[56]. Also, there is a study regarded 1.00 D or less as an appropriate exclusion criterion[80].Besides, if patients show unreasonable expectations, then they are unsuited to these lenses. As for the surgeon, it is helpful for the accommodative ability to prevent vitreous loss during the surgery and continuous curvilinear capsulorhexis is important to enhance the postoperative efficiency. These demand the surgeon highly skilled. Individualized weighing of benefits and side-effects for patients is also crucial[81-82]. Accurate and precise biometry is crucial in ensuring the correct lens power selection[83].
The long-term trial of AIOLs is insufficient, and the assessments of postoperative effect have no uniform standards yet. And because of the influence on pupil diameter and high-order aberration, it is unable to distinguish the real accommodation from pseudo accommodation[84]. To evaluate the benefit after implanted AIOL still needs further research.
METHODS OF POSTOPERATIVE EFFECT EVALUATION IN ACCOMMODATING INTRAOCULAR LENSES
Evaluating the postoperative effect in AIOLs has various methods, including subjective measurement and objective measurement. Subjective measurement is depending on patient’s own perception, like accommodative ability,reading accuracy, reading speed, defocus curve and patient’s satisfaction. These are not enough to reflect real situation. It is necessary to use objective measurement which including retinoscopy[85], aberration analysis[23], autorefractor and measurement of optic movement[86].
Measurement of Refraction Using the standard visual acuity chart to test postoperative patients’ distance vision and near vision is simple and convenient. And defocus curve is a kind of useful subjective measurement which mainly assessed depth of field, it inducing the blurred vision through positive and negative lens and measuring vision acuity in different distance[87-89]. However, there were memory effect[90] and different definition of clearly image[91-92], so the measuring error existed. The objective measurements are more valuable to reflect reality. Retinoscopy was used to obtain objective measurement of refraction, and in well trained testers, it was considered an accurate and effective method[93-94]. It could visualized the refraction intuitively, but the results relied on the handler’s subjective explanation which made the repeatability at a low level[95]. Autorefractoras another objective measurement, could provide fast and objective test on refraction, it was be used widely in study and clinic[94,96].In addition, infrared refractometer and wavefront analysis are objective measurement to detect refractive change[47]. During the test, these instruments had light source and may induced luminous effect which could increase complexity of test in high myopia or microcoria patients[4,47].
Measurement of Accommodative Amplitude Assessment the amplitude of accommodation is a significant item for postoperative patients with AIOL, because providing accommodation is a superiority of AIOLs. Subjective measurements had disadvantages in overestimation and low repeatability, although objective measurements overcame these.
Subjective methods for accommodative amplitude Subjective methods for accommodative ability are push-up method, pushdown method and minus lens method.
Push-up method[97] should be implemented under standard room illumination and correct refractive error totally. Using a near vision acuity chart placed at 40 cm initially in front of patient and the non-dominant eye was covered. The chart was moved at a speed around 4 cm/s towards the patient’s plane of spectacle. Patient needs to point out when the letter on the chart started to become blurred, then the examiner need to move the chart back to regain a clear image. When the patient reported the first sustained blur, the tester stopped moving the chart, and the reciprocal of this distance (in meters) between the point of sustained blurring and the plane of the spectacles was the accommodative amplitude.
Push-down method[98] is similar to push-up method, the near vision acuity chart was moved away from the plane of the spectacles at a speed of about 4 cm/s, the patient need to hint until the letter on the chart becomes clear, reciprocal of the distance (in meters) from the target to plane of the spectacles was the accommodative amplitude. Modified push-down method was putting a -4.00 D spherical lens on the basis of refractive correction, the final result should plus +4.00 D.
Minus lens method[97-98] be implemented by adding minus lenses in 0.25 D steps when the patient focus on the previous line of a clear line on a near visual acuity chart placed at 40 cm or a distance visual acuity chart placed at 5 m. The patient has to be refractive corrected, and should hint to the examiner when the target became and remained blurred. The sum of absolute value of the added negative lens plus 2.50 D was the accommodative amplitude.
Agreement between the three techniques was poor[97], but minus lens method may be superior in repeatability and approaching to true value[99], even the result of this method is lower than the other two[100].
