Dear Editor,
Idiopathic choroidal neovascularization (CNV) is described as a rare but potentially blinding fundus disease wherein a choroidal neovascular membrane develops in the apparent absence of any coexistent ocular or systemic disease[1-2].Idiopathic CNV is most commonly seen in young patients who are below 50 years old and without any signs of age-related macular degeneration (AMD). Recent studies on the use of anti-vascular endothelial growth factor (VEGF) therapies like bevacizumab and ranibizumab in treating CNV have demonstrated better results than conventional photodynamic therapy in terms of visual gain and maintenance[2-4]. However,retinal pigment epithelium (RPE) tear and RPE atrophy are two major ocular complications of anti-VEGF drugs, especially in patients with neovascular AMD, and could potentially outweigh the benefits of the treatment[5-7]. Early detection of these RPE changes is of crucial importance. To the best of our knowledge, this is the first case report of using multispectral imaging (MSI) as a new modality to visualize the RPE atrophy at an early stage after anti-VEGF therapy.
CASE PRESENTATION
A 48-year-old Chinese male presented with a history of blurred vision in his right eye for at least the past 2mo. Best corrected visual acuity (BCVA) was 20/100 in the right eye and 20/20 in the left eye. Intraocular pressures (IOPs) and the anterior segments were within normal limits in both eyes.Dilated fundus examination of the left eye was quite normal;however, his right eye showed a significant deep red subretinal hemorrhage in the macula (Figure 1A). He denied medical history of any systemic or ocular disease. Written informed consent was obtained from this patient.
Optical coherence tomography (OCT; Carl Zeiss Cirrus HDOCT 4000), using the macular cube scan, showed a hyperreflective area between RPE and neuroepithelium layer and discontinuous signal of corresponding RPE layer. The existence of foveal subretinal fluid was also revealed by OCT(Figure 1A). Fluorescein angiography images (Carl Zeiss VISUCAM 500) presented a well-defined hyper-fluorescent area in the early phase and diffuse leakage in the late phase(Figure 2A, 2B).The final diagnosis was idiopathic CNV(Type 2) in the right eye and the patient was referred to retinal specialists where he received an intravitreal ranibizumab (IVR)injection one week after diagnosis.
Patient came back for follow-up 4mo post-surgery. BCVA in the right eye was improved to 20/50 and remained 20/20 in the left eye. The color fundus photography indicated the absorption of subretinal hemorrhage in the macula and this was further confirmed by OCT wherein only a small pigment epithelial detachment (PED) existed in the previous hyperreflective area (Figure 1B). Fluorescein angiography showed no remarkable difference between early and late phase (Figure 2C, 2D).
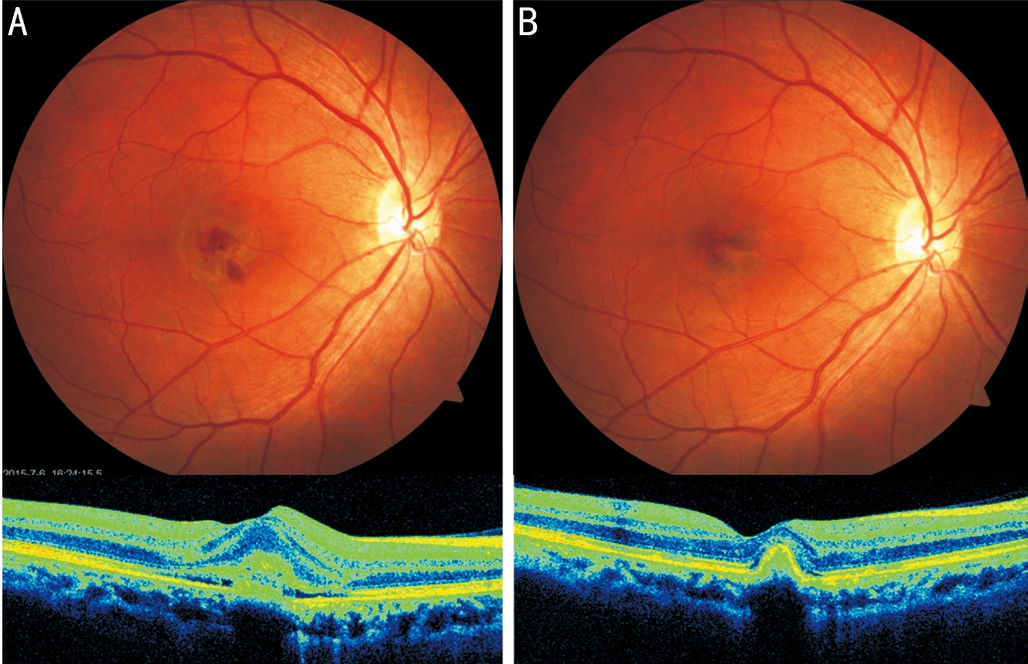
Figure 1 Color fundus images and OCT of this patient before and 4mo after IVB treatment for idiopathic CNV A: Fundus photograph and OCT at baseline. Note the hyper-reflective area between RPE and neuroepithelium layer on OCT; B: Four months after IVB treatment.Note the absorption of subretinal hemorrhage in the macula.
