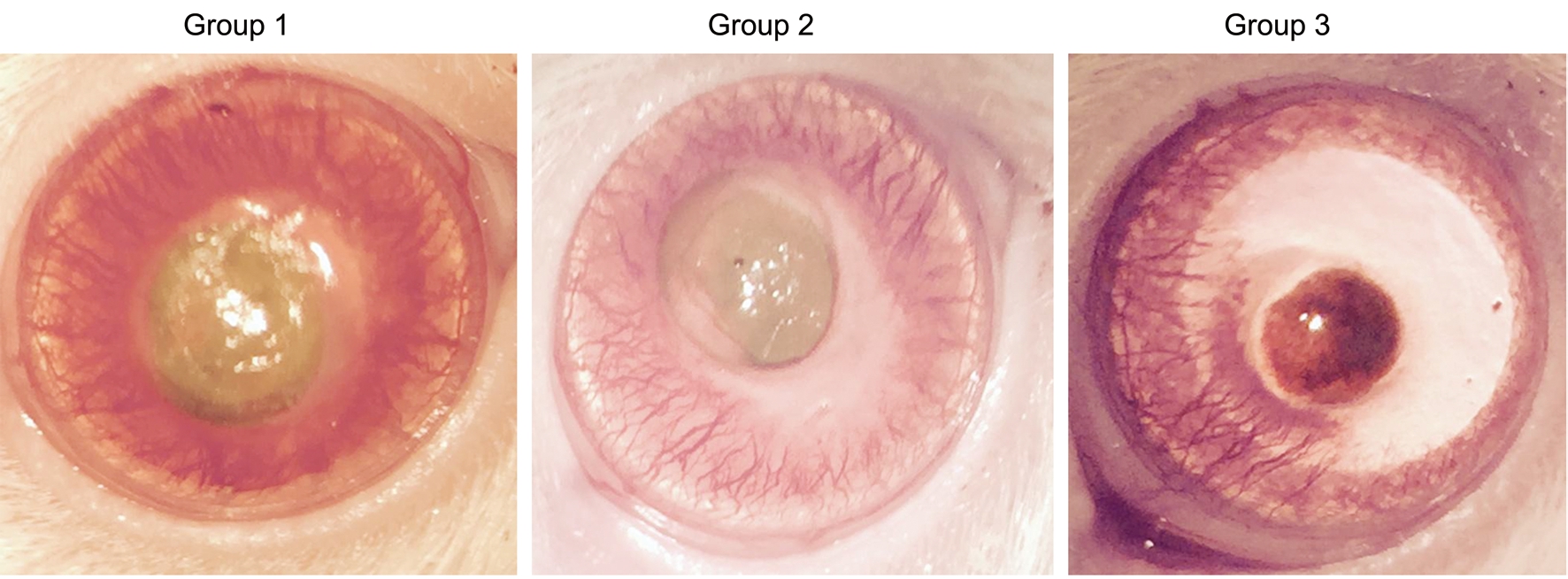
Figure 1 The degree of corneal neovascularization according to the groups.
Burak Ulas1, Rana Altan-Yaycioglu1, Nebil Bal2
1Department of Ophthalmology, Baskent University School of Medicine, Adana 01250, Turkey
2Department of Pathology, Baskent University School of Medicine, Adana 01250, Turkey
Cornea is a transparent connective tissue, as it’s clarity is related to the anatomic factors including avascularity of cornea, regular arrangement of the collagen fibers and balance between different layers and cellular components[1]. Corneal neovascularization is one of the reasons that may cause loss of optimal vision by damaging its transparency[2]. In flammatory,infectious, traumatic and degenerative disorders may lead to new vessel formations in cornea resulting in decreased visual acuity. Therefore, in related corneal disorders new treatment options are needed to maintain corneal avascularity[3].
The avascular character of cornea results from a balance between angiogenic and anti-angiogenic factors[3-4]. Vascular endothelial growth factor (VEGF) has shown to be associated with corneal neovascularization[3,5]. Expression of VEGF receptors(Flt-1 and Flk-1) was also increased on endothelial cells of newly formed vessels in the stroma of in flamed corneas[5]. The secreted VEGF-A growth factor peptides are generated by alternative splicing into some isoforms including VEGF115, VEGF121,VEGF165, VEGF189, and VEGF206[6]. The other VEGF members include VEGF-B, VEGF-C, and VEGF-D, which bind differentially to VEGF receptors[6]. VEGF members stimulate both angiogenesis and lymphangiogenesis[5-6].
