Early expression of PTX3 in Aspergillus fumigatus infected rat cornea
Jie Zhang1, Gui-Qiu Zhao1, Jing Qu2, Jing Lin1, Cheng-Ye Che1, Xue-Jiao Yang1
1Department of Ophthalmology, the Affiliated Hospital of Qingdao University, Qingdao 266003, Shandong Province,China
2Department of Administrative Of fice, the Affiliated Hospital of Qingdao University, Qingdao 266003, Shandong Province,China
INTRODUCTION
Fungal keratitis (FK) is a sight-threatening ocular infection,with high incidence in tropical, subtropical areas and developing countries. It accounts for 39%-65.2% of microbial keratitis. Trauma is the most common predisposing factor that happened in 42%-74.5% of patients[1-3]. Aspergillus fumigatus(A. fumigatus) was reported as one of the main etiologic agents of fungal keratitis[3-5]. The innate immune system is the first line of human to defend microbial invasion. This is initiated by the recognition of highly conservative consensus sequence in pathogens through different pattern recognition receptors(PRRs)[6-7]. The pentraxin family is an important member of pattern recognition receptors and composes the humoral arm of innate immunity[8-9]. The pentraxins, characterized by a pentraxin domain with pentraxin signature (HxCxS/TWxS),are divided into short and long[8]. Pentraxin 3 (PTX3) belongs to the long pentraxin family and is believed as the prototype of it. Besides immune cells, like mononuclear phagocytes[10-11],dendritic cells[12]and neutrophils[13]. PTX3 is also constitutively expressed by resident cells, such as epithelial cells[14]as well as endothelial cells[15]. Researches have demonstrated that PTX3 was significantly increased in various inflammatory disease[8,16-17], indicating an intimate relationship with the immune response. Recent studies have shown that PTX3 has a non-redundant role in the resistance against A. fumigatus infection[18]. Garlanda et al[18]found that PTX3 could bind A. fumigatus conidia but not A. fumigatus hyphae, and that this could be abolished by the presence of galactomannan. In that study, PTX3 was shown to facilitated the ingestion of conidia in macrophages[18], and PTX3-/- mice showed defective resistance to A. fumigatus, correlated with an increase in colonization[18]. In this study, we investigated the expression of PTX3 in cornea epithelium and explored the role of it during A. fumigatus invasion.
MATERIALS AND METHODS
ReagentsPrimers were designed and synthesized by TaKaRa(Dalian, Liaoning Province, China). RNAiso Plus, reverse transcriptase polymerase chain reaction (RT-PCR) kits with gDNA Eraser (Perfect Real Time) and SYBR Premix ExTaqTM (Tli RNaseH Plus) were purchased from TaKaRa (Dalian,Liaoning Province, China). Polyclonal rabbit anti-rat PTX3 was obtained from Enzo Life Sciences (Enzo Life Sciences,Switzerland). Polyclonal rabbit anti-rat GAPDH was obtained from CWBIO (CWBIO, Beijing, China). Phenylmethylsulfonyl fluoride (PMSF) and cell lysis buffer (RIPA) were purchased from Solarbio (Beijing, China). ECL Western blotting detection Reagent was purchased from Beyotime (Shanghai, China).
Table 1 Visual scoring for fungal keratitis rat models
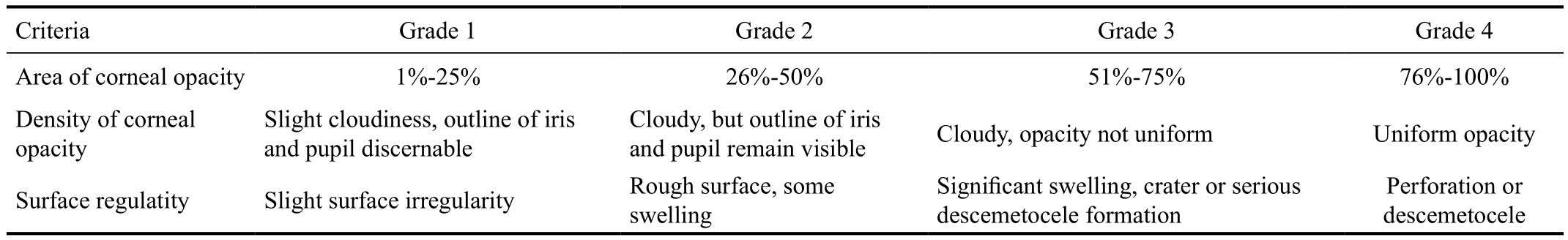
Criteria Grade 1 Grade 2 Grade 3 Grade 4 Area of corneal opacity 1%-25% 26%-50% 51%-75% 76%-100%Density of corneal opacity Slight cloudiness, outline of iris and pupil discernable Cloudy, but outline of iris and pupil remain visible Cloudy, opacity not uniform Uniform opacity Surface regulatity Slight surface irregularity Rough surface, some swelling Significant swelling, crater or serious descemetocele formation Perforation or descemetocele
Table 2 Prime list used for qRT-PCR
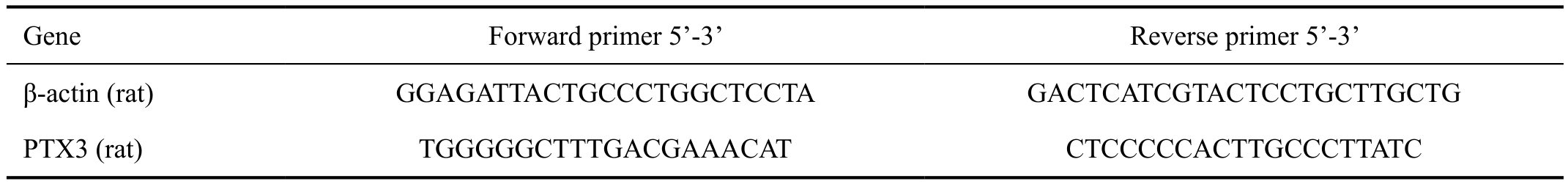
Gene Forward primer 5’-3’ Reverse primer 5’-3’β-actin (rat) GGAGATTACTGCCCTGGCTCCTA GACTCATCGTACTCCTGCTTGCTG PTX3 (rat) TGGGGGCTTTGACGAAACAT CTCCCCCACTTGCCCTTATC
Preparation of Aspergillus Fumigatus AntigensA. fumigatus strains (NO3. 0772) was purchased from China General Microbiological Culture Collection Center. A. fumigatus conidia was inoculated and grown on Sabouraud media at 28℃ for 5-7d. Then the conidia were harvested by centrifugation and washed with sterile phosphate buffer saline (PBS) for three times.
