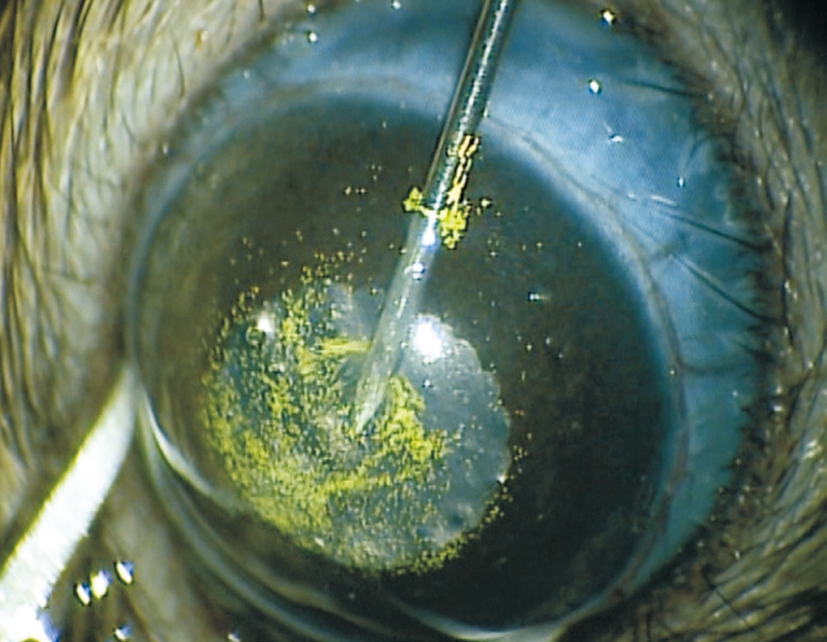
Figure 1 Injection of green fluorescent microbeads and then viscoelastic into the anterior chamber of the eye.
Lin Fu1,2, Jimmy Shiu Ming Lai2, Amy Cheuk Yin Lo2, Kendrick Co Shih2
1Affiliated Eye Hospital, School of Ophthalmology and Optometry, Wenzhou Medical University, Wenzhou 325035,Zhejiang Province, China
2Department of Ophthalmology, Li Ka Shing Faculty of Medicine, University of Hong Kong, Pokfulam, Hong Kong SAR, China
Globally, glaucoma is the leading cause of irreversible visual loss, affecting an estimated 60.5 million of the world’s population in 2010 with almost 15% suffering bilateral blindness as a result of the disease. The World Health Organization (WHO) estimates that by 2020 there will be 79.6 million people with glaucoma, making it an important global health concern[1]. Although it consists of a heterogenous group of optic neuropathies, intraocular pressure (IOP) remains the most important and only treatable risk factor for glaucoma[2-3].
There is accumulating evidence that patients with a higher variation of IOP over the twenty-four hour day or over multiple clinic visits may be independently at more risk for glaucomatous progression[4-6]and may be related to the ocular anterior segment anatomy in primary angle closure glacuoma[7-8]. Such findings, if conclusive, would necessitate a review of current standard of care in glaucoma treatment.However, there are a number of studies, most notably the Early Manifest Glaucoma Trial study, that have found no association between IOP fluctuation and visual field progression long-term and hence current published clinical literature remains divided on the issue of IOP fluctuation as an independent risk factor for glaucoma progression[9]. As methods for monitoring a more complete IOP profile exist, and newer treatments including prostaglandin analogues and selective laser trabeculoplasty have proven benefits in decreasing the amplitude of IOP fluctuation, this issue deserves further investigation[10-11]. There is currently however a limited number of experimental models in animals to investigate the effect of diurnal IOP fluctuation on retinal ganglion cell (RGC) death. One such technique is photocoagulation of the trabecular meshwork, which requires specialized and expensive equipment as well as technical expertise[12].