Objective methods for accommodative amplitude A series of objective measurements have been used currently to assess the displacement of optic[47], for the purpose of estimating accommodative amplitude indirectly. To measure the shift of an AIOL induced by ciliary muscle contraction after application of pilocarpine could reflect accommodative ability[101]. For example, ultrasound biomiroscopy (UBM)[101], partial coherence interferometry[39,102], scheimp flug photography by IOL Master[19,103]and anterior segment optical coherence tomography[104]. There is also a new optical low coherence reflectometry device could detect the minimum displacement of 0.01 mm[105-106]. Most of study used 2% pilocarpine to stimulate the ciliary muscles for purpose of simulating accommodation, this method could measure the maximum amplitude of accommodation but could not represent normal physiological response[107-108].
The objective measures of accommodative amplitude are dynamic retinoscopy, autorefractometer, hartinger coincidence refractometer (HCR) and optical quality analysis system(OQAS; Visiometrics, Spain).
Dynamic retinoscopy[98] is using a similar procedure to the minus lens method described above. The visual acuity chart was placed close to the corrected optic with added -4.00 D spherical lens of patient, then pushed the target away until the letters on chart became clear. Fixing the visual acuity chart at this location, then examiner used the retinoscope to observed the retinoscopy reflex, if a ‘toward’ movement was seen, the examiner moved retinoscopy far from the eye until a neutral reflex was found. Then the reciprocal distance between the spectacle plane and the retinoscope added +4.00 D was the amplitude of accommodation. This method was high repeatability but relied on proficient skill.
Autorefractometer can be used in dynamic or static state[109],common model including Shin-Nippon SRW-5000[110], Grand Seiko WAM-5500[109,111] and Grand Seiko WR-5100K[112]and so on. The device is an open-field autorefractor with an infrared pupillometry function. The patient asked to fixate on a target placed 5 m, 50 cm, 33 cm and 20 cm, and diopter was obtained automatically, the maximum transformation of diopter is the amplitude of accommodation[113]. Comparing to dynamic retinoscopy and HCR, this method has lower demand for patients, but if the pupil diameter was smaller than 2 mm it could not be implemented[109,114-115].
HCR is based on Scheiner principle[114]. Non-dominant eye was covered and measured three times of baseline refraction, while patient viewed the distant vision acuity chart reflected off the beam splitter in front of the instrument. Then simulate the accommodation by minus lens or mydriatics and measure the refraction. The difference between initial and eventual diopter was the amplitude of accommodation[114,116]. HCR is capable of measuring through pupils as small as 1.1 mm, but the difficulty in measurement increases with the increase of diopter of minus lens, so the examiner needs higher skill[114,116].
OQAS based on the double-pass technique, it can provides parameters such as modulation transfer function curve(MTF curve) and point spread function image, depends on these parameters the objective visual quality could be calculated[117-118]. As a new type of instrument, the repeatability and feasibility demand more study.
Other Tests Contrast sensitivity is the ability to detect differences in luminance between adjacent areas and reflects the quality of vision[119]. Testing methods including sinewave gratings[119] and contrast sensitivity unit[120-121]. About the measurement for reading speed, yet has no unified standards.
CONCLUSION
People with a desire of both good distance vision and near vision, especially those who still have good function of ciliary muscles, AIOLs may be an appropriate choice. In the meantime, they may take risk of undesirable amplitude of accommodation, image magnification and PCO or ACO. The ultimate goal of cataract treatment is let the patients have approving visual outcomes which nearly reach the level of youth. Before such a treatment becomes available, further work is demanded. For now, some of the IOL designs described in this article might provide patients a better visual performance.
ACKNOWLEDGEMENTS
Conflicts of Interest: Liang YL, None; Jia SB, None.
REFERENCES
1 Finkelman YM, Ng JQ, Barrett GD. Patient satisfaction and visual function after pseudophakic monovision. J Cataract Refract Surg 2009; 35(6):998-1002.
2 Ong HS, Evans JR, Allan BD. Accommodative intraocular lens versus standard monofocal intraocular lens implantation in cataract surgery.Cochrane Database Syst Rev 2014(5):CD009667.
3 Charman WN. The eye in focus: accommodation and presbyopia. Clin Exp Optom 2008;91(3):207-225.
4 Glasser A. Restoration of accommodation: surgical options for correction of presbyopia. Clin Exp Optom 2008;91(3):279-295.
5 Strenk SA, Strenk LM, Guo S. Magnetic resonance imaging of aging,accommodating, phakic, and pseudophakic ciliary muscle diameters. J Cataract Refract Surg 2006;32(11):1792-1798.