AnimalsWistar rats (both male and female) were purchased from Qingdao Institute of Drug Control (Qingdao, Shandong Province, China). All the rats were weighed between 200-300 g and allowed to acclimatize to the laboratory conditions for 3d.Corneal diseases were excluded with slit-lamp examination before animal models were made. All animal procedures complied with the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research.
Animal Models of Fungal KeratitisFifty Wistar rats were randomly divided into the following groups: 5 rats for control group (10 corneas) and 45 rats for experiment group. The right eyes of 45 rats were chosen for FK group (45 corneas)while the left eyes were chosen for Sham group (45 corneas).Levo floxacin eyedrop was given to eyes three times per day.Chloral hydrate 10% 3 mL/kg for intraperitoneal injection and 0.4% oxybuprocaine hydrochloride eyedrop for surface anesthesia before animal models were made. Conjunctival sac was cleaned with 0.5% Yasuji iodine and washed with sterile water. Central epithelium of the right cornea was scraped about 2 mm in diameter and then A. fumigatus conidia antigen(about 3-4 mm) was given. After covering with contact lens,the eyelid was sutured with 5-0 silk. The left eye was treated as the right eye except for giving A. fumigatus conidia antigen.The control group was not given any scrape of treatment.Totally 45 rats were executed at 8, 16 and 24h randomly after the experimental model being established. The diagnoses of FK models were confirmed by corneal scrapings staining or confocal microscopy. The eyeball was taken under sterile conditions. Cornea was divided into two parts preserving in-80℃: one half was used for quantitative RT-PCR and the other half was for Western blot analysis.
Evaluation of InflammationSeverity of FK in animal models was scored visually as well as the aid of sit lamp before executed[19]. A grade of 0 to 4 was assigned to each of the following criteria: area of opacity, density of opacity and surface regularity. The normal, unscarified cornea was scored by 0 in each category and had a summation score of 0. A total score of 5 or less was considered mild, 6 to 9 was considered moderate and more than 9 was considered severe. All the evaluations were performed by the same person (Table 1).
Quantitative Reverse Transcription-polymerase Chain ReactionTotal RNA was extracted from cornea samples using RNAiso plus reagent (TaKaRa, Dalian, Liaoning Province,China) according to the manufacturer’s protocol and quantified by spectrophotometry. Complementary DNA (cDNA) was generated by reverse transcription with 2 μg total RNA and then 2 μL cDNA was used for quantitative PCR with SYBR Green and specific primers. The reaction parameters were performed as follows: 95℃ for 30s, followed by 40 cycles of 95℃ for 5s, 60℃ for 30s, followed by a final stage of 95℃for 15s, 60℃ for 30s, 95℃ for 15s. The primers used were as follows. Quantification was performed using the 2-Δ Δ Ctmethod.Each experiment was repeated at least for three separate times(Table 2).
Western Blot AnalysisTotal proteins were extracted from cornea samples using RIPA lysis buffer and PMSF (100:1)mixture for 2h. After centrifuged at 12 000 rpm for 15min, the supernatants were quantified by bicinchoninic acid assay and denatured with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample loading buffer (5×) in boiled water for 10min. Proteins (50 μg/well) were separated by 12% SDS-PAGE in Tris/glycine/SDS buffer and transferred to polyvinylidene fluoride membranes (Millipore, billerica,MA, USA). The membranes were blocked with Western blocking Buffer at 37℃ for 2h before incubating with PTX3 or GAPDH primary antibody at 4℃ overnight. Then membranes were incubated with secondary antibody at 37℃ for 1h and detected with BeyoECL Plus (Beyotime, Shanghai, China).
