Dear Editor,
The cornea is the ocular structure most commonly affected by fungi. In general, fungal organisms infiltrate into cornea following ocular injuries, such as the vegetative trauma,leading to infectious fungal keratitis, which is characterized by epithelial defects, corneal edema and ulceration, satellite lesions in the surrounding cornea, and white fluffy hypopyon[1].Secondary attenuation of cell defense mechanisms after prolonged use of topical or systemic corticosteroid or immunosuppressive agents may also result in endogenous fungal infection[2-4].Isolated corneal endotheliitis is less common, and has mainly been attributed to various viral species, including herpes simplex virus (HSV), varicella-zoster virus (VZV), and cytomegalovirus (CMV), among others[5-8]; however, there are few published reports about fungal corneal endotheliitis. Here,we present a series of cases of fungal corneal endotheliitis and details of their unusual clinical characteristics. This study was approved by the Ethical Committee of Shaanxi Provincial Institute of Ophthalmology. The informed consents were obtained from all of patients. All of the treatments in this study were followed the Declaration of Helsinki.
Case 1
A 51-year-old male complained of repeated episodes of blurred vision and irritative symptoms in the left eye over 2y. His medical history was negative for ocular trauma. He denied using corticosteroids or immunosuppressive agents by any route. Slit-lamp biomicroscope examination revealed mild stromal edema in the central cornea. The epithelium was intact. The endothelial layer in the area of the lesion had the appearance of ground glass. There was poor clinical response to a one-week course of topical antiviral agents.Confocal biomicroscopy revealed numerous well-defined,hyperreflective linear, and bifurcating branch-like hyphae concentrated around Descemet’s membrane in the lesion area, suggestive of fungal endotheliitis. Topical voriconazole solution (4%, 100 mg of voriconazole powder reconstituted for injection with 1 mL sterile water and added to 1.5 mL artificial tears stored at 4℃) was applied into the affected eye every 2h. Seven days later, the corneal edema had resolved and the topical voriconazole was tapered off over the next 4wk; however, six months later, the patient relapsed, and the condition responded well to another course of topical voriconazole. In total, he had five relapses over a period of two years, resulting in a corneal scar necessitating penetrating keratoplasty. The initial diagnosis was confirmed by histopathological examination of the excised corneal button,which revealed numerous fungal hyphae and spores in smears from the corneal lesion. The results of pathogenic culture of the excised corneal button and aqueous humor failed to show any growth of fungal pathogens. PAS staining of the excised corneal button was negative for fungus. Topical voriconazole combined with 1% cyclosporine treatment was maintained for 4wk after surgery. In the 12mo of follow-up, the corneal graft remained clear, with no further recurrences (Figures 1-3).
Case 2
A 64-year-old male complained of blurred vision and irritation in his left eye for the past three months. He had no history of ocular trauma or risk factors for immune-compromise. Several cotton-wool-like keratic precipitates (KPs) were noted on the endothelial layer of the central cornea, with moderate stromal edema in the area of the lesion. The epithelium was intact.Confocal biomicroscopy showed numerous highly reflective,branching filaments in close proximity to Descemet’s membrane in the lesion area, accompanied by some highly reflective inflammatory cells. A presumed diagnosis of fungus corneal endotheliitis was made and topical voriconazole initiated, as described for Case 1 above. Despite remarkable improvements in clinical signs after initiation of antifungal therapy, the corneal lesion relapsed and worsened three months later. Penetrating keratoplasty was performed on the affected eye. Fungal hyphae and spores were found in smears from the excised corneal button, but not on pathogenic culture or PAS staining. We were unable to identify any specific pathogens in cultures from the aqueous humor. As in the first case, the patient was maintained on topical voriconazole combined with 1% cyclosporine until 4wk after surgery. Over the following year, the corneal graft remained clear, with no further disease recurrence (Figures 1-3).