Weber and Zelenak[13]first introduced a technique of injecting latex microspheres into the anterior chamber of primate eyes in order to achieve raised IOP through iridocorneal angle occlusion. In the published study, different magnitudes and durations of IOP elevation can be obtained by different injection frequencies as well as volume of microsphere solution[13]. Thereafter, Sappington et al[14]applied it into mice and rats and achieved a modest increase in mean IOP with associated RGC death. In order to maximize the proportion of injected microbeads to block the outflow of aqueous humor, the procedure was further modified to include intracameral injection of dispersive viscoelastic solution to push the microbeads peripherally during surgery as well as to use magnetic beads, which could be directed towards the iridocorneal angle via a magnet[15-16]. In this study, we investigated the effect of a single injection of microbead solution,using a modified approach, on diurnal IOP fluctuation in rats.
AnimalsMale Dark Agouti (DA) rats, 8-10 weeks old, were kept in a temperature-controlled animal room. The animals were subjected to a 12-h light/12-h dark cycle and provided with suf ficient food and water supply. All animal experiments were conducted in accordance with the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of Animals in Ophthalmic and Vision Research and also approved by the Committee on the Use of Live Animals in Teaching and Research, the University of Hong Kong.
Intraocular Pressure MeasurementRats were gently packed with a towel leaving the head exposed. A portable rebound tonometer (Tonolab; Icare, Espoo, Finland) was used to measure the IOP of the rats by an experienced investigator. It was handheld near the rat’s eye at a 5-8 mm distance to the central cornea and the IOP was measured without general and topical anesthesia. After six consecutive measurements, the machine itself calculated a mean result and this was regarded as one reading. Three readings for each eye were collected for each time point. Light and dark time IOP measurements were performed at 10 a.m.-11 a.m. and 8 p.m.-9 p.m. respectively.Furthermore, dark time IOP measurement was carried out in a dark room. Two days before the microbeads injection surgery,the baseline IOP was taken as the mean of two readings on two separate days. After microbeads injection, twice-daily measurements of IOP were conducted in the first week and then repeated every three days in the follow weeks. IOP fluctuation was calculated as the value of dark IOP minus light IOP.

Figure 1 Injection of green fluorescent microbeads and then viscoelastic into the anterior chamber of the eye.
Microbeads Injection SurgeryIntraperitoneal injection 7 mg xylazine combined with 70 mg/kg ketamine was used to anesthetize the rats at the beginning of surgery. After the rats lost consciousness, a drop of 0.5% proparacaine hydrochloride was applied to the surgical eye for tropical anesthesia and a drop of 1% tropicamide was then used for mydriasis. Under microscope, 10 μL 15-μm green fluorescent (450/480)polystyrene microbeads (Thermo Fisher Scientific, MA, USA)were injected into the anterior chamber through a trans-corneal incision via a 30-gauge needle which was connecting to a 1 mL syringe. This was followed by 10 μL Healon D viscoelastic solution (AMO, Santa Ana, CA, USA) injection to push the microbeads towards the iridocorneal angle. The needle was then kept in position for 1min and slowly withdrawn to minimize the leakage of microbeads and sudden collapse of the anterior chamber (Figure 1).
Hematoxylin and eosin stainingTo identify the location of the microbeads in the iridocorneal angle, histological analysis of the eye was performed by the hematoxylin and eosin (H&E)staining. Three weeks after microbeads injection, the animals were euthanized by 20% dorminal (240 mg/kg; Alfasan International BV, Woerden, Holland) intraperitoneal injection.Eyes were enucleated rapid and fixed in 4% paraformaldehyde(PFA) overnight. They were then washed in 1× phosphate buffer saline (PBS) three times and prepared for dehydration.
DehydrationThe eyes were put into plastic cassettes first and marked by pencil. The gradient concentration of ethanol was used to dehydrate the eyes. They were incubated in the gradient concentrations of ethanol and chloroform in order of 50%, 70%and 80% ethanol 30min one time each, 95% ethanol 30min two times and 100% ethanol 30min three times and finally chloroform one hour two times followed by the third time overnight.