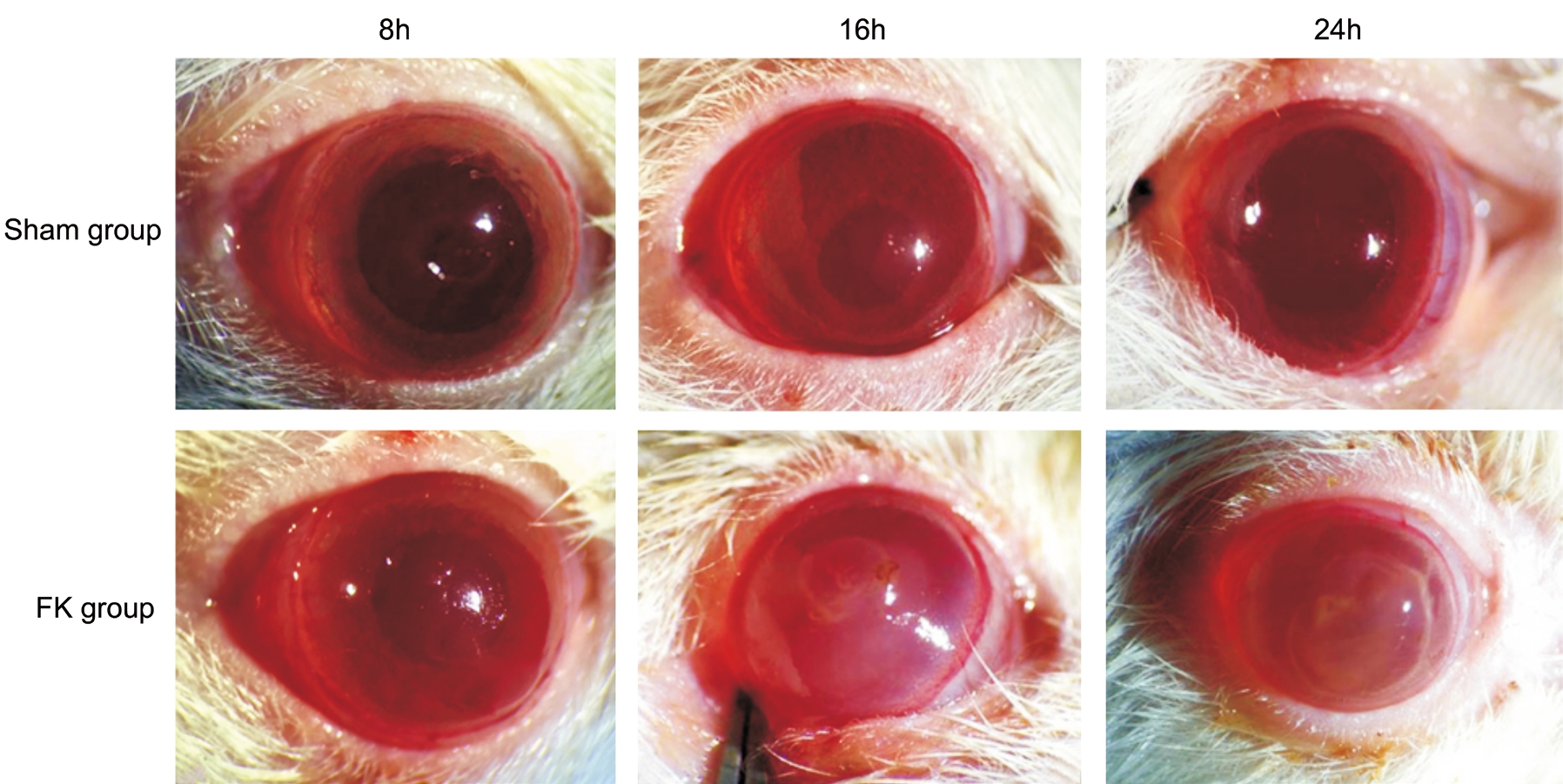
Figure 1 Progression of FK in rat models Inflammation was aggravated gradually with time in the FK group while relieved in the Sham group.
Statistical AnalysisAll the data were presented as mean±SD.Data analysis was performed by one-way analysis of variance and further pairwise comparisons were made by using SPSS17.0 software (SPSS, Chicago, IL, USA). Data were considered significant at P<0.05.
RESULTS
Progression of Fungal Keratitis in Rat ModelsIn FK group, different manifestations were found at three time points after infection. At 8h post infection, corneal edema was found around the defect area with a rough surface in corneal stroma.There was even a slight enlargement of the defect size in some cases. However, no obvious infection was found in all rats. At 16 and 24h post infection, beside corneal edema, a significant defect enlargement in corneal epithelium as well as tissue necrosis in epithelium and stroma could be seen.Compared with 16h, corneal ulcer was aggravated in 24h. On the contrary, defect in corneal epithelium recovered gradually.The defect was covered by fragile epithelium at 16h and nearly transparent at 24h (Figure 1).
Inflammation Evaluation of Fungal KeratitisCorneal in flammation scores in FK group were higher than that in the Sham group. Scores increased gradually after infection in the FK group. Compared with 8h group, scores in 16h as well as 24h group were significantly higher and the differences were significant (P<0.001). However, the difference between the 16 and 24h group was not significant (P>0.05). Meanwhile,scores in Sham group decreased along with the recovery of corneal defect. Compared with 8h, differences at 16 and 24h were significant in Sham group (P<0.05, P<0.001 respectively)(Figure 2).