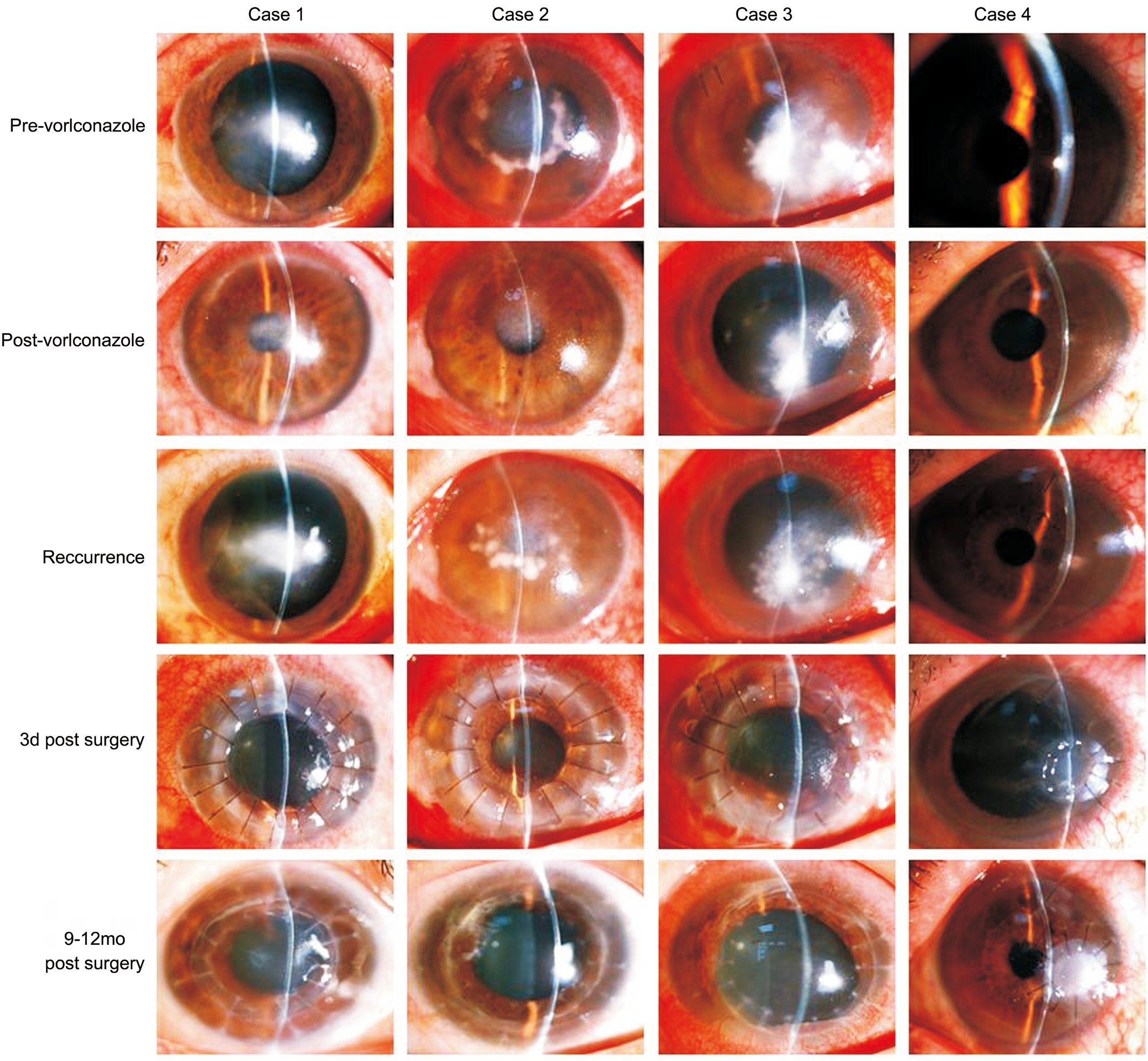
Figure 1 Photographs obtained by slit-lamp biomicroscopy, showing mild to moderate edema of the stroma in the lesion area, without in filtration, ulcer, or epithelial defect The endothelial layer appears blurred and contains cotton-wool-like keratic precipitates. Clinical signs improved after antifungal therapy, but recurred once treatment was terminated. There was no relapse after a corneal graft.
Case 3
A 43-year-old male had blurred vision and pain in his left eye 20d prior to seeking medical attention. He had no significant risk factors for fungal infection. A cluster of cotton-wool-like KPs was observed on the paracentral corneal endothelium,surrounded by moderate stromal edema; the epithelium was otherwise intact. On confocal biomicroscopy, high contrast,fibrous hyphae were found above and below Descemet’s membrane in the lesion area. The patient was treated for fungal corneal endotheliitis. The clinical signs improved remarkably after two-hourly topical voriconazole treatment for 3d; however, on the 13th day, due to arbitrary termination of treatment with the antifungal agent by the patient himself, the corneal lesion suddenly deteriorated. Penetrating keratoplasty was performed in the affected eye. Numerous fungal hyphae grew in pathogenic culture from the excised corneal button,but not in that from the aqueous humor. The results of PAS staining of the corneal button did not identify any fungal pathogens. The patient experienced graft rejection three months post-surgery, which was successfully treated. At one year post surgery, the corneal graft remained clear and the corneal endotheliitis has never relapsed (Figures 1-3).