Some medical therapies including steroids, cyclosporin A,methotrexate, thalidomide have been used in the treatment of corneal neovascularization[7-10]. However, currently there is no proven available therapeutic method for corneal neovascularization[7-11]. Bevacizumab is a recombinant humanized monoclonal IgG1 antibody that specifically binds to and neutralizes biological activity of VEGF-A and inhibits corneal neovascularization by binding all isoforms of VEGF-A[11-12]. VEGF inhibition has been shown to reduce corneal neovascularization[12-13]. In several studies both clinically and experimentally, bevacizumab has been used effectively for the treatment of corneal neovascularizaton[11-13].However, there is no treatment protocol agreed upon[12-13].
Hence in this study, we aimed to evaluate and compare the inhibitory effects of single-dose and multiple-dose subconjunctival bevacizumab injection on corneal neovascularization in a rat model.
Thirty eyes of thirty adult male Sprague-Dawley rats weighing 350-450 gram were used in this study. Approval of the experimental protocol was obtained from the Baskent University Medical School Research and Ethics Committee(DA 15/11). The animals were treated and maintained in accordance with the tenets of the Association for Research in Vision and Ophthalmology (ARVO) Statement for Use of Animals in Ophthalmic and Vision Research. The rats were placed in individual plastic cages in temperature-controlled room (22℃) where 12-12h light-dark circle was maintained.Proper food and water was provided for rats.
For general anesthesia ketamine hydrochloride (50 mg/kg body weight) was administered intraperitoneally. Following topical anesthesia by 0.5% proparacaine hydrochloride,corneal neovascularization was induced with silver nitrate cauterization. In all right eyes of 30 rats, central area of each cornea was cauterized by the same investigator (Ulas B)via pressing a stick coated with 75% silver nitrate and 25%potassium nitrate for 10s under operating microscope. Cornea and fornices were then rinsed with 10 mL of balanced salt solution to remove excess silver nitrate and potassium nitrate.The rats were randomly enrolled into three groups, and all rats received subconjunctival injection at the first day following cauterization. In Group 1 (control group, n=10), 0.05 mL 0.9%NaCl solution was injected. In Group 2 (single-dose group,n=10), 0.05 mL bevacizumab (1.25 mg) was injected. In Group 3 (multiple-dose group, n=10), four doses of 0.05 mL bevacizumab (1.25 mg) were injected subconjunctivally on the third, fifth and seventh day, in addition to the first day. The subconjunctival injections were performed 1-mm behind the limbus at the same time, of the day by a 30-gauge needle.
Slit-lamp examination of all rats was performed on the third and ninth day. On the third day, using similar method of Monzano et al[14], the extent of burn stimulus response was graded for each cornea via slit-lamp as: grade 0 (no blister, not raised above the corneal surface); grade 1 (small blister, raised slightly above the surface); grade 2 (medium blister, raised moderately above the surface); grade 3 (large blister); grade 4(much wider and larger blister).
At the examination on the ninth day, corneal edema and corneal opacity grades were evaluated based on biomicroscopic examination by the way described by Yoeruek et al[15]. Corneal opacity was graded for each cornea as: grade 0 (transparent);grade 1 (minimal haze, details of iris and pupil distinct); grade 2 (mild haze, iris and pupil detectable); grade 3 (moderate haze, iris and pupil hardly visible); grade 4 (opaque, iris and pupil not discernable)[15]. Corneal edema was graded for each cornea as: grade 0 (no edema); grade 1 (mild to moderate edema); grade 2 (severe edema)[15].
On the tenth day, digital images of the corneas were taken and analyzed using image-analysis software (Topcon Image Net 2000 Itabashik, Tokyo, Japan) to calculate corneal neovascularization area. The area of neovascularization was measured in terms of pixels, and its ratio to the entire corneal area was determined as the percentage of corneal neovascularization[14].
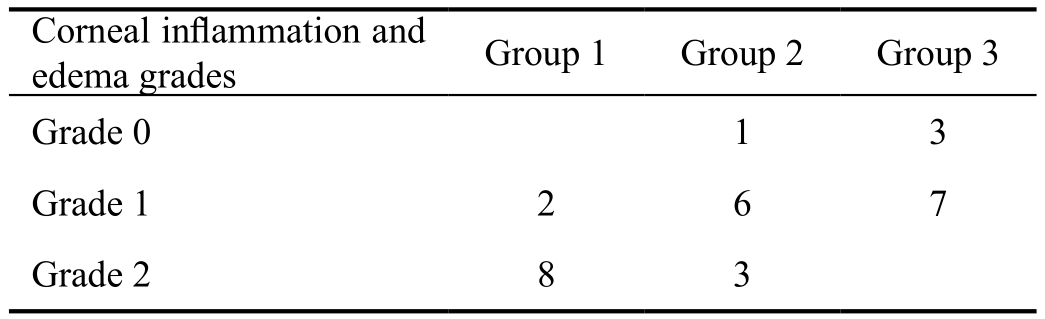
All rats were sacrificed on tenth day, and globes were enucleated for histopathologic examination by same investigator(Ulas B) as described by Oner et al[16]. Eyes were kept in 10% formaldehyde for 24h. Corneas were embedded into paraf fin and stained with hematoxylin and eosin and Masson trichrome[16]. Five-micron sections were taken from each cornea[16]. The corneal quadrants were evaluated with 400 times magnification, and the number of blood vessels, degree of in flammation and collagen formation were compared[16]. In addition, the pathologist of our study (Bal N), described and graded corneal inflammation (hematoxylin and eosin) and corneal collagen formation (Masson trichrome) as following.Inflammation was graded for each cornea as: grade 0 (no inflammation); grade 1 (mild to moderate inflammation);grade 2 (severe inflammation). Collagen formation was also graded for each cornea as: grade 0 (regular); grade 1 (minimal separation and disruption); grade 2 (severe disruption).