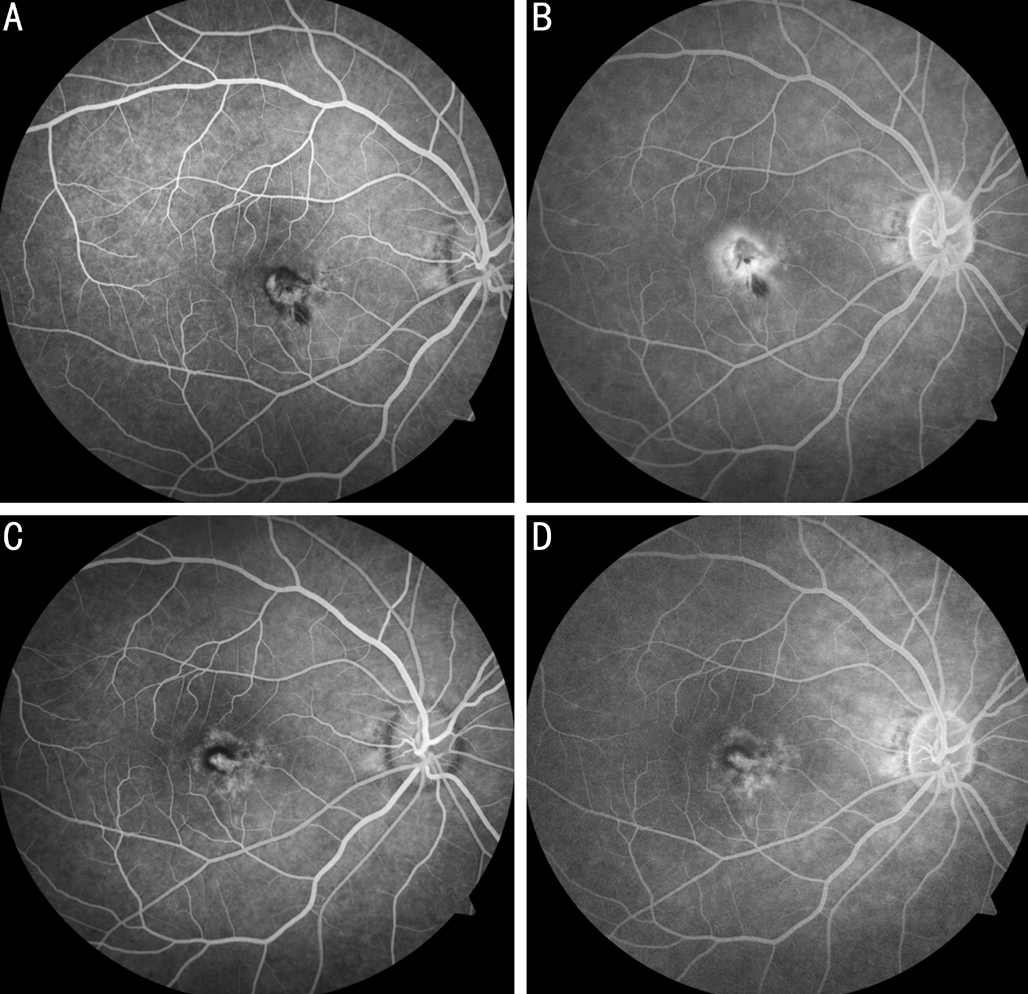
Figure 2 Fluorescein angiography before and 4mo after IVB treatment for idiopathic CNV A: Early phase at baseline. Note the well-defined hyper- fluorescent area; B: Late phase of fluorescein angiography showing the diffuse leakage in the macula; C: Early phase at 4mo; D: No leakage in the late phase after IVB treatment.