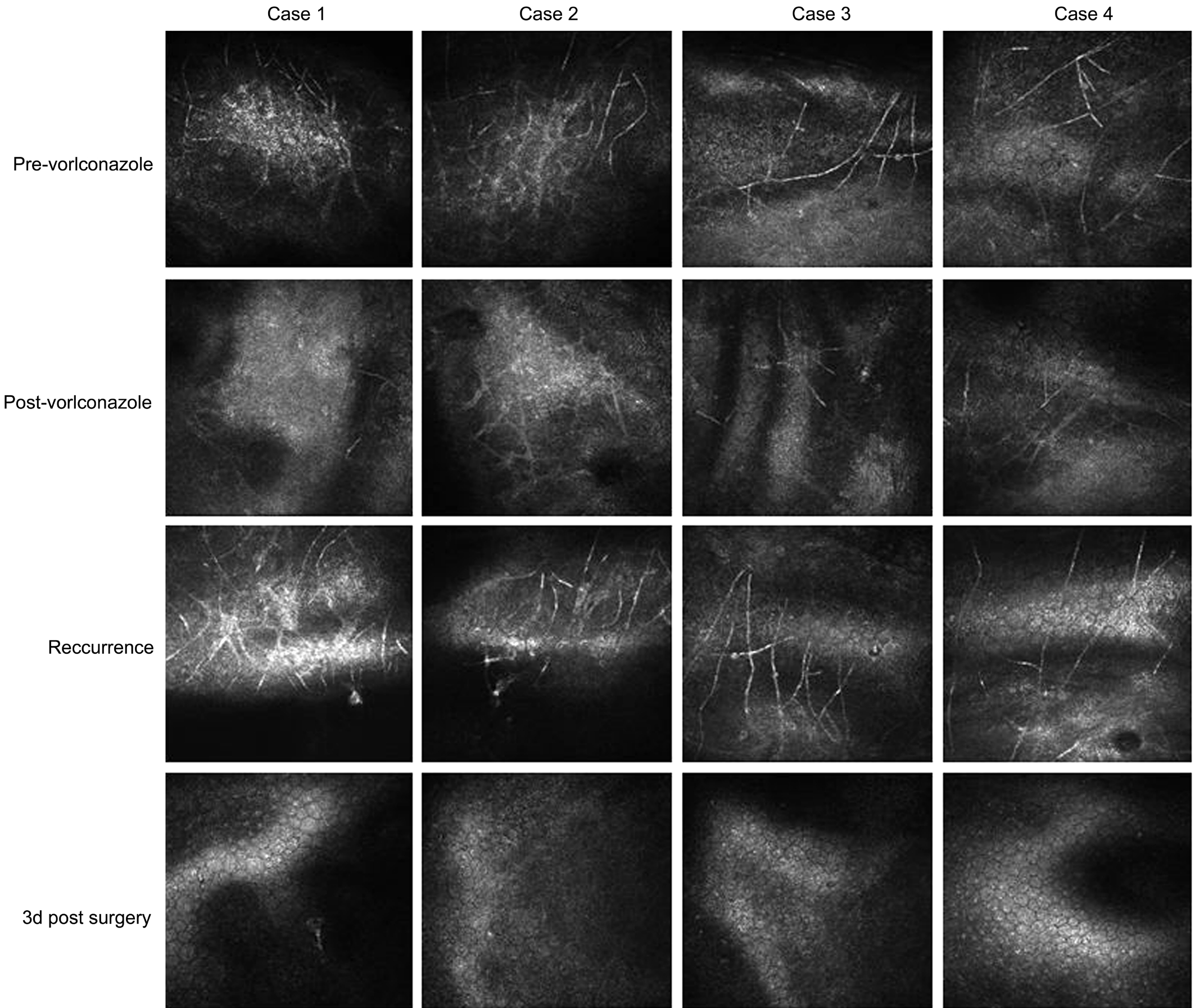
Figure 2 Photographs obtained by confocal biomicroscopy, showing numerous highly reflective, filament-like hyphae in close proximity to Descemet’s membrane in the corneal lesion area Consistent with the improvement in clinical symptoms, the hyphae decreased after antifungal therapy, but multiplied in the relapse stage. In the corneal graft, no hyphae were observed.