All statistical analysis was performed using SPSS (Statistical Package for Social Sciences, SPSS 17.0 for Windows, USA).For the comparison of categorical variables, the Chi-square test or Fisher’s exact test was used. Kruskal-Wallis and Mann-Whitney U test was used to compare the continuous measurements between groups, P<0.05 was considered statistically significant.
The degree of corneal neovascularization is shown in Figure 1.All groups had corneal burn grades of 3 and 4. The corneal burn grade was not statistically different among the groups(P=0.873).
Corneal opacity grade was statistically significantly lower in multiple-dose bevacizumab treatment group (Group 3) than single-dose bevacizumab treatment group (Group 2), and control group (Group 1) (P=0.004, and P=0.0001, respectively).Although there was no statistical significance, corneal opacity grade of single-dose bevacizumab group (Group 2) was lower than control group (Group 1, P=0.218; Table 1).

Figure 1 The degree of corneal neovascularization according to the groups.
The corneal edema grade was least in multiple-dose bevacizumab treatment group, followed by single-dose bevacizumab treatment group, and control group (Table 2).Corneal edema grade was statistically significantly lower in multiple-dose bevacizumab treatment group than single-dose bevacizumab treatment group, and control group (P=0.035,and P=0.02, respectively).
The mean±standard deviation (SD) percentage of neovascularized area was 68±5 percent in control group, 59±7 percent in singledose bevacizumab treatment group, and 47±10 percent in multiple-dose treatment group (Figure 2). The mean percentage of neovascularized area was significantly lower in single and multiple-dose bevacizumab treatment groups than control group (P=0.004, and P=0.0001, respectively). Additionally,the percentage of neovascularization was significantly lower in multiple-dose bevacizumab treatment group compared with single-dose bevacizumab treatment group (P=0.005).
Histopathological examination displayed the average numbers of blood vessels (Figure 3). The average number of blood vessels was 145.1±55.0 in control group, 69.5±20.6 in single-dose treatment group, and 43.7±22.6 in multipledose treatment group. Multiple and single-dose bevacizumab treatment groups had significantly fewer blood vessels than the control group (P=0.001, and P=0.005, respectively).Additionally, multiple-dose bevacizumab treatment group had significantly fewer blood vessels compared to singledose bevacizumab treatment group (P=0.019). Regarding to evaluation of collagen formation evaluation with Masson trichrome staining, multiple and single-dose bevacizumab treatment groups showed more regular collagen formation than control group, however there was no statistical significance(P=0.159; Figure 4).
Corneal in flammation and edema evaluation on histopathological examination were shown on Figure 5 and Table 3. The lowest corneal inflammation and edema grade was in multipledose bevacizumab treatment group, followed by single-dosebevacizumab treatment group, and control group. Corneal inflammation grade was statistically lower in multiple-dose bevacizumab treatment group than control group (P=0.001).Although there was no statistical significance, corneal inflammation grade in multiple-dose bevacizumab treatment group was lower than single dose bevacizumab treatment group (P=0.130).