In filtration and embeddingThe next day, the eyeballs were sent for infiltration and embedded with paraffin to support their structure. They were first allowed to equilibrate in melted paraf fin in a 58℃ incubator for 1h three times with new melted paraf fin changed each time. And the third time in filtration was carried out in a vacuum station. For embedding, fine forceps were used to transfer the eyeball blocks to the metal molds,the covers of the plastic cassette were removed and the bases were put into the metal molds and then the molds were filled with liquid paraffin from the station (Shandon Histocentre2 Embedding Station, Midwest, USA). They were solidified on ice and stored in 4℃ fridge.
SectioningThe embedded blocks were cut into 5 µm thick by a microtome and the paraffin sections were flatted in a 37℃water bath. Glass slides were applied to pick up them after 15-20min floating and subsequently dried in a 37℃ incubator.Before staining, the sections should be removed with the paraf fin and rehydrated. This process was done by incubating the sections for 5min in the solutions in following sequence:Toluene twice, 100% ethanol three times, 95% ethanol twice,70% ethanol once. Then these sections were rinsed with distilled water before putting into hematoxylin for 5min,differentiated in 0.3% acid alcohol for three seconds and counterstained with eosin for 10s. During the interval of each step the sections were rinsed in running tap water. Thereafter these sections were dehydrated in a reverse sequence of solutions in rehydration. They were 70% ethanol once, 95%ethanol twice, 100% ethanol three times and toluene twice with 5min each time again. At last, the sections were mounted with 2-3 drops resin by glass cover slips. Ocular section images were captured by an upright microscope (Eclipse80i, Nikon,Japan) and the imaging software SPOT AdvancedTM (SPOT Imaging Solutions, Diagnostic Instruments, Inc. USA).
Retrograde retinal ganglion cells labelingThe RGC labeling by intracranial fluorogold injection was performed according to the methodology outlined by Chiu et al[17]. After intraperitoneal anesthesia with xylazine (8 mg/kg) and ketamine (75 mg/kg),a drill was used to make a 1/8-inch hole in the skull 2 mm from the lambda and 6 mm from the bregma at each side. After carefully removing the cortex above the superior colliculus,a smooth light yellow area exposed. Then fluorogold soaked with a piece of gelatin sponge was insert into the hole. Skin covering the skull was then sutured and the animals were put into the intensive care unit (ICU) for recovery from anesthesia.
Retinal flat mount preparation and retinal ganglion cells countingOne week after RGCs labeling, when the fluorogold dye fully penetrates the retina, the animal was sacrificed by dorminal overdose as previously described. The eye was rapidly enucleated and fixed in 4% PFA at room temperature for half an hour with a hole created in the corneas. Fine forceps were used to gently separate the retina from the sclera with thorough elimination of the vitreous. Two hours after 4% PFA incubation again, it was rinsed with 1×PBS solution three times. Finally the retina was flat mounted with antifading fluorescent mounting medium (DAKO, Denmark)and stored in a 4℃ fridge waiting for RGCs counting. RGCs were counted using a fluorescence microscope under 400×magnification and the areas at the midline of each quadrant were captured from seven fields at fixed distance from the optic disc.
Statistical AnalysisData are presented as mean±standard error of mean (SEM). The significance of differences was analyzed by the Student’s unpaired t-test. The statistical significance level was set at P<0.05.
Equivalent Baseline Intraocular Pressure Between GroupsTo ensure a lack of significant differences in baseline IOP between groups, IOP measurement was initiated two days before surgery. The mean baseline IOP during light hours in the control eye was 12.000±0.925 mm Hg (n=5) and 11.332±0.274 mm Hg (n=5) in the eyes for injection (Figure 2).During dark hours, the IOP was 15.500±0.462 mm Hg (n=5)and 15.968±0.225 mm Hg (n=5) respectively (Figure 2). There was no significant difference in baseline mean IOP and diurnal fluctuation magnitude between the two groups.