MSI (RHA; Annidis Corp., Canada), a new imaging method in diagnosing fundus diseases, was performed to observe the RPE changes before and after anti-VEGF treatment.The short-wavelength images (MSI-580 nm; Figure 3A)gave better visibility of subretinal hemorrhage absorption in the macula compared to color fundus photography. As longer MSI wavelengths penetrate deeper into the retina,the pigment stacking close to the fibrotic CNV lesion was revealed from postsurgical images, especially with the nearinfrared wavelength (MSI-780 nm; Figure 3B). Interestingly,we found in the case that MSI scan in near-infrared spectrum clearly evidenced a bull’s-eye pattern of RPE atrophy that was particularly prominent at the wavelength of 850 nm (MSI-850 nm; Figure 3C). Also, we noticed that the subretinal hemorrhage in the baseline didn’t block the MSI view of deeper retinal structures, because the MSI longer wavelengths have the ability to penetrate through the obstructions from the inner retina. Meanwhile, choroidal oxy-deoxy map, used to detect variations in the distribution of choroidal blood,indicated decreased perfusion (dark in appearance) around the macula after IVR injection (Figure 4). This was most likely caused by loss of RPE and choriocapillaris. This patient required close follow-up over the next 6mo and would be reexamined by MSI and other modalities to evaluate the ongoing RPE atrophy.
DISCUSSION
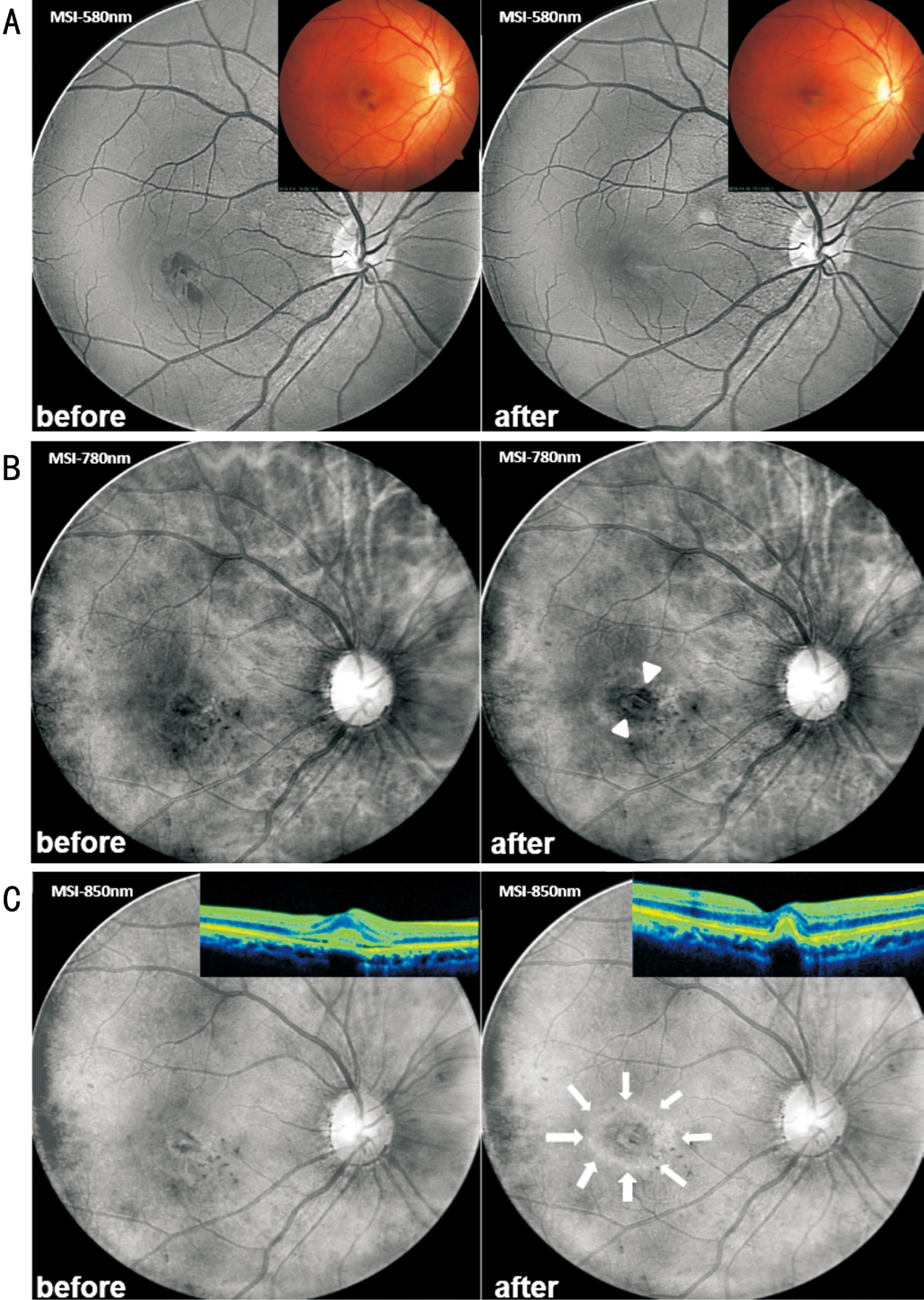
Figure 3 MSI before and 4mo after IVB treatment for idiopathic CNV A: MSI-580 nm clearly showing the absorption of subretinal hemorrhage in the macula. Note better visualization by MSI than color fundus images. B: MSI-780 nm demonstrating the pigment stacking close to the fibrotic CNV lesion after IVB treatment (white arrowheads). C: MSI-850 nm representing a bull’s-eye pattern of RPE atrophy (bright circle in appearance pointed by white arrows)compared with the image at the baseline. Note the invisibility of this RPE change from OCT scans.