Table 1 Corneal opacity grades according to groups as grade 0(none) to grade 4 (most intense)
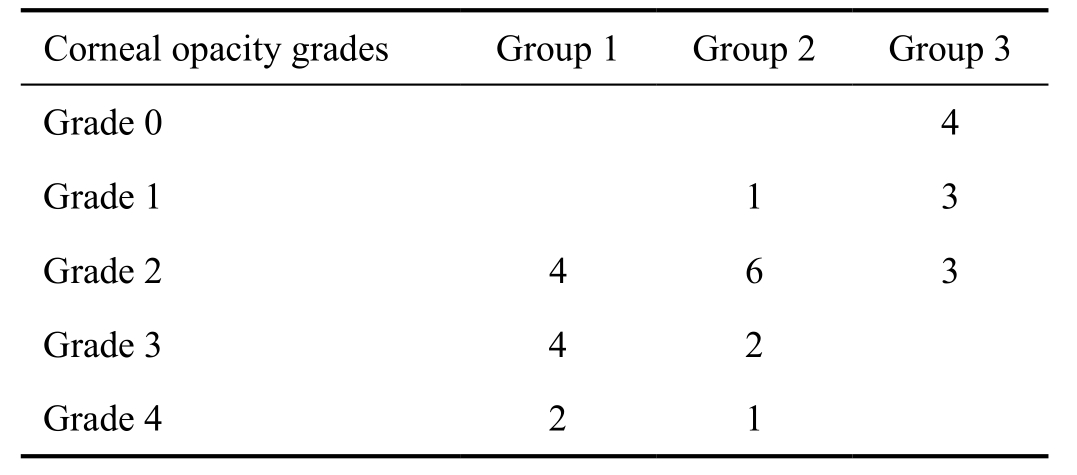
Table 2 Corneal edema grades according to groups as grade 0 (no edema), grade 1 (mild to moderate edema) and grade 2 (severe edema)
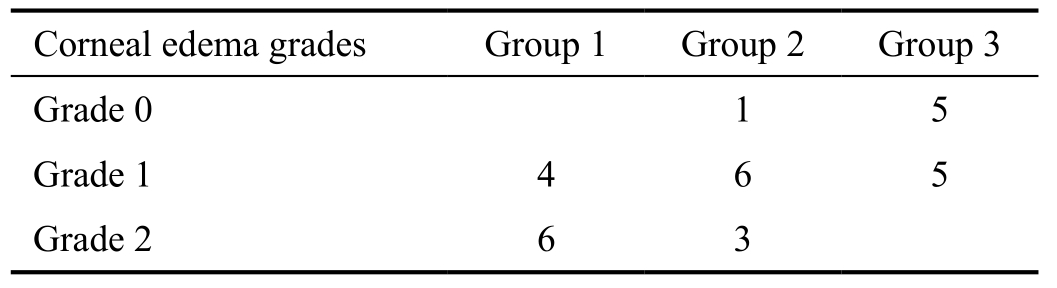
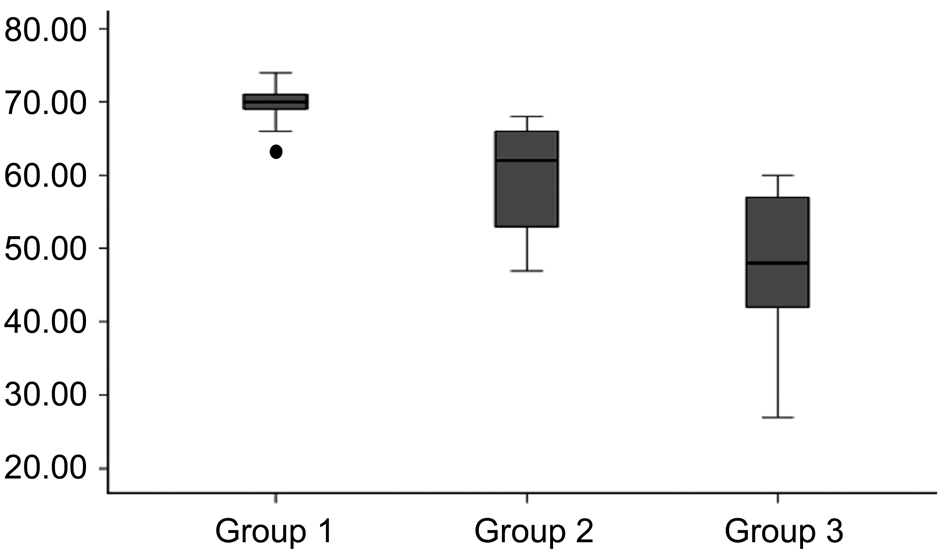
Figure 2 The histogram showing the mean percentage of corneal neovascularization area, which was significantly lower in multiple-dose bevacizumab treatment group than single-dose bevacizumab treatment group and control group.