6 Marchini G, Pedrotti E, Sartori P, Tosi R. Ultrasound biomicroscopic changes during accommodation in eyes with accommodating intraocular lenses: pilot study and hypothesis for the mechanism of accommodation.J Cataract Refract Surg 2004;30(12):2476-2482.
7 Sadoughi MM, Einollahi B, Roshandel D, Sarimohammadli M, Feizi S.Visual and refractive outcomes of phacoemulsification with implantation of accommodating versus standard monofocal intraocular lenses. J Ophthalmic Vis Res 2015;10(4):370-374.
8 Ostrin L, Kasthurirangan S, Win-Hall D, Glasser A. Simultaneous measurements of refraction and A-scan biometry during accommodation in humans. Optom Vis Sci 2006;83(9):657-665.
9 Menapace R, Findl O, Kriechbaum K, Leydolt-Koeppl Ch. Accommodating intraocular lenses: a critical review of present and future concepts.Graefes Arch Clin Exp Ophthalmol 2007;245(4):473-489.
10 Ossma IL, Galvis A, Vargas LG, Trager MJ, Vagefi MR, McLeod SD. Synchrony dual-optic accommodating intraocular lens. Part 2: pilot clinical evaluation. J Cataract Refract Surg 2007;33(1):47-52.
11 Ale JB, Manns F, Ho A. Paraxial analysis of the depth of field of a pseudophakic eye with accommodating intraocular lens. Optom Vis Sci 2011;88(7):789-794.
12 Legeais JM, Werner L, Werner L, Abenhaim A, Renard G.Pseudoaccommodation: BioComFold versus a foldable silicone intraocular lens. J Cataract Refract Surg 1999;25(2):262-267.
13 Leyland M, Bloom P. Intraocular lens design for pseudoaccommodation. J Cataract Refract Surg 1999;25(8):1038-1039.
14 Ramón ML, Piñero DP, Blanes-Mompó FJ, Pérez-Cambrodí RJ. Clinical and quality of life data correlation with a single-optic accommodating intraocular lens. J Optom 2013;6(1):25-35.
15 Findl O, Leydolt C. Meta-analysis of accommodating intraocular lenses. J Cataract Refract Surg 2007;33(3):522-527.
16 Doane JF, Jackson RT. Accommodative intraocular lenses: considerations on use, function and design. Curr Opin Ophthalmol 2007;18(4):318-324.17 Alió JL, Piñero DP, Plaza-Puche AB. Visual outcomes and optical performance with a monofocal intraocular lens and a new-generation single-optic accommodating intraocular lens. J Cataract Refract Surg 2010;36(10):1656-1664.
18 Pepose JS, Burke J, Qazi MA. Benefits and barriers of accommodating intraocular lenses. Curr Opin Ophthalmol 2017;28(1):3-8.
19 Küchle M, Nguyen NX, Langenbucher A, Gusek-Schneider GC, Seitz B, Hanna KD. Implantation of a new accommodative posterior chamber intraocular lens. J Refract Surg 2002;18(3):208-216.
20 Harman FE, Maling S, Kampougeris G, Langan L, Khan I, Lee N, Bloom PA. Comparing the 1CU accommodative, multifocal, and monofocal intraocular lenses: a randomized trial. Ophthalmology 2008;115(6):993-1001.e2.
21 Küchle M, Seitz B, Langenbucher A, Martus P, Nguyen NX; Erlangen Accommodative Intraocular Lens Study Group. Stability of refraction,accommodation, and lens position after implantation of the 1CU accommodating posterior chamber intraocular lens. J Cataract Refract Surg 2003;29(12):2324-2329.
22 Dogru M, Honda R, Omoto M, Toda I, Fujishima H, Arai H,Matsuyama M, Nishijima S, Hida Y, Yagi Y, Tsubota K. Early visual results with the 1CU accommodating intraocular lens. J Cataract Refract Surg 2005;31(5):895-902.
23 Wolffsohn JS, Hunt OA, Naroo S, Gilmartin B, Shah S, Cunliffe IA,Benson MT, Mantry S. Objective accommodative amplitude and dynamics with the 1CU accommodative intraocular lens. Invest Ophthalmol Vis Sci 2006;47(3):1230-1235.