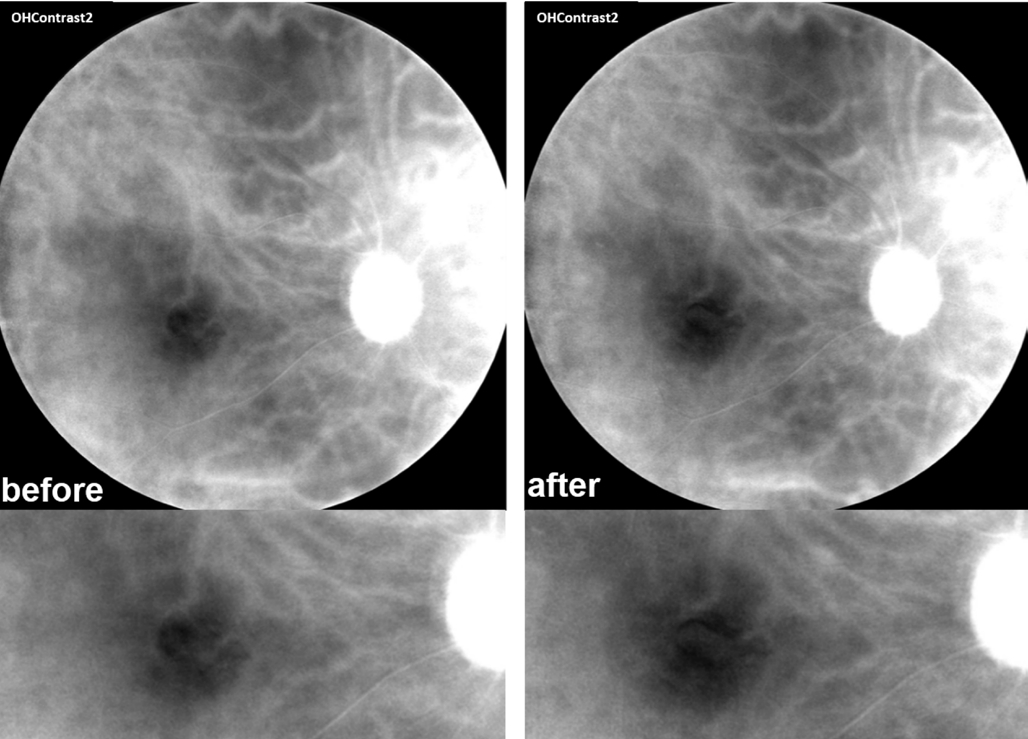
Figure 4 Choroidal oxy-deoxy map indicating enlarged dark area in the macula after IVB treatment.
RPE is responsible for many retinal functions and is diseased in many conditions, frequently observed in patients with AMD and CNV. The diseased RPE can lead to geographic atrophy, pigment epithelial mottling, drusen or PED[8].These RPE changes can be exacerbated by anti-VEGF treatment and are associated with the amount of anti-VEGF injections that patients receive[6]. Choriocapillaris may also be vulnerable during the treatment because of the reduced RPE-produced VEGF which plays an essential role in maintaining normal functions of subretinal vasculature[7]. Recent studies demonstrated that in mice, the absence of VEGFa and VEGF isoforms could lead to the development of focal choroidal atrophy and RPE attenuation and loss, in line with the results of clinical studies[9].
The earliest method to observe RPE changes was color fundus photography. With the development of more advanced imaging modalities such as short wavelength fundus auto fluorescence(FAF), near-infrared reflectance (NIR), and spectral domain OCT (SD-OCT), the boundaries of atrophic areas have been more precisely identified and researchers can utilize these tools to determine the rate of progression of geographic atrophy[10-11].However, due to the low resolution and noisy images provided by FAF (Figure 5) and NIR, more sensitive technology is required for early detection of RPE atrophy and quantifiable CNV progression. OCT also has limitations with respect to demonstrating subtle alternations in RPE morphology, because normal RPE and adjacent atrophic RPE or melanin disruption share similar reflectivity in OCT (Figure 6)[12].