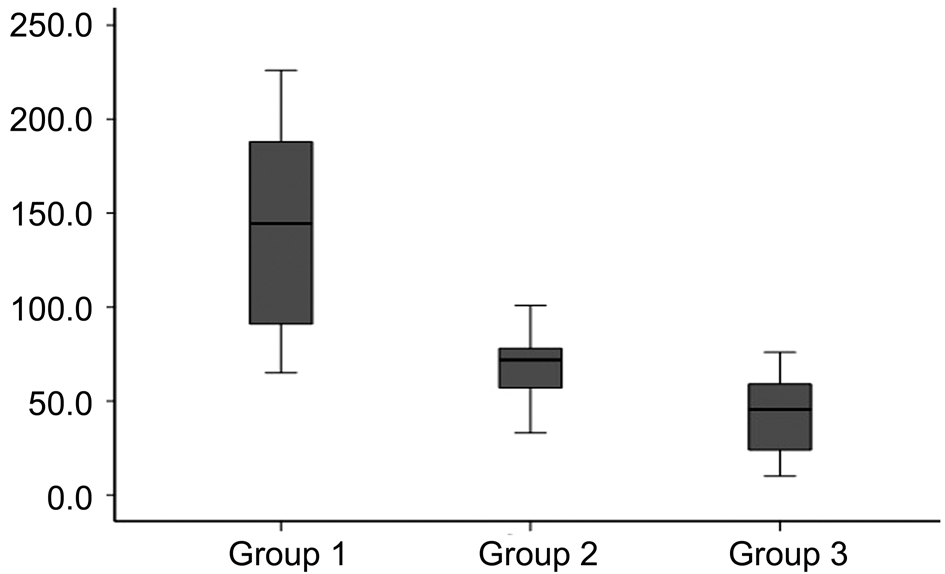
Figure 3 The average numbers of blood vessels was more prominent in Group 1 than Groups 2 and 3.
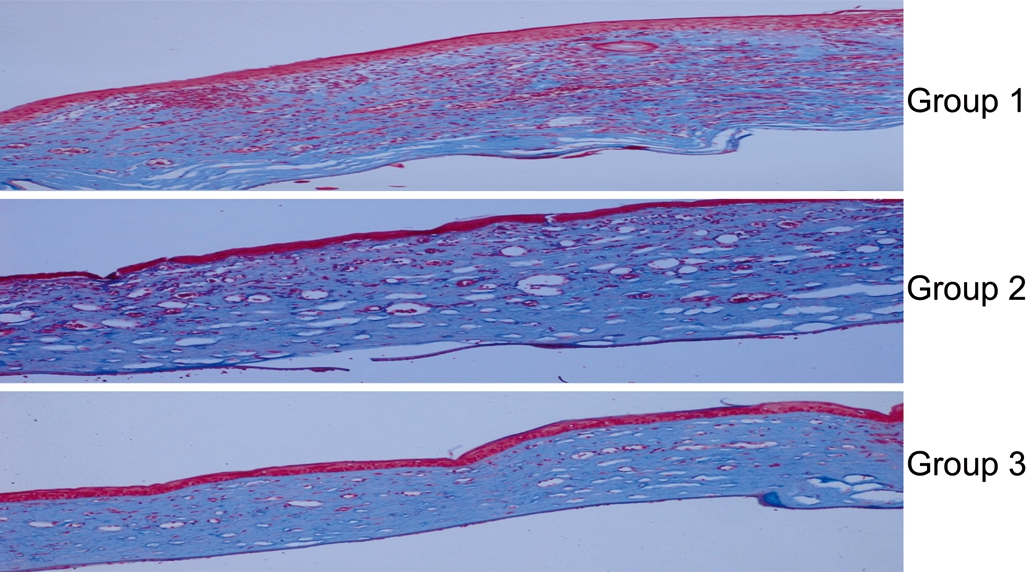
Figure 4 Collagen formation evaluation with Masson trichrome staining In subconjunctival bevacizumab treatment groups (Groups 2 and 3), collagen fibers were in more regular order.
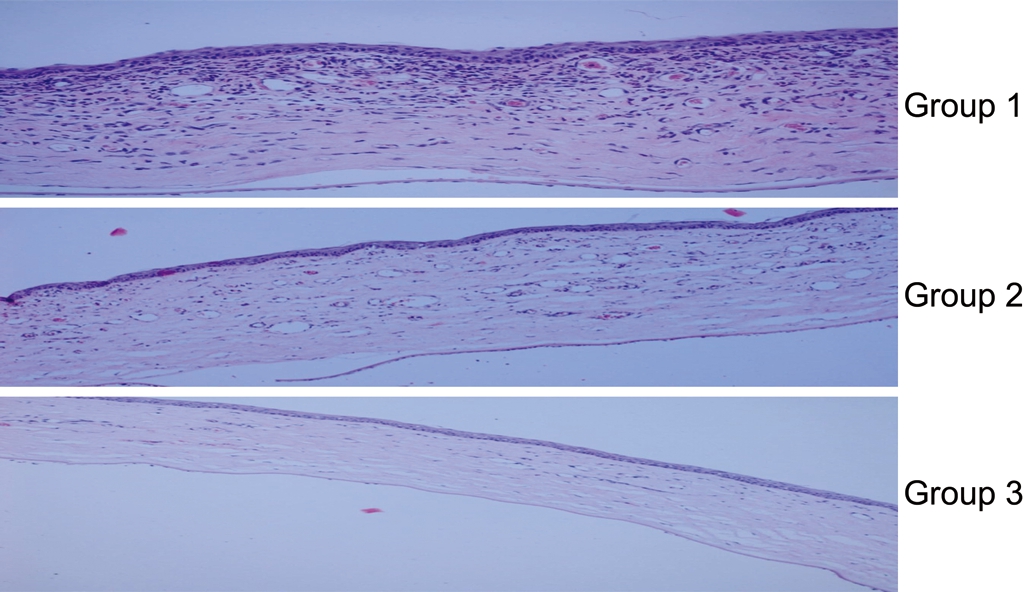
Figure 5 Corneal inflammation and edema evaluation on histopathological examination In the control group, edema and in flammation were more intense compared to the treatment groups.