24 Hancox J, Spalton D, Heatley C, Jayaram H, Yip J, Boyce J, Marshall J. Fellow-eye comparison of posterior capsule opacification rates after implantation of 1CU accommodating and AcrySof MA30 monofocal intraocular lenses. J Cataract Refract Surg 2007;33(3):413-417.
25 Sanders DR, Sanders ML. Visual performance results after Tetra flex accommodating intraocular lens implantation. Ophthalmology 2007;114(9):1679-1684.
26 Sheppard AL, Bashir A, Wolffsohn JS, Davies LN. Accommodating intraocular lenses: a review of design concepts, usage and assessment methods. Clin Exp Optom 2010;93(6):441-452.
27 Brown D, Dougherty P, Gills JP, Hunkeler J, Sanders DR, Sanders ML. Functional reading acuity and performance: Comparison of 2 accommodating intraocular lenses. J Cataract Refract Surg 2009;35(10):1711-1714.
28 Ferko J, Ferkova A. IOL Tetra flex, KH 3500--presbyopia treatment.Oftalmologia 2009;53(4):72-73.
29 Cumming JS, Colvard DM, Dell SJ, Doane J, Fine IH, Hoffman RS, Packer M, Slade SG. Clinical evaluation of the Crystalens AT-45 accommodating intraocular lens: results of the U.S. Food and Drug Administration clinical trial. J Cataract Refract Surg 2006;32(5):812-825.
30 Stachs O, Schneider H, Beck R, Guthoff R. Pharmacological-induced haptic changes and the accommodative performance in patients with the AT-45 accommodative IOL. J Refract Surg 2006;22(2):145-150.
31 Koeppl C, Findl O, Menapace R, Kriechbaum K, Wirtitsch M, Buehl W, Sacu S, Drexler W. Pilocarpine-induced shift of an accommodating intraocular lens: AT-45 Crystalens. J Cataract Refract Surg 2005;31(7):1290-1297.
32 Jardim D, Soloway B, Starr C. Asymmetric vault of an accommodating intraocular lens. J Cataract Refract Surg 2006;32(2):347-350.
33 Alió JL, Plaza-Puche AB, Montalban R, Ortega P. Near visual outcomes with single-optic and dual-optic accommodating intraocular lenses. J Cataract Refract Surg 2012;38(9):1568-1575.
34 Karavitaki AE, Pallikaris IG, Panagopoulou SI, Kounis GA, Kontadakis G, Kymionis GD. Long-term visual outcomes after Crystalens(®) HD intraocular lens implantation. Clin Ophthalmol 2014;8:937-943.
35 Pérez-Vives C, Montés-Micó R, López-Gil N, Ferrer-Blasco T, García-Lázaro S. Crystalens HD intraocular lens analysis using an adaptive optics visual simulator. Optom Vis Sci 2013;90(12):1413-1423.
36 Zamora-Alejo KV, Moore SP, Parker DG, Ullrich K, Esterman A,Goggin M. Objective accommodation measurement of the Crystalens HD compared to monofocal intraocular lenses. J Refract Surg 2013;29(2):133-139.
37 Takakura A, Iyer P, Adams JR, Pepin SM. Functional assessment of accommodating intraocular lenses versus monofocal intraocular lenses in cataract surgery: metaanalysis. J Cataract Refract Surg 2010;36(3):380-388.
38 Beiko GH. Comparison of visual results with accommodating intraocular lenses versus mini-monovision with a monofocal intraocular lens. J Cataract Refract Surg 2013;39(1):48-55.
39 Hancox J, Spalton D, Heatley C, Jayaram H, Marshall J. Objective measurement of intraocular lens movement and dioptric change with a focus shift accommodating intraocular lens. J Cataract Refract Surg 2006;32(7):1098-1103.
40 Heatley CJ, Spalton DJ, Hancox J, Kumar A, Marshall J. Fellow eye comparison between the 1CU accommodative intraocular lens and the Acrysof MA30 monofocal intraocular lens. Am J Ophthalmol 2005;140(2):207-213.
41 Marchini G, Mora P, Pedrotti E, Manzotti F, Aldigeri R, Gandolfi SA. Functional assessment of two different accommodative intraocular lenses compared with a monofocal intraocular lens. Ophthalmology 2007;114(11):2038-2043.
42 Nawa Y, Ueda T, Nakatsuka M, Tsuji H, Marutani H, Hara Y, Uozato H.Accommodation obtained per 1.0 mm forward movement of a posterior chamber intraocular lens. J Cataract Refract Surg 2003;29(11):2069-2072.