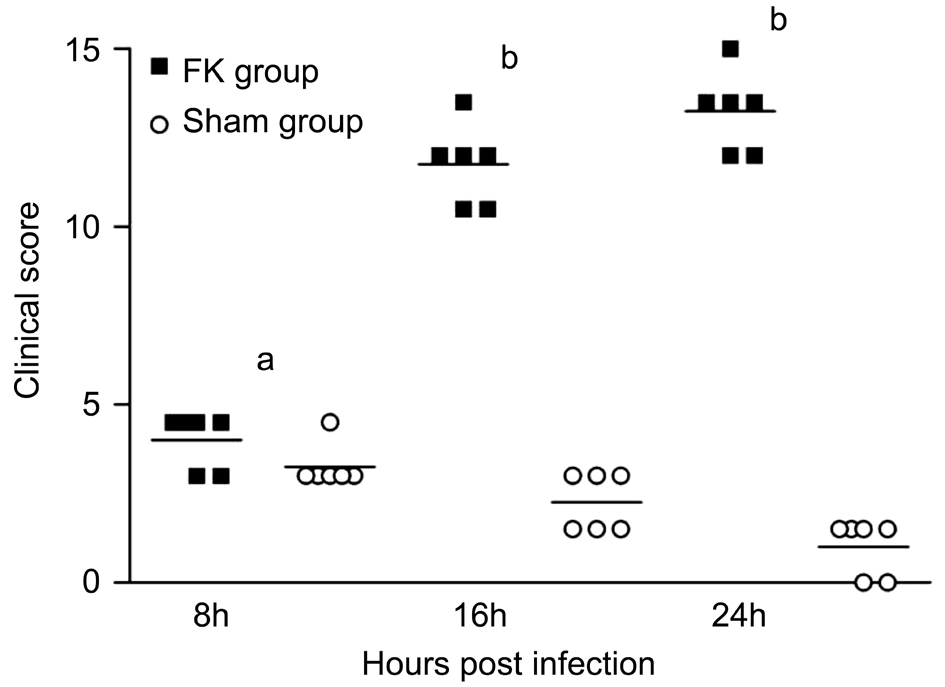
Figure 2 Inflammation at different time points in keratitis rat models Scores gradually increased over time in FK group while they decreased over time in Sham group (P<0.001). The difference between FK group and Sham group was significant at each time point post infection (aP<0.05,bP<0.001).
Expression of Pentraxin 3 mRNAAs shown in Figure 3, PTX3 expression was low in normal rat cornea. When infected by A. fumigatus, PTX3 mRNA began to increase at 8h, peaked at 16h and declined at 24h. Compared with 0h,the differences were significant at each time point in FK group(P<0.05 at 8 and 24h, P<0.001 at 16h). Furthermore, differences between 8 and 16h, 16 and 24h were also significant (P<0.05).However, compared with 0h, no significant elevation was found at 8, 16 and 24h in the Sham group (P>0.05). Differences between 8, 16 and 24h in Sham group were also not significant(P>0.05). Meanwhile, differences between FK group and Sham group were significant at each time point (P<0.05 at 8 and 24h, P<0.001 at 16h).
Expression of Pentraxin 3 Protein A constant change of PTX3 protein expression is shown in Figure 4. The change in PTX3 protein expression is consistent with changes in the mRNA expression at the different time points. PTX3 protein expression increased significantly at 8h and peaked at 16h after A. fumigatus infection (P<0.05, P<0.001 respectively).The difference of PTX3 protein level between 8 and 16h was also significant (P<0.05). Although a slight decline was seen at 24h compared with 16h, the difference was not significant(P>0.05).
DISCUSSION
The immune system composed by innate and adaptive immunity plays a vital role in resisting and eliminating various invasion microorganisms. The innate immune response was first activated after the recognition of pathogens by different PRRs. As a soluble PRR, PTX3 participates in the process against pathogen infection. A. fumigatus is one of the selected pathogen that could be recognized by PTX3[18]and is made locally at the in flammatory sites[9]. However, the relationship between PTX3 and FK is not fully understand.Our previous study in immortalized human corneal epithelial cells (HCECs) found that PTX3 played a proin flammatory role in the pathogenesis of FK[20]. However, the immune system is a complex network, which means the immune results in vivo maybe different from that in vitro. To further explore the impact of PTX3 during fungi invasion, we resorted to animal models. Rat is one of the common animals used for keratitis models[21-22]and we have successfully established rat FK model before[23]. On this basis, we investigated the expression of PTX3 in A. fumigatus infected rat model in this study. FK was reproduced in the rat model. As time prolonged, the clinical score of keratitis got higher while corneal infection progressed.This is according with the development of the disease in clinic.In line with previous findings on the induction of PTX3 by fungi[18,24], PTX3 expression in corneal epithelium was also up-regulated after A. fumigatus infection both from mRNA and protein level. PTX3 expression was almost correlated with the severity of keratitis on the whole in the models, and this phenomenon was also found in human clinical samples.However, the clinical score of Sham group without fungi infection decreased at the same time. We also found that PTX3 was expressed in cornea epithelium and kept at a low level under normal condition. No obvious increase of PTX3 at both mRNA and protein level was found in the Sham group.The clinical manifestation of rat cornea is consistent with our previous finding in vitro that PTX3 plays its role against fungi invasion by amplifying the inflammatory response. These results indicate that PTX3 is a key component in corneal antifungi immune response and may be a marker of corneal injury.The diagnostic value or as a marker of PTX3 has been proved in both in infectious and non-infectious diseases[25-28]. Compared with 16h, an aggravated corneal inflammation was found in FK rat models at 24h post A. fumigatus infection. Meanwhile,expression of PTX3 was decreased at 24h both in mRNA and protein levels comparing with 16h. Although there was no significant difference between 16 and 24h in inflammation score and PTX3 expression, the aggravated corneal ulcer in 24h maybe a comprehensive result of the activations of other PRRs, such as dendritic cell-associated C-type lectin-1(Dectin-1)[29], mannose receptor (MR)[30]and triggering receptor expressed on myeloid cells-1 (TREM-1)[31]. Levels of these PRRs continued to increase at 24h and activations of them up-regulated the expression of proin flammatory cytokines like tumor necrosis factor-α (TNF-α) and interleukin-1β(IL-1β)[30,32-33].