MSI employs up to 12 monochromatic, discrete light-emitting diodes (LED) ranging from 520 to 940 nm in wavelength as the illuminants, and is used to create discrete spectral slices of fundus from the internal limiting membrane to the choroid, particularly of the RPE[13]. The in vivo observation of pathological changes to RPE, especially those related to melanin clumping, stacking or migration, was hard to image with traditional modalities. Given the possibility to use range of MSI wavelengths, researchers could now observe melanin in the RPE directly and early detect RPE disruption noninvasively. Also, we demonstrated here in this case that MSI is able to view deeper retinal structure through obstructions from inner layer[14]. MSI with broad range of wavelengths and high resolution images (2000×2000 pixels per wavelength),appears to be a promising tool to more precisely quantify the atrophic areas at baseline and after every injection of anti-VEGF drugs (Figure 5). Although anti-VEGF drugs are mainstream treatment for idiopathic CNV and exudative AMD, it’s necessary for clinicians to notice the progression of atrophy in time and adjust the monthly treatment to pro re nata treatment which is less likely to develop geographic atrophy[7].In addition, RPE atrophy is more frequently observed in exudative AMD than idiopathic CNV due to more injections.It is very important to inform patients that prolonged treatment may increase the risk of geographic atrophy and could greatly affect the prognosis[15]. Although we have no alternatives for anti-VEGF treatment today, doctors should be aware of all major complications especially for patients who already have RPE atrophy at baseline.