43 Marcos S, Ortiz S, Pérez-Merino P, Birkenfeld J, Durán S, Jiménez-Alfaro I. Three-dimensional evaluation of accommodating intraocular lens shift and alignment in vivo. Ophthalmology 2014;121(1):45-55.
44 Cleary G, Spalton DJ, Marshall J. Anterior chamber depth measurements in eyes with an accommodating intraocular lens: agreement between partial coherence interferometry and optical coherence tomography. J Cataract Refract Surg 2010;36(5):790-798.
45 Pérez-Merino P, Birkenfeld J, Dorronsoro C, Ortiz S, Durán S,Jiménez-Alfaro I, Marcos S. Aberrometry in patients implanted with accommodative intraocular lenses. Am J Ophthalmol 2014;157(5):1077-1089.
46 Wolffsohn JS, Naroo SA, Motwani NK, Shah S, Hunt OA, Mantry S,Sira M, Cunliffe IA, Benson MT. Subjective and objective performance of the Lenstec KH-3500 "accommodative" intraocular lens. Br J Ophthalmol 2006;90(6):693-696.
47 McLeod SD. Optical principles, biomechanics, and initial clinical performance of a dual-optic accommodating intraocular lens (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc 2006;104:437-452.
48 Duman R, Karel F, Özyol P, Ateş C. Effect of four different intraocular lenses on posterior capsule opacification. Int J Ophthalmol 2015;8(1):118-121.
49 Dewey S. Posterior capsule opacification. Curr Opin Ophthalmol 2006;17(1):45-53.
50 Nguyen NX, Seitz B, Reese S, Langenbucher A, Küchle M. Accommodation after Nd: YAG capsulotomy in patients with accommodative posterior chamber lens 1CU. Graefes Arch Clin Exp Ophthalmol 2005;243(2):120-126.
51 Langenbucher A, Reese S, Jakob C, Seitz B. Pseudophakic accommodation with translation lenses-dual optic vs mono optic.Ophthalmic Physiol Opt 2004;24(5):450-457.
52 McLeod SD, Portney V, Ting A. A dual optic accommodating foldable intraocular lens. Br J Ophthalmol 2003;87(9):1083-1085.
53 Ale J, Manns F, Ho A. Evaluation of the performance of accommodating IOLs using a paraxial optics analysis. Ophthalmic Physiol Opt 2010;30(2):132-142.
54 McLeod SD, Vargas LG, Portney V, Ting A. Synchrony dual-optic accommodating intraocular lens. Part 1: optical and biomechanical principles and design considerations. J Cataract Refract Surg 2007;33(1):37-46.
55 Werner L, Pandey SK, Izak AM, Vargas LG, Trivedi RH, Apple DJ,Mamalis N. Capsular bag opacification after experimental implantation of a new accommodating intraocular lens in rabbit eyes. J Cataract Refract Surg 2004;30(5):1114-1123.
56 Peris-Martínez C, Díez-Ajenjo A, García-Domene C. Short-term results with the Synchrony lens implant for correctionof presbyopia following cataract surgery. J Emmetropia 2013;4:137-143.
57 Ho A, Manns F, Therese, Parel JM. Predicting the performance of accommodating intraocular lenses using ray tracing. J Cataract Refract Surg 2006;32(1):129-136.
58 Fernández-Buenaga R, Alio JL, Pérez-Ardoy AL, Larrosa-Quesada A,Pinilla-Cortés L, Barraquer R, Alio JL 2nd, Muñoz-Negrete FJ. Late inthe-bag intraocular lens dislocation requiring explantation: risk factors and outcomes. Eye (Lond) 2013;27(7):795-801.
59 Ale JB, Manns F, Ho A. Magnifications of single and dual element accommodative intraocular lenses: paraxial optics analysis. Ophthalmic Physiol Opt 2011;31(1):7-16.
60 García M, González C, Pascual I, Fimia A. Magnification and visual acuity in highly myopic phakic eyes corrected with an anterior chamber intraocular lens versus by other methods. J Cataract Refract Surg 1996;22(10):1416-1422.
61 Montés-Micó R, España E, Bueno I, Charman WN, Menezo JL.Visual performance with multifocal intraocular lenses: mesopic contrast sensitivity under distance and near conditions. Ophthalmology 2004;111(1):85-96.