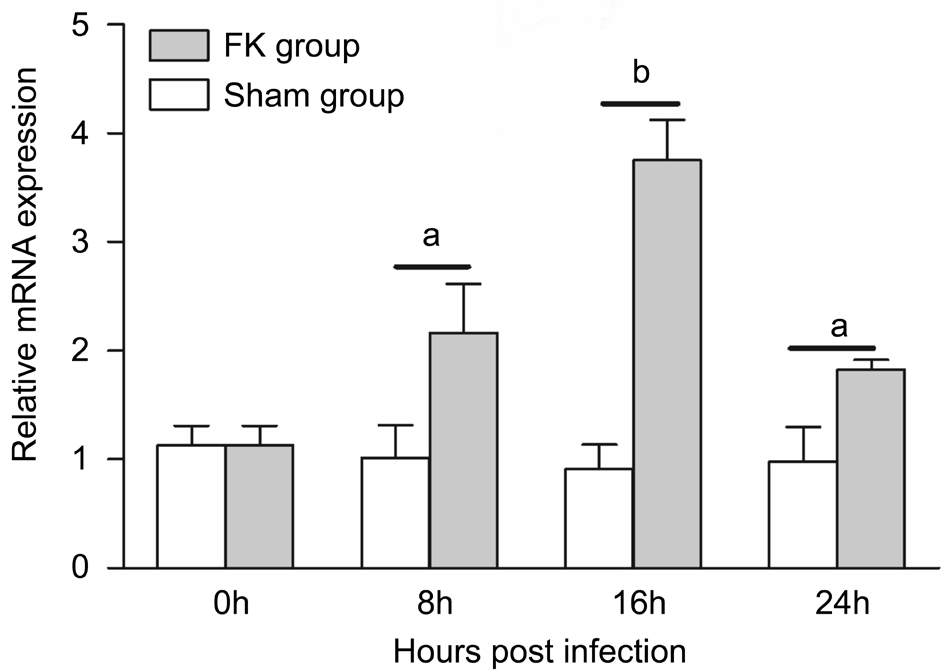
Figure 3 Relative mRNA expression of PTX3 in rat cornea PTX3 mRNA was evaluated at 8, 16 and 24h after A. fumigatus infection.A gradually increase of PTX3 mRNA was detected from 0 to 16h.Compared with Sham group, the difference was significant at each time point after infection (aP<0.05,bP<0.001). The difference between 8 and 16h was significant (P<0.05).