Table 3 The recorded corneal inflammation and edema grades were lower in multiple-dose bevacizumab treatment group
In this study, we designed an experimental corneal neovascularization model in rats and applied single- or multipledose subconjunctival bevacizumab injections to examine their effect on newly formed vessels, also compared their effect with control group. We observed that, in experimental animal model subconjunctival bevacizumab injections inhibited corneal neovascularization. Additionally, multipledose bevacizumab injections were superior to the single-dose bevacizumab treatment in regards of effectiveness in inhibiting neovascularization and in flammation.
Inflammation is seen in the cornea due to the etiology of neovascularization[17]. Inflammation is accompanied by cell chemotaxis, migration and proliferation in a controlled manner with proinflammatory and anti-inflammatory molecules[18].Inflammation and angiogenesis are parallel at many points[17-19]. In the study of Amano et al[20], in flammation and VEGF increased with trauma in rat corneas and it was seen that inflammatory neovascularization was associated with VEGF. Several studies focused on the effects of anti-VEGF treatment in corneal neovascularization in either human or animal studies[11-15,21]. In an experimental study conducted by Bock et al[21], a model of corneal neovascularization with sutures was prepared in mice. In this study, inflammationinduced angiogenesis and lymphangiogenesis were suppressed with bevacizumab[21]. In a rat corneal neovascularization model, Oh et al[17]showed that subconjunvtival bevacizumab reduced the in flammatory cell in filtration and proin flammatory cytokines such as interleukin (IL)-2, interferon (IFN) gamma and IL-6. In our study, where bevacizumab was evaluated for its effectiveness against inflammation, histopathologically it was observed that there was a statistically significant reduction in the intensity of inflammation in the bevacizumab-treated groups compared to the control group.
Some experimental studies evaluated the treatment timing of subconjunctival bevacizumab. Papathanissou et al[22]produced a corneal burn model in rabbits. In their study,3.75 mg subconjunctival bevacizumab injection was administered immediately and fourteen days after corneal burn, separately. They found early treatment group was more effective in suppressing corneal neovascularization than the late treatment group[22]. Hurmeric et al[13]showed the similar result that inhibition of corneal neovascularization in the early subconjunctival bevacizumab treatment group was significantly more successful than late treatment group. Henceforth, we designed our study to start the treatment immediately after creating chemical corneal burn.
Yoeruek et al[15]created an experimental study in rabbit corneal burn. Corneal edema and opacity were evaluated with topical bevacizumab treatment in this study. When the corneal opacity was assessed, the treatment group had a lower grade of edema compared to the control group. However, no statistical difference was observed between the control group and treatment group when corneal edema was evaluated[15].Similarly in the neovascularization model with chemical cauterization in rats, Dursun et al[23]reported that corneal edema and opacity grades in the bevacizumab treated groups were statistically lower than those in the control group. Our study showed that, the treatment group receiving multipledoses of bevacizumab had statistically significantly lower grades in corneal opacity and edema than the single-dose bevacizumab treatment group and control group. There was no statistically significant difference between single-dose bevacizumab treatment group and the control group, although corneal opacity and edema grades were lower in the singledose bevacizumab treatment group.
Edelman et al[24]found that the levels of VEGF mRNA and protein following chemical burns on rat corneas reached their highest levels after 48h, and their levels decreased to control levels by 7d. In an experimental study on rats, Kim et al[25]investigated the half-life of subconjunctivally administered bevacizumab, and observed that following injection,bevacizumab was first detected at 12thhour in aqueous humor,and the approximate half-life was 27h. So in our present study,instead of administering a single high-dose of bevacizumab at one time, we preferred to administer the agent in multiple doses. In this respect, we aimed to increase the duration of anti-VEGF effect in the tissue with repeated administration.
Lopes et al[26]investigated subconjunctival and topical bevacizumab efficacy in rabbits with corneal neovascularization.In this experimental study, both subconjunctival and topical bevacizumab showed an inhibitory effect on corneal neovascularization in rabbits’ eyes after chemical burning of the cornea[26]. In our experimental study, we observed that subconjunctival bevacizumab treatment, either as a singleor multiple-dose, resulted in better results compared to the controls in decreasing corneal edema, corneal opacity,area of corneal neovascularization, number of vessels, and in flammation status. When we compared Group 2 with Group 3, we found the multiple-dose group to be more effective in decreasing corneal edema, corneal opacity, area of corneal neovascularization, and number of vessels, resulting in better suppression of corneal neovascularization.