62 Alió JL, Ben-nun J, Rodríguez-Prats JL, Plaza AB. Visual and accommodative outcomes 1 year after implantation of an accommodating intraocular lens based on a new concept. J Cataract Refract Surg 2009;35(10):1671-1678.
63 McCafferty SJ, Schwiegerling JT. Deformable surface accommodating intraocular lens: second generation prototype design methodology and testing. Transl Vis Sci Technol 2015;4(2):17.
64 Ben-Nun J, Alió JL. Feasibility and development of a highpower real accommodating intraocular lens. J Cataract Refract Surg 2005;31(9):1802-1808.
65 Kohl JC, Werner L, Ford JR, Cole SC, Vasavada SA, Gardiner GL,Noristani R, Mamalis N. Long-term uveal and capsular biocompatibility of a new accommodating intraocular lens. J Cataract Refract Surg 2014;40(12):2113-2119.
66 Alfonso JF, Carones F, Cionni RJ, Claoué C, Cochener B, Goes FJ,Pepose JS, Vukich JA. Annual IOL Issue Focusing on the available lens options in Europe and the United States. CATARACT & REFRACTIVE SURGERY TODAY EUROPE 2010.
67 Dhital A, Spalton DJ, Gala KB. Comparison of near vision, intraocular lens movement, and depth of focus with accommodating and monofocal intraocular lenses. J Cataract Refract Surg 2013;39(12):1872-1878.
68 Nakazawa M, Ohtsuki K. Apparent accommodation in pseudophakic eyes after implantation of posterior chamber intraocular lenses. Am J Ophthalmol 1983;96(4):435-438.
69 Altan-Yaycioglu R, Gözüm N, Gücükoglu A. Pseudo-accommodation with intraocular lenses implanted in the bag. J Refract Surg 2002;18(3):271-275.
70 Elder MJ, Murphy C, Sanderson GF. Apparent accommodation and depth of field in pseudophakia. J Cataract Refract Surg 1996;22(5):615-619.
71 Verzella F, Calossi A. Multifocal effect of against-the-rule myopic astigmatism in pseudophakic eyes. Refract Corneal Surg 1993;9(1):58-61.
72 Wolffsohn JS, Davies LN, Gupta N, Naroo SA, Gibson GA, Mihashi T,Shah S. Mechanism of action of the tetra flex accommodative intraocular lens. J Refract Surg 2010;26(11):858-862.
73 Coleman DJ, Fish SK. Presbyopia, accommodation, and the mature catenary. Ophthalmology 2001;108(9):1544-1551.
74 Dick HB. Accommodative intraocular lenses: current status. Curr Opin Ophthalmol 2005;16(1):8-26.
75 Kubal AA. Multifocal versus accommodating intraocular lenses:a review of the current technology, outcomes, and complications. Int Ophthalmol Clin 2011;51(2):131-141.
76 Mastropasqua L, Toto L, Falconio G, Nubile M, Carpineto P,Ciancaglini M, Di Nicola M, Ballone E. Longterm results of 1CU accommodative intraocular lens implantation: 2-year follow-up study.Acta Ophthalmol Scand 2007;85(4):409-414.
77 Strenk SA, Semmlow JL, Strenk LM, Munoz P, Gronlund-Jacob J, DeMarco JK. Age-related changes in human ciliary muscle and lens: a magnetic resonance imaging study. Invest Ophthalmol Vis Sci 1999;40(6):1162-1169.
78 Tahir HJ, Tong JL, Geissler S, Vedamurthy I, Schor CM. Effects of accommodation training on accommodation and depth of focus in an eye implanted with a crystalens intraocular lens. J Refract Surg 2010;26(10):772-779.
79 Tan DK, Tay WT, Chan C, Tan DT, Mehta JS. Postoperative ocular higher-order aberrations and contrast sensitivity: femtosecond lenticule extraction versus pseudo small-incision lenticule extraction. J Cataract Refract Surg 2015;41(3):623-634.
80 Bohórquez V, Alarcon R. Long-term reading performance in patients with bilateral dual-optic accommodating intraocular lenses. J Cataract Refract Surg 2010;36(11):1880-1886.
81 Pepose JS. Maximizing satisfaction with presbyopia-correcting intraocular lenses: the missing links. Am J Ophthalmol 2008;146(5):641-648.
82 Lichtinger A, Rootman DS. Intraocular lenses for presbyopia correction:past, present, and future. Curr Opin Ophthalmol 2012;23(1):40-46.