Injected Microbeads Partially Blocked the Out flow of the Aqueous HumorTo confirm the microbead position at the iridocorneal angle, the injected eye was investigated under slit lamp with cobalt blue light every week and later enucleated for at 3wk after surgery for evaluation under H&E staining (Figure 3).Our assessment found that most of the green microbeads accumulated at the angle with only a small number of them remaining suspended in the aqueous humor. And under histological observation, microbeads were noted to be partially occluding the trabecular meshwork.
Intraocular Pressure Elevated 2wk After Microbeads InjectionAfter microbeads injection, the IOP was measured twice daily (morning and evening) in the first week and every three days thereafter. There was no significant difference in mean IOP during light hours between the two groups during the entire observation period. The mean light time IOP readings in the first, second and third week after injection were 14.50±1.786 mm Hg (n=5), 13.42±0.854 mm Hg (n=5)and 12.93±0.800 mm Hg (n=5) in the surgical eyes and were 12.28±0.473 mm Hg (n=5), 14.51±1.108 mm Hg (n=5) and 12.76±0.627 mm Hg (n=5) in the control eyes (Figure 4).
Conversely, the dark time IOP gradually increased 2wk after injection and remained significantly higher than the control eyes. From the first week to the third week, the mean IOP in the injected eyes were 21.70±1.690 