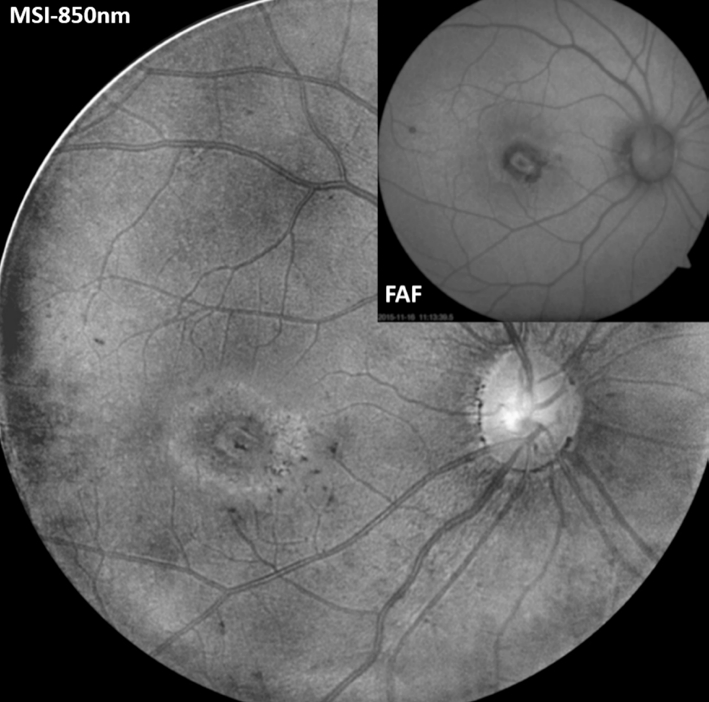
Figure 5 The comparison of images from MSI-850 nm and FAF(Carl Zeiss VISUCAM 500) 4mo after IVB treatment Note the clear boundary of RPE atrophic area in MSI image.
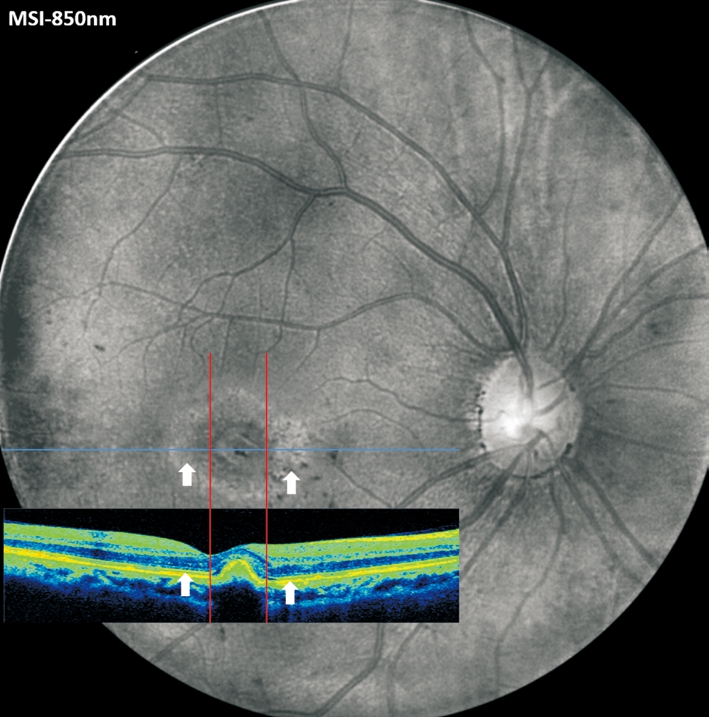
Figure 6 The aligned images from MSI-850 nm and OCT scan 4mo after IVB treatment Note the invisibility of corresponding RPE changes on OCT.
In conclusion, MSI can aid detection of early secondary RPE atrophy after anti-VEGF treatment of idiopathic CNV patients.To date, by clearly showing the melanin disruption and the boundary of atrophic area, MSI is a promising tool to monitor the RPE changes and may help regulating the amount of anti-VEGF injections to a more beneficial way while causing less damage to RPE.