83 Kane JX, Van Heerden A, Atik A, Petsoglou C. Intraocular lens power formula accuracy: Comparison of 7 formulas. J Cataract Refract Surg 2016;42(10):1490-1500.
84 Thornton SP. Restoring accommodation: what is real and what is pseudo? J Cataract Refract Surg 2005;31(10):1851-1852.
85 Langenbucher A, Huber S, Nguyen NX, Seitz B, Gusek-Schneider GC, Küchle M. Measurement of accommodation after implantation of an accommodating posterior chamber intraocular lens. J Cataract Refract Surg 2003;29(4):677-685.
86 Findl O, Kriechbaum K, Menapace R, Koeppl C, Sacu S, Wirtitsch M, Buehl W, Drexler W. Laserinterferometric assessment of pilocarpineinduced movement of an accommodating intraocular lens: a randomized trial. Ophthalmology 2004;111(8):1515-1521.
87 Alio JL, Plaza-Puche AB, Javaloy J, Ayala MJ, Moreno LJ, Piñero DP. Comparison of a new refractive multifocal intraocular lens with an inferior segmental near add and a diffractive multifocal intraocular lens.Ophthalmology 2012;119(3):555-563.
88 Cillino S, Casuccio A, Di Pace F, Morreale R, Pillitteri F, Cillino G,Lodato G. One-year outcomes with new-generation multifocal intraocular lenses. Ophthalmology 2008;115(9):1508-1516.
89 Pieh S, Kellner C, Hanselmayer G, Lackner B, Schmidinger G,Walkow T, Sticker M, Weghaupt H, Fercher AF, Skorpik C. Comparison of visual acuities at different distances and defocus curves. J Cataract Refract Surg 2002;28(11):1964-1967.
90 Gupta N, Naroo SA, Wolffsohn JS. Is randomisation necessary for measuring defocus curves in pre-presbyopes? Cont Lens Anterior Eye 2007;30(2):119-124.
91 Ogle KN, Schwartz JT. Depth of focus of the human eye. J Opt Soc Am 1959;49(3):273-280.
92 Schwartz JT, Ogle KN. The depth of focus of the eye. AMA Arch Ophthalmol 1959;61(4):578-588.
93 Prabakaran S, Dirani M, Chia A, Gazzard G, Fan Q, Leo SW, Ling Y,Au Eong KG, Wong TY, Saw SM. Cycloplegic refraction in preschool children: comparisons between the hand-held autorefractor, table-mounted autorefractor and retinoscopy. Ophthalmic Physiol Opt 2009;29(4):422-426.
94 Sheppard AL, Davies LN. Clinical evaluation of the Grand Seiko Auto Ref/Keratometer WAM-5500. Ophthalmic Physiol Opt 2010;30(2):143-151.
95 Rutstein RP, Fuhr PD, Swiatocha J. Comparing the amplitude of accommodation determined objectively and subjectively. Optom Vis Sci 1993;70(6):496-500.
96 Mallen EA, Gilmartin B, Wolffsohn JS, Tsujimura S. Clinical evaluation of the Shin-Nippon SRW-5000 autorefractor in adults: an update. Ophthalmic Physiol Opt 2015;35(6):622-627.
97 Antona B, Barra F, Barrio A, Gonzalez E, Sanchez I. Repeatability intraexaminer and agreement in amplitude of accommodation measurements. Graefes Arch Clin Exp Ophthalmol 2009;247(1):121-127.
98 León AÁ, Medrano SM, Rosen field M. A comparison of the reliability of dynamic retinoscopy and subjective measurements of amplitude of accommodation. Ophthalmic Physiol Opt 2012;32(2):133-141.
99 Iyamu E, Iyamu JE, Oghovwerha L. Anthropometry, amplitude of accommodation, and spherical equivalent refractive error in a nigerian population. ISRN Ophthalmol 2012;2012:295613.
100 Fitch RC. Procedural effects on the manifest human amplitude of accommodation. Am J Optom Arch Am Acad Optom 1971;48(11):918-926.
101 Auffarth GU, Martin M, Fuchs HA, Rabsilber TM, Becker KA,Schmack I. Validity of anterior chamber depth measurements for the evaluation of accommodation after implantation of an accommodative Humanoptics 1CU intraocular lens. Ophthalmologe 2002;99(11):815-819.