mm Hg (n=5),22.92±1.631 mm Hg (n=5) and 23.59±1.494 mm Hg (n=5) and were 16.96±0.775 mm Hg (n=5), 17.35±0.751 mm Hg (n=5)and 17.73±0.592 mm Hg (n=5) in the control eyes. The IOP in the surgical eye was 5.57±1.692 mm Hg and 5.86±1.547 mm Hg higher than the control eyes in the second and third week(Figure 4).
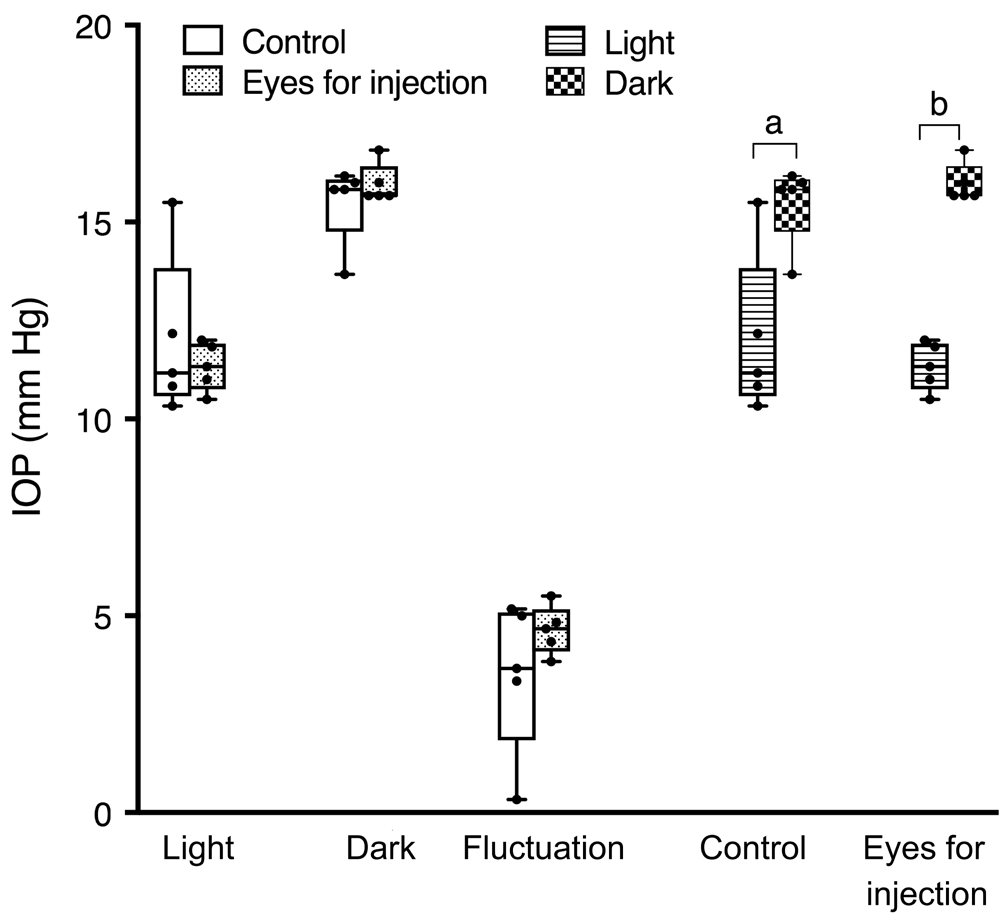
Figure 2 There were no differences of the baseline IOP in both groups during both light and dark period The IOP in the dark time was significantly higher than in the light time in both groups but the fluctuation level between groups was similar.aP<0.05,bP<0.01.
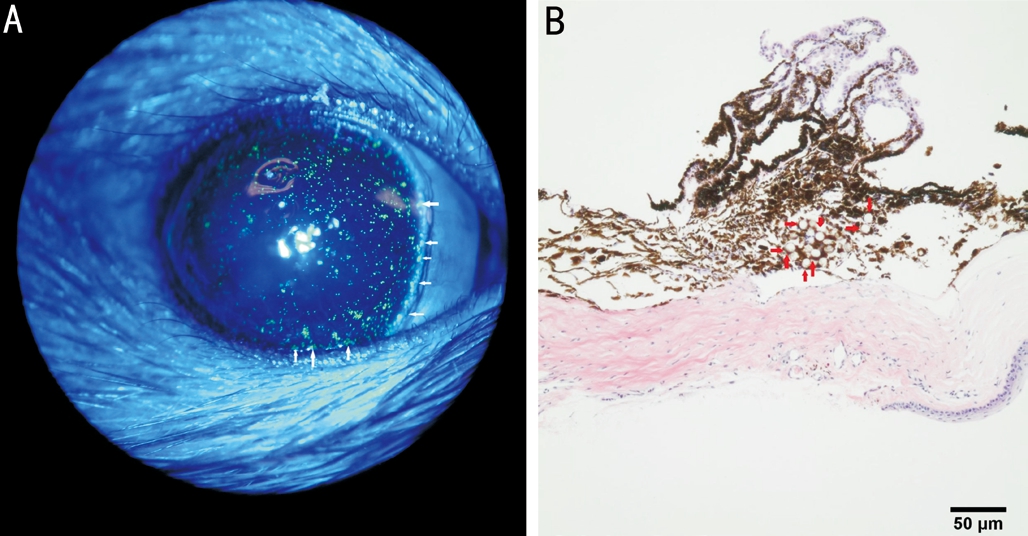
Figure 3 The out flow of the aqueous humor was partially blocked by injected microbeads Green fluorescent microbeads (white arrow)occlude the iridocorneal angle under slit lamp investigation using cobalt blue light (A) immediately after injection. A cross section of the H&E staining of the iridocorneal angle showing the microbeads (red arrow) obstructing the trabecular meshwork (B) 3wk after injection.
After averaging out dark and light time IOPs, the mean IOP was compared. Surgical eyes had statistically higher readings only at the 3rdpostoperative week. The recorded values were 18.13±0.917 mm Hg (n=5) in surgical eyes and 15.24±0.431 mm Hg(n=5) in controls respectively. It can be seen in the graph that there was still a tendency of higher IOP in