Case 4
A 48-year-old male was referred to our clinic because of recurrent eye redness and discomfort in his left eye. He had a history of left eye ocular trauma with a metal foreign body at 4 o’clock in the paracentral cornea three months earlier; the foreign body had been removed in a local hospital. On slitlamp biomicroscopy, snowflake-like KPs were observed on the endothelial layer, just below the injured corneal site, with mild stromal edema. There was no epithelial defect. Confocal biomicroscopy revealed some highly reflective, filamentlike hyphae in close proximity to Descemet’s membrane in the lesion area. The initial diagnosis was fungal corneal endotheliitis. After anterior chamber washout, intracameral injection of voriconazole (20 mg in 0.1 mL sterile water)was performed. The patient was also started on topical fluconazole drops. In the following two months, the patient received another intracameral injection of voriconazole. The KPs gradually disappeared; however, eye symptoms and KPs recurred one month after the second intracameral injection.Penetrating keratoplasty was performed in the affected eye. As described above, voriconazole, combined with 1%cyclosporine, was used topically after surgery. Pathogenic assay confirmed the growth of fungi in culture from the excised corneal button; however, the same phenomena did not occur in culture from the aqueous humor. No pathogens were identified by PAS staining of the corneal button. The patient experienced a graft rejection at nine months post-surgery,resulting in failure of the graft despite anti-rejection treatment;however, the corneal endotheliitis never relapsed (Figures 1-3).
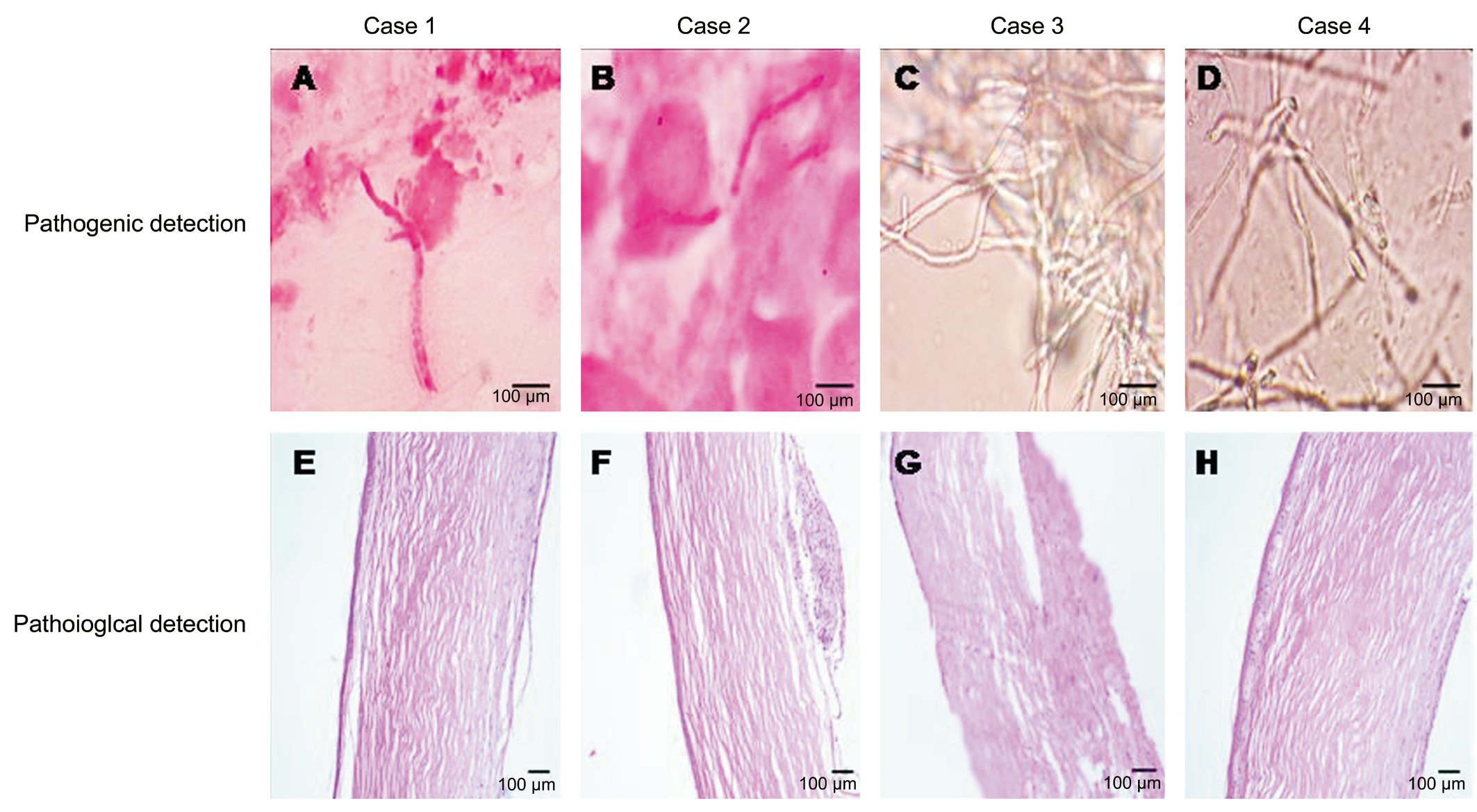
Figure 3 Pathogenic assay of the excised corneal button Many fungal hyphae and spores were found in the smears from the corneal lesions from cases 1 and 2 (A and B). Pathogenic cultures from cases 3 and 4 (C and D) showing the growth of fungi. PAS staining of the corneal lesion failed to identify the fungal pathogens (E, F, G, and H).