In conclusion, we observed that subconjunctival bevacizumab administration was effective in suppressing and controlling experimental corneal neovascularization. We believe that, it should be administered as soon as possible following chemical burn. We observed that the results in inhibiting in flammation and neovascularization were better with multiple-dose application compared to the single-dose, probably by achieving a standard basal anti-VEGF level in the first few days. Thus,we consider giving bevacizumab in multiple doses should be preferred in the treatment protocol. However, there is still need for further prospective studies, in regards of determination of long term effects of bevacizumab in larger groups, and the proper dosing regimen.
Foundation:Supported by Baskent University.
Conflicts of Interest: Ulas B,None;Altan-Yaycioglu R,None;Bal N,None.
REFERENCES
1 Al-Debasi T, Al-Bekairy A, Al-Katheri A, Al Harbi A, Mansour M.Topical versus subconjunctival anti-vascular endothelial growth factor(Bevacizumab, Ranibizumab and Aflibercept) for treatment of corneal neovascularization.Saudi J Ophthalmol2017;31(2):99-105.
2 Azimzade Y, Hong J, Mashaghi A. Immunophysical analysis of corneal neovascularization: mechanistic insights and implications for pharmacotherapy.Sci Rep2017;7(1):12220.
3 Lee JE, Kim KL, Kim D, Yeo Y, Han H, Kim MG, Kim SH, Kim H, Jeong JH, Suh W. Apatinib-loaded nanoparticles suppress vascular endothelial growth factor-induced angiogenesis and experimental corneal neovascularization.Int J Nanomedicine2017;12:4813-4822.
4 Sarah B, Ibtissam H,Mohammed B, Hasna S, Abdeljalil M. Intrastromal enjection of Bevacizumab in the management of corneal neovascularization:about 25 eyes.J Ophthalmol2016;2016:6804270.
5 Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas.Invest Ophthalmol Vis Sci2000;41(9):2514-2522.
6 Azar DT. Corneal angiogenic privilege: angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis).Trans Am Ophthalmol Soc2006;104:264-302.
7 Joussen AM, Kruse FE, Völcker HE, Kirchhof B. Topical application of methotrexate for inhibition of corneal angiogenesis.Graefes Arch Clin Exp Ophthalmol1999;237(11):920-927.
8 Benelli U, Ross JR, Nardi M, Klintworth GK. Corneal neovascularization induced by xenografts or chemical cautery. Inhibition by cyclosporin A.Invest Ophthalmol Vis Sci1997;38(2):274-282.
9 Abdelfattah NS, Amgad M, Zayed AA, Salem H, Elkhanany AE,Hussein H, Abd El-Baky N. Clinical correlates of common corneal neovascular diseases: a literature review.Int J Ophthalmol2015;8(1):182-193.
10 Lee YK, Chung SK. The inhibitory effect of thalidomide analogue on corneal neovascularization in rabbits.Cornea2013;32(8):1142-1148.
11 Kim TI, Kim SW, Kim S, Kim T, Kim EK. Inhibition of experimental corneal neovascularization by using subconjunctival injection of bevacizumab (Avastin).Cornea2009;27(3):349-352.
12 Hashemian MN, Mahrjerdi HZ, Mazloumi M, Sa fizadeh MS, Shakiba Y, Rahimi F, Afarideh M, Zare MA, Tafti MF, Sepidan BB, Abtahi MA,Abtahi SH. Comparison of different doses of subconjunctival sunitinib with bevacizumab in the treatment of corneal neovascularization in experimental rats.J Res Med Sci2017;22:16.
13 Hurmeric V, Mumcuoglu T, Erdurman C, Kurt B, Dagli O, Durukan AH. Effect of subconjunctival bevacizumab (Avastin) on experimental corneal neovascularization in guinea pigs. Cornea2008;27(3):357-362.
14 Manzano RP, Peyman GA, Khan P, Carvounis PE, Kivilcim M,Ren M, Lake JC, Chévez-Barrios P. Inhibition of experimental corneal neovascularisation by bevacizumab (Avastin).Br J Ophthalmol2007;91(6):804-807.
15 Yoeruek E, Ziemssen F, Henke-Fahle S, Tatar O, Tura A, Grisanti S, Bartz-Schmidt KU, Szurman P. Safety, penetration and efficacy of topically applied bevacizumab: evaluation of eyedrops in corneal neovascularization after chemical burn.Acta Ophthalmol2008;86(3):322-328.
16 Öner V, Küçükerdönmez C, Akova YA, Çolak A, Karalezli A. Topical and subconjunctival bevacizumab for corneal neovascularization in an experimental rat model.Ophthalmic Res2012;48(3):118-123.