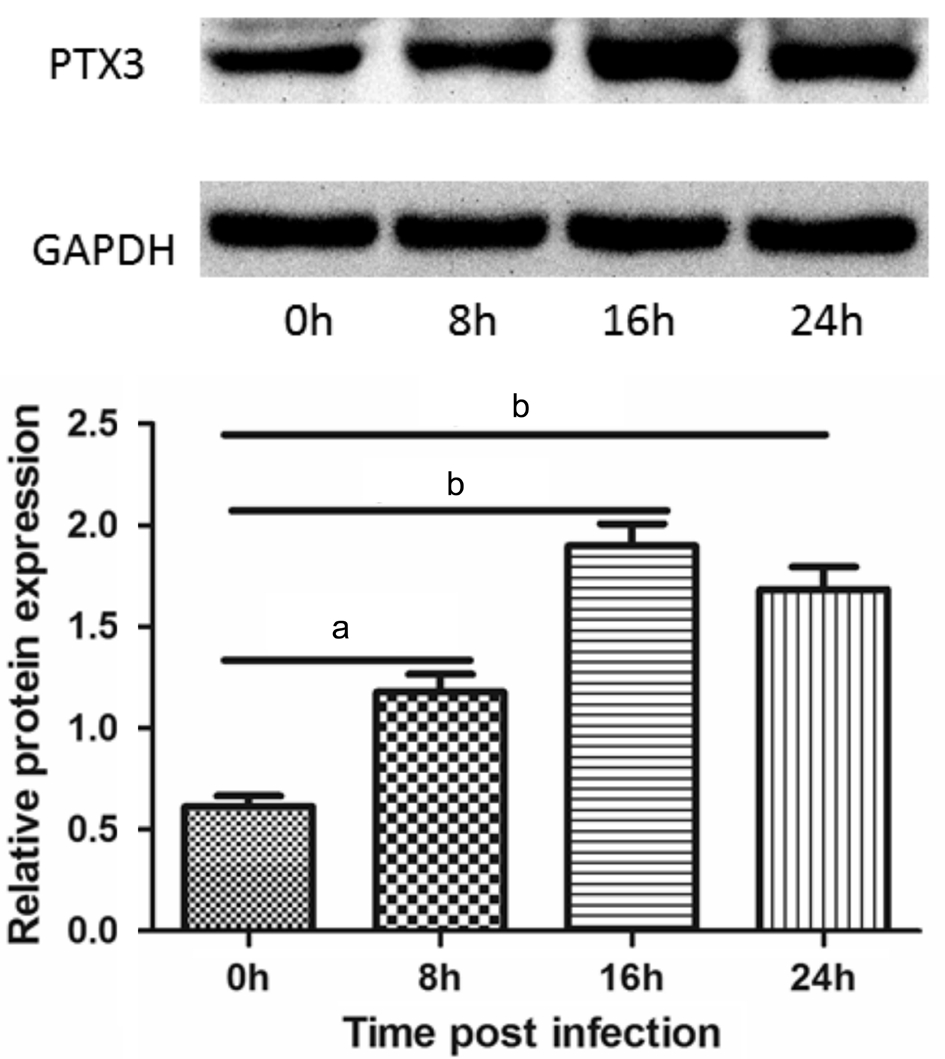
Figure 4 Relative protein expression of PTX3 in rat cornea Western blot confirmed an induction of PTX3 by A. fumigatus at 8,16 and 24h. PTX3 expression was increased after infection (aP<0.05,bP<0.001). Compared with 8h group, PTX3 was further up-regulated at 16h (P<0.05).
As a long pentraxin, PTX3 has a common structural motif with the short pentraxins. C-reactive protein (CRP) and serum amyloid P component (SAP) are the two classic short pentraxins and CRP is known as an early marker of in flammation[8]. In our study, we also found an early induction of PTX3 by A. fumigatus in infected cornea. PTX3 expression was visible at 8h and peaked at 16h after A. fumigatus infection, indicating an early role of PTX3 during cornea antifungi immune response. The early rapid expression of PTX3 was similar to that in HCECs[20]. The occurrence of FK was initiated from the invasion of fungi spores in damaged cornea tissues. PTX3 plays an important role in the recognition and ingestion of A. fumigatus spores, so the immediate upregulation of PTX3 maybe count for the elimination of invading spores. Similar results were proved by other previous studies. PTX3 expression was up-regulated at 1h with a peak between 2 and 6h in IL-1β stimulated human umbilical vein endothelial cells[15]. At the presence of A. fumigatus stimulation, a rapid detectable immunoreactivity of PTX3 in macrophages was also demonstrated at 1h[18]. Reasons that PTX3 expression in vivo was prolonged compare to in vitro results may be due to the complexity of the immune system.It is known that the innate immune response occurred with 4 to 96h after infection[34], so PTX3 participated in the innate immune process against A. fumigatus invasion.
The protective role of PTX3 has been proved both by in vivo and in vitro experiments. Compared with A. fumigatus conidia alone, the production of monocyte chemotactic protein-1(MCP-1 or CCL-2) in mononuclear phagocytes is up-regulated by conidia and exogenous PTX3, resulting in the migration of monocytes and macrophages to infection sites. PTX3 also restores the ability of PTX3-/- dendritic cells to respond to A. fumigatus conidia[18]. Other research with bone marrow transplantation mice also found that PTX3 administration cured mice with A. fumigatus infection and increased resistance to reinfection in the lung[35]. These findings imply that up-regulation of PTX3 by corneal epithelial cells may protect cornea from A. fumigatus infection by regulation the ability of immune cells and corneal resistance. As Erreni et al[36]concluded, PTX3 may act as a sensor of tissue injury.In conclusion, we provided experimental evidence that PTX3 may be induced by A. fumigatus to protect cornea from infection. As a member of corneal early innate immunity,PTX3 contributes to the inflammatory response in FK rat models.
ACKNOWLEDGEMENTS
Foundations:Supported by National Natural Science Foundation of China (No.81170825; No.81470609);Specialized Research Fund for the Doctoral Program of Higher Education (No.20123706110003); the Youth Natural Science Foundation of Shandong Province (No.ZR2013HQ007);the Key Project of Natural Science Foundation of Shandong Province (No.ZR2012HZ001).
Conflicts of Interest: Zhang J,None;Zhao GQ,None;Qu J,None;Lin J,None;Che CY,None;Yang XJ,None.
REFERENCES
1 Nath R, Baruah S, Saikia L, Devi B, Borthakur AK, Mahanta J. Mycotic corneal ulcers in upper Assam.Indian J Ophthalmol2011;59(5):367-371.