ACKNOWLEDGEMENTS
Conflicts of Interest: Zhang J, None; Chen YB, None; Yu ZK, None; Liu L, None.
REFERENCES
1 Ho AC, Yannuzzi LA, Pisicano K, DeRosa J. The natural history of idiopathic subfoveal choroidal neovascularization. Ophthalmology 1995;102(5):782-789.
2 Sudhalkar A, Yogi R, Chhablani J. Anti-vascular endothelial growth factor therapy for naive idiopathic choroidal neovascularization: a comparative study. Retina 2015;35(7):1368-1374.
3 Kang HM, Koh HJ. Intravitreal anti-vascular endothelial growth factor therapy versus photodynamic therapy for idiopathic choroidal neovascularization. Am J Ophthalmol 2013;155(4):713-719.
4 Matsuo M, Honda S, Matsumiya W, Kusuhara S, Tsukahara Y, Negi A.Comparison between anti-vascular endothelial growth factor therapy and photodynamic therapy for myopic choroidal neovascularization. Eur J Ophthalmol 2012;22(2):210-215.
5 Coco RM, Sanabria MR, Hernandez AG, Fernandez Munoz M. Retinal pigment epithelium tears in age-related macular degeneration treated with antiangiogenic drugs: a controlled study with long follow-up.Ophthalmologica 2012;228(2):78-83.
6 Lois N, McBain V, Abdelkader E, Scott NW, Kumari R. Retinal pigment epithelial atrophy in patients with exudative age-related macular degeneration undergoing anti-vascular endothelial growth factor therapy.Retina 2013;33(1):13-22.
7 Young M, Chui L, Fallah N, Or C, Merkur AB, Kirker AW, Albiani DA, Forooghian F. Exacerbation of choroidal and retinal pigment epithelial atrophy after anti-vascular endothelial growth factor treatment in neovascular age-related macular degeneration. Retina 2014;34(7):1308-1315.
8 Punjabi OS, Huang J, Rodriguez L, Lyon AT, Jampol LM, Mirza RG.Imaging characteristics of neovascular pigment epithelial detachments and their response to anti-vascular endothelial growth factor therapy. Br J Ophthalmol 2013;97(8):1024-1031.
9 Kurihara T, Westenskow PD, Bravo S, Aguilar E, Friedlander M.Targeted deletion of VEGFa in adult mice induces vision loss. J Clin Invest 2012;122(11):4213-4217.
10 Holz FG, Bindewald-Wittich A, Fleckenstein M, Dreyhaupt J,Scholl HP, Schmitz-Valckenberg S; FAM-Study Group. Progression of geographic atrophy and impact of fundus auto fluorescence patterns in agerelated macular degeneration. Am J Ophthalmol 2007;143(3):463-472.
11 Yehoshua Z, Rosenfeld PJ, Gregori G, Feuer WJ, Falcao M, Lujan BJ,Puliafito C. Progression of geographic atrophy in age-related macular degeneration imaged with spectral domain optical coherence tomography.Ophthalmology 2011;118(4):679-686.
12 Domalpally A, Danis RP, Zhang B, Myers D, Kruse CN. Quality issues in interpretation of optical coherence tomograms in macular diseases.Retina 2009;29(6):775-781.
13 Zhang J, Yu Z, Liu L. Multimodality imaging in diagnosing polypoidal choroidal vasculopathy. Optom Vis Sci 2015;92(1):e21-26.
14 Dugel PU, Zimmer CN. Imaging of melanin disruption in age-related macular degeneration using multispectral imaging. Ophthalmic Surg Lasers Imaging Retina 2016;47(2):134-141.
15 Kim M, Kim ES, Seo KH, Yu SY, Kwak HW. Change of retinal pigment epithelial atrophy after anti-vascular endothelial growth factor treatment in exudative age-related macular degeneration. Indian J Ophthalmol 2016;64(6):427-433.