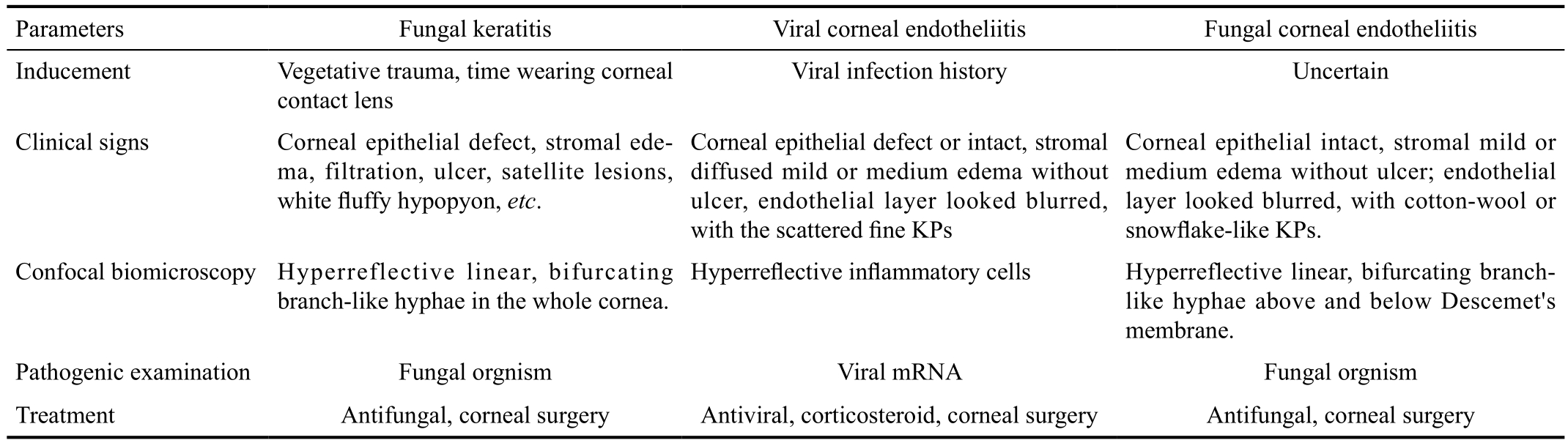
Table 1 Comparison of the clinical characteristics of patients included in this study
DISCUSSION
Herein, we present four cases of fungal corneal endotheliitis and their unusual clinical characteristics (Table 1). First,common predisposing factors associated with fungal in filtration were not identified in these cases, except for one,who experienced ocular trauma with a corneal foreign body;the others did not disclose any ocular trauma or immune compromise. Zapata et al[9] postulated that the presence of recurrent onychomycosis was a risk factor for fungal corneal endotheliitis; however, none of the cases described in this report suffered from onychomycosis. Moreover, the lesions primarily involved Descemet’s membrane and endothelia.The endothelial layer appeared blurred in the lesion area and contained cotton-wool or snowflake-like KPs, unlike the scattered, fine KPs observed in viral endotheliitis. Corneal stroma in the lesion area showed mild to moderate edema, but without epithelial defect, stromal infiltration, or ulcer; hence differing from the signs of fungal keratitis.