102 Findl O, Kiss B, Petternel V, Menapace R, Georgopoulos M, Rainer G, Drexler W. Intraocular lens movement caused by ciliary muscle contraction. J Cataract Refract Surg 2003;29(4):669-676.
103 Langenbucher A, Seitz B, Huber S, Nguyen NX, Kuchle M.Theoretical and measured pseudophakic accommodation after implantation of a new accommodative posterior chamber intraocular lens. Arch Ophthalmol 2003;121(12):1722-1727.
104 Németh G, Tsorbatzoglou A, Módis L, Berta A. Examination of accommodation in pseudophakic eyes. Orv Hetil 2009;150(20):943-948.
105 Holzer MP, Mamusa M, Auffarth GU. Accuracy of a new partial coherence interferometry analyser for biometric measurements. Br J Ophthalmol 2009;93(6):807-810.
106 Buckhurst PJ, Wolffsohn JS, Shah S, Naroo SA, Davies LN, Berrow EJ. A new optical low coherence reflectometry device for ocular biometry in cataract patients. Br J Ophthalmol 2009;93(7):949-953.
107 Kriechbaum K, Findl O, Koeppl C, Menapace R, Drexler W.Stimulus-driven versus pilocarpine-induced biometric changes in pseudophakic eyes. Ophthalmology 2005;112(3):453-459.
108 Uthoff D, Holland D, Hepper D, Gulati A, Koch L, Haigis W.Laserinterferometric measurements of accommodative changes in the position of an optic-shift intraocular lens. J Refract Surg 2009;25(5):416-420.
109 Win-Hall DM, Houser J, Glasser A. Static and dynamic accommodation measured using the WAM-5500 Autorefractor. Optom Vis Sci 2010;87(11):873-882.
110 Wolffsohn JS, Sheppard AL, Vakani S, Davies LN. Accommodative amplitude required for sustained near work. Ophthalmic Physiol Opt 2011;31(5):480-486.
111 Anderson HA, Stuebing KK. Subjective versus objective accommodative amplitude: preschool to presbyopia. Optom Vis Sci 2014;91(11):1290-1301.
112 Durr NJ, Dave SR, Vera-Diaz FA, Lim D, Dorronsoro C, Marcos S,Thorn F, Lage E. Design and clinical evaluation of a handheld wavefront autorefractor. Optom Vis Sci 2015;92(12):1140-1147.
113 Nemeth G, Lipecz A, Szalai E, Berta A, Modis L Jr. Accommodation in phakic and pseudophakic eyes measured with subjective and objective methods. J Cataract Refract Surg 2013;39(10):1534-1542.
114 Win-Hall DM, Ostrin LA, Kasthurirangan S, Glasser A. Objective accommodation measurement with the Grand Seiko and Hartinger coincidence refractometer. Optom Vis Sci 2007;84(9):879-887.
115 Singman E, Matta N, Tian J, Silbert D. Association between accommodative amplitudes and amblyopia. Strabismus 2013;21(2):137-139.
116 Wold JE, Hu A, Chen S, Glasser A. Subjective and objective measurement of human accommodative amplitude. J Cataract Refract Surg 2003;29(10):1878-1888.
117 Leonard AP, Gardner SD, Rocha KM, Zeldin ER, Tremblay DM,Waring GO 4th. Double-pass retina point imaging for the evaluation of optical light scatter, retinal image quality, and staging of keratoconus. J Refract Surg 2016;32(11):760-765.
118 Wang YJ, Yang YN, Huang LY, Wang B, Han YC, Yan JB. Optical quality and related factors in ocular hypertension: preliminary study. J Ophthalmol 2016;2016:3071036.
119 Feizi S, Karimian F. Effect of higher order aberrations on contrast sensitivity function in myopic eyes. Jpn J Ophthalmol 2009;53(4):414-419.
120 Nassiri N, Sheibani K, Azimi A, Khosravi FM, Heravian J, Yekta A, Moghaddam HO, Nassiri S, Yasseri M, Nassiri N. Refractive outcomes, contrast sensitivity, hoas, and patient satisfaction in moderate myopia: wavefront-optimized versus tissue-saving PRK. J Refract Surg 2015;31(10):683-690.
121 Kamiya K, Shimizu K, Iijima A, Kobashi H. Factors influencing contrast sensitivity function in myopic eyes. PLoS One 2014;9(11):e113562.