2 Chowdhary A, Singh K. Spectrum of fungal keratitis in north India.Cornea2005;24(1):8-15.
3 Xie L, Zhong W, Shi W, Sun S. Spectrum of fungal keratitis in north China.Ophthalmology2006;113(11):1943-1948.
4 Tilak R, Gupta M, Chandra A, Prakash P, Banerjee T, Maurya O. Fungal keratitis in north India; Spectrum and diagnosis by Calco fluor white stain.Indian J Med Microbiol2015;33(3):462-463.
5 Srinivasan M. Fungal keratitis.Curr Opin Ophthalmol2004;15(4):321-327.6 Li N, Zhao GQ. Mechanism of the immune response to keratomycosis.Zhonghua Yan Ke Za Zhi2011;47(4):378-381.
7 Romani L. Immunity to fungal infections.Nat Rev Immunol2004;4(1):1-23.
8 Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition,and female fertility.Annu Rev Immunol2005;23:337-366.
9 Bottazzi B, Garlanda C, Salvatori G, Jeannin P, Manfredi A, Mantovani A. Pentraxins as a key component of innate immunity.Curr Opin Immunol2006;18(1):10-15.
10 Vouret-Craviari V, Matteucci C, Peri G, Poli G, Introna M, Mantovani A. Expression of a long pentraxin, PTX3, by monocytes exposed to the mycobacterial cell wall component lipoarabinomannan.Infect Immun1997;65(4):1345-1350.
11 Alles VV, Bottazzi B, Peri G, Golay J, Introna M, Mantovani A.Inducible expression of PTX3, a new member of the pentraxin family, in human mononuclear phagocytes.Blood1994;84(10):3483-3493.
12 Doni A, Peri G, Chieppa M, Allavena P, Pasqualini F, Vago L, Romani L, Garlanda C, Mantovani A. Production of the soluble pattern recognition receptor PTX3 by myeloid, but not plasmacytoid, dendritic cells.Eur J Immunol2003;33(10):2886-2893.
13 Jaillon S, Peri G, Delneste Y,et al. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps.J Exp Med2007;204(4):793-804.
14 Han B, Mura M, Andrade CF, Okutani D, Lodyga M, dos Santos CC, Keshavjee S, Matthay M, Liu M. TNFalpha-induced long pentraxin PTX3 expression in human lung epithelial cells via JNK.J Immunol2005;175(12):8303-8311.
15 Breviario F, d’Aniello EM, Golay J, Peri G, Bottazzi B, Bairoch A, Saccone S, Marzella R, Predazzi V, Rocchi M. Interleukin-1-inducible genes in endothelial cells. Cloning of a new gene related to C-reactive protein and serum amyloid P component.J Biol Chem1992;267(31):22190-22197.
16 Kunes P, Holubcova Z, Kolackova M, Krejsek J. Pentraxin 3(PTX 3): an endogenous modulator of the in flammatory response.Mediators In flamm2012;2012:920517.
17 Wisniewski HG, Vilcek J. Cytokine-induced gene expression at the crossroads of innate immunity, inflammation and fertility: TSG-6 and PTX3/TSG-14.Cytokine Growth Factor Rev2004;15(2-3):129-146.
18 Garlanda C, Hirsch E, Bozza S, Salustri A, De Acetis M, Nota R,Maccagno A, Riva F, Bottazzi B, Peri G, Doni A, Vago L, Botto M,De Santis R, Carminati P, Siracusa G, Altruda F, Vecchi A, Romani L,Mantovani A. Non-redundant role of the long pentraxin PTX3 in antifungal innate immune response.Nature2002;420(6912):182-186.
19 Wu TG, Wilhelmus KR, Mitchell BM. Experimental keratomycosis in a mouse model.Invest Ophthalmol Vis Sci2003;44(1):210-216.
20 Zhang J, Zhao G, Lin J, Che C, Li C, Jiang N, Hu L, Wang Q. Role of PTX3 in corneal epithelial innate immunity against Aspergillus fumigatus infection.Exp Eye Res2018;167:152-162.
21 Abou Shousha M, Santos AR, Oechsler RA, Iovieno A, Maestre-Mesa J, Ruggeri M, Echegaray JJ, Dubovy SR, Perez VL, Miller D, Alfonso EC, Bajenaru ML. A novel rat contact lens model for Fusarium keratitis.Mol Vis2013;19:2596-2605.
22 Hu J, Hu Y, Chen S, Dong C, Zhang J, Li Y, Yang J, Han X, Zhu X,Xu G. Role of activated macrophages in experimental Fusarium solani keratitis.Exp Eye Res2014;129:57-65.
23 Zhang J, Zhao GQ, Qu J, Che CY, Lin J, Jiang N, Zhao H, Wang XJ.Expression of S100B during the innate immune of corneal epithelium against fungi invasion.Int J Ophthalmol2016;9(2):191-197
24 Cortez KJ, Lyman CA, Kottilil S, Kim HS, Roilides E, Yang J,Fullmer B, Lempicki R, Walsh TJ. Functional genomics of innate host defense molecules in normal human monocytes in response to Aspergillus fumigatus.Infect Immun2006;74(4):2353-2365.