17 Oh JY, Kim MK, Shin MS, Lee HJ, Lee JH, Wee WR. The antiinflammatory effect of subconjunctival bevacizumab on chemically burned rat corneas.Curr Eye Res2009;34(2):85-91.
18 Philip M, Rowley DA, Schreiber H. In flammation as a tumor promoter in cancer induction.Semin Cancer Biol2004;14(6):433-439.
19 Costa C, Incio J, Soares R. Angiogenesis and chronic in flammation:cause or consequence?Angiogenesis2007;10(3):149-166.
20 Amano S, Rohan R, Kuroki M, Tolentino M, Adamis AP.Requirement for vascular endothelial growth factor in wound- and inflammation-related corneal neovascularization.Invest Ophthalmol Vis Sci1998;39(1):18-22.
21 Bock F, Onderka J, Dietrich T, Bachmann B, Kruse FE, Paschke M,Zahn G, Cursiefen C. Bevacizumab as a potent inhibitor of in flammatory corneal angiogenesis and lymphangiogenesis.Invest Ophthalmol Vis Sci2007;48(6):2545-2552.
22 Papathanassiou M, Theodossiadis PG, Liarakos VS, Rouvas A, Giamarellos-Bourboulis EJ, Vergados IA. Inhibition of corneal neovascularization by subconjunctival bevacizumab in an animal model.Am J Ophthalmol2008;145(3):424-431.
23 Dursun A, Arici MK, Dursun F, Ozec AV, Toker MI, Erdogan H,Topalkara A. Comparison of the effects of bevacizumab and ranibizumab injection on corneal angiogenesis in an alkali burn induced model. Int J Ophthalmol2012;5(4):448-451.
24 Edelman JL, Castro MR, Wen Y. Correlation of VEGF expression by leukocytes with the growth and regression of blood vessels in the rat cornea.Invest Ophthalmol Vis Sci1999;40(6):1112-1123.
25 Kim MJ, Han ES, Kim J, Kim TW. Aqueous humor concentration of bevacizumab after subconjunctival injection in rabbit.J Ocul Pharmacol Ther2010;26(1):49-53.
26 Lopes GJA, Casella AMB, Oguido AP, Matsuo T. Effects of topical and subconjunctival use of bevacizumab on corneal neovascularization in rabbits’ eyes.Arq Bras Oftalmol2017;80(4):252-256.
Correspondence to:Burak Ulas. Ataturk Cad. No:186 Ulas Eczanesi Ceyhan, Adana 01250, Turkey. drburakulas@gmail.com
Received:2017-06-23 Accepted: 2017-12-05
Abstract ● AlM: To evaluate the inhibitory effect of subconjunctival bevacizumab as single- and multiple-dose application, and compare their effects on corneal neovascularization in a rat model.● METHODS: Thirty adult Sprague-Dawley rats were used in this experimental study. The central cornea of the rats was cauterized chemically. The rats were randomly enrolled into three groups. All groups received subconjunctival injections. ln Group 1 (control group, n=10), 0.05 mL 0.9%NaCl solution was injected on the first day. ln Group 2(single-dose group, n=10), 0.05 mL bevacizumab (1.25 mg)was injected on the first day. ln Group 3 (multiple-dose group, n=10), four doses of 0.05 mL bevacizumab (1.25 mg) were injected on the first, third, fifth and seventh day.Slit-lamp examination of all rats was performed at the third and ninth day. Digital images of the corneas were taken and analyzed using image analysis software to calculate corneal neovascularization area. All rats were sacrificed on the tenth day. ln corneal sections, the number of blood vessels, state of in flammation and collagen formation was evaluated histopathologically.● RESULTS: ln Group 3, corneal edema grades were significantly lower than Group 1 and Group 2 (P=0.02,and P=0.035, respectively). The mean percentage of neovascularized corneal area in Group 3 was significantly lower than Group 2 (P=0.005). On histopathological examination, Group 2 and Group 3 showed significantly less number of blood vessels than Group 1 (P=0.005, and P=0.001,respectively). Additionally, Group 3 showed significantly less number of blood vessels compared to Group 2 (P=0.019).ln flammation and edema grades were significantly lower in Group 3 compared to Group 1 (P=0.001).● CONCLUSlON: Subconjunctival bevacizumab injection is effective in inhibition of newly formed corneal neovascularization. The multiple-dose bevacizumab treatment seems to be more effective compared to single-dose treatment.
● KEYWORDS:cornea; corneal neovascularization; bevacizumab;in flammation; rat
DOl:10.18240/ijo.2018.07.03
Citation:Ulas B, Altan-Yaycioglu R, Bal N. Comparison of the inhibitory effect of different doses of subconjunctival bevacizumab application in an experimental model of corneal neovascularization.Int J Ophthalmol 2018;11(7):1090-1095