Confocal biomicroscopy is a reliable technique for the diagnosis of deep fungal keratitis[10]. In the current study,confocal biomicroscopy revealed the presence of numerous highly reflective, bifurcating branch-like hyphae, primarily deposited in close proximity to Descemet’s membrane,which contributed to establishment of the diagnosis. In the remission stage, confocal biomicroscopy also demonstrated that a few fungal hyphae remained in the lesion area,with the potential to drive an endotheliitis relapse.During post-surgery fungal hyphae were not observed in the corneal grafts by confocal biomicroscopy. Overall,confocal biomicroscopy played a pivotal role in the initial diagnosis and determination of prognosis of fungal corneal endotheliitis in these cases.
The results of pathogenic examination further confirmed the diagnosis of fungal corneal endotheliitis. In addition, the response to antifungal therapy in these cases also supported this diagnosis; however, although this therapy improved the clinical signs in the short term, it did not prevent progression or relapse of the corneal lesion in any of the current cases;thus, alternative treatment strategies should be explored in the future, including the selection of medication (single or combination, time course, etc.), the route of administration(topical eye drop, or intrastromal or intracameral injection,etc.), and the time window of surgical intervention.
In conclusion, the clinical characteristics, results of auxiliary examinations, and responses to the antifungal agents indicated a diagnosis of fungal corneal endotheliitis in all cases described in this report; however, some uncertainties remained to be clarified. These uncertainties include why the fungalrelated lesion primarily involved Descemet’s membrane and the endothelial layer; why those fungi preferred to reside in close proximity to Descemet’s membrane; and what biological characteristics the fungi had that resulted in a different clinical presentation from the classical fungal keratitis.
ACKNOWLEDGEMENTS
Foundation: Supported by the Shaanxi Provincial Natural Science Basic Research Program (No.2016JM8017).
Conflicts of Interest: Wang SY, None; Zhu HF, None;Cheng Y, None; Wu J, None; Tian Y, None.
REFERENCES
1 Ansari Z, Miller D, Galor A. Current thoughts in fungal keratitis:diagnosis and treatment. Curr Fungal Infect Rep 2013;7(3):209-218.
2 Yildiz EH, Abdalla YF, Elsahn AF, Rapuano CJ, Hammersmith KM,Laibson PR, Cohen EJ. Update on fungal keratitis from 1999 to 2008.Cornea 2010;29(12):1406-1411.
3 Keay LJ, Gower EW, Iovieno A, Oechsler RA, Alfonso EC, Matoba A, Colby K, Tuli SS, Hammersmith K, Cavanagh D, Lee SM, Irvine J, Stulting RD, Mauger TF, Schein OD. Clinical and microbiological characteristics of fungal keratitis in the United States, 2001-2007: a multicenter study. Ophthalmology 2011;118(5):920-926.
4 Kalkanci A, Ozdek S. Ocular fungal infections. Curr Eye Res 2011;36(3):179-189.
5 Suzuki T, Ohashi Y. Corneal endotheliitis. Semin Ophthalmol 2008;23(4):235-240.
6 Koizumi N, Suzuki T, Uno T, Chihara H, Shiraishi A, Hara Y, Inatomi T,Sotozono C, Kawasaki S, Yamasaki K, Mochida C, Ohashi Y, Kinoshita S. Cytomegalovirus as an etiologic factor in corneal endotheliitis.Ophthalmology 2008;115(2):292-297.e3.
7 Koizumi N, Yamasaki K, Kawasaki S, Sotozono C, Inatomi T, Mochida C, Kinoshita S. Cytomegalovirus in aqueous humor from an eye with corneal endotheliitis. Am J Ophthalmol 2006;141(3):564-565.
8 Ohashi Y, Yamamoto S, Nishida K, Okamoto S, Kinoshita S, Hayashi K, Manabe R. Demonstration of herpes simplex virus DNA in idiopathic corneal endotheliopathy. Am J Ophthalmol 1991;112(4):419-423.
9 Zapata LF, Paulo JD, Restrepo CA, Velásquez LF, Montoya AE, Zapata MA. Infectious endotheliitis: a rare case of presumed mycotic origin. Clin Ophthalmol 2013;7:1459-1461.
10 Das S, Samant M, Garg P, Vaddavalli PK, Vemuganti GK. Role of confocal microscopy in deep fungal keratitis. Cornea 2009;28(1):11-13.