25 Kabbani D, Bhaskaran A, Singer LG, Bhimji A, Rotstein C, Keshavjee S, Liles WC, Husain S. Pentraxin 3 levels in bronchoalveolar lavage fluid of lung transplant recipients with invasive aspergillosis.J Heart Lung Transplant2017;36(9):973-979.
26 Hamed S, Behnes M, Pauly D, Lepiorz D, Barre M, Becher T, Lang S, Akin I, Borggrefe M, Bertsch T, Hoffmann U. Diagnostic value of Pentraxin-3 in patients with sepsis and septic shock in accordance with latest sepsis-3 definitions.BMC Infect Dis2017;17(1):554.
27 Guo T, Huang L, Liu C, Shan S, Li Q, Ke L, Cheng B. The clinical value of in flammatory biomarkers in coronary artery disease: PTX3 as a new in flammatory marker.Exp Gerontol2017;97:64-67.
28 Sharma A, Khan R, Gupta N, Sharma A, Zaheer MS, Abbas M, Khan SA. Acute phase reactant, pentraxin 3, as a novel marker for the diagnosis of rheumatoid arthritis.Clin Chim Acta2018;480:65-70.
29 Che CY, Li C, Gao A, Lin J, Zhang LL, Xu Q, Wang Q, Zhao GQ.Dectin-1 expression at early period of Aspergillus fumigatus infection in rat’s corneal epithelium.Int J Ophthalmol2013;6(1):30-33.
30 Wang Q, Zhao G, Lin J, Li C, Jiang N, Xu Q, Wang Q, Zhang J. Role of the mannose receptor during Aspergillus fumigatus infection and interaction with Dectin-1 in corneal epithelial cells.Cornea2016;35(2):267-273.
31 Hu LT, Du ZD, Zhao GQ, Qiu S, Jiang N, Lin J, Wang Q, Xu Q.TREM-1 expression in rat corneal epithelium with Aspergillus fumigatus infection.Int J Ophthalmol2015;8(2):222-227.
32 Xu Q, Zhao G, Lin J, Wang Q, Hu L, Jiang Z. Role of Dectin-1 in the innate immune response of rat corneal epithelial cells to Aspergillus fumigatus.BMC Ophthalmol2015;15:126.
33 Hu LT, Du ZD, Zhao GQ, Jiang N, Lin J, Wang Q, Xu Q, Cong L,Qiu S. Role of TREM-1 in response to Aspergillus fumigatus infection in corneal epithelial cells.Int Immunopharmacol2014;23(1):288-293.
34 Che CY, Li XJ, Jia WY, Li N, Xu Q, Lin J, Wang Q, Jiang N, Hu LT,Zhao GQ. Early expression of surfactant proteins D in Fusarium solani infected rat cornea.Int J Ophthalmol2012;5(3):297-300.
35 Gaziano R, Bozza S, Bellocchio S, Perruccio K, Montagnoli C, Pitzurra L, Salvatori G, De Santis R, Carminati P, Mantovani A, Romani L. Anti-Aspergillus fumigatus efficacy of pentraxin 3 alone and in combination with antifungals.Antimicrob Agents Chemother2004;48(11):4414-4421.
36 Erreni M, Manfredi AA, Garlanda C, Mantovani A, Rovere-Querini P. The long pentraxin PTX3: a prototypical sensor of tissue injury and a regulator of homeostasis.Immunol Rev2017;280(1):112-125.
Correspondence to:Gui-Qiu Zhao. Department of Ophthalmology, the Affiliated Hospital of Qingdao University,Qingdao 266003, Shandong Province, China. zhaoguiqiu_good@126.com
Received:2018-02-22 Accepted: 2018-04-12
Abstract ● AlM: To investigate the expression of pentraxin 3 (PTX3)in rat corneal epithelium at the early stage of Aspergillus fumigatus (A. fumigatus) infection.● METHODS: A total of 50 Wistar rats were randomly divided into control group, Sham group and experimental group (fungal keratitis group, FK group). The right eye was chosen as the experiment one and infected by A.fumigatus. Rats were executed at 8, 16 and 24h after the experimental models being established. Corneal epithelia were collected to assess the expression of PTX3 by quantitative reverse transcription polymerase chain reaction (qRT-PCR) and Western blot analysis.● RESULTS: Corneal inflammation scores increased as infection prolonged (P<0.05, P<0.001). PTX3 mRNA expression was low in normal and Sham group rats' corneas.Level of PTX3 mRNA in infected rat cornea was elevated at 8h and peaked at 16h. The difference was significant compared with control group (P<0.001). Western blot analysis also showed a significant increase of PTX3 protein in experimental group at 8h and peaked at 16h(P<0.001). The synchronous expression of control group and experimental group were also in significant difference(P<0.001).● CONCLUSlON: PTX3 exists in cornea epithelium and is significantly increased after A. fumigatus infection. PTX3 plays an important role in the early stage of cornea innate immunity against A. fumigatus.
● KEYWORDS:fungal keratitis; Aspergillus fumigates;pentraxin 3; innate immunity
DOl:10.18240/ijo.2018.07.02
Citation:Zhang J, Zhao GQ, Qu J, Lin J, Che CY, Yang XJ. Early expression of PTX3 in Aspergillus fumigatus infected rat cornea. Int J Ophthalmol 2018;11(7):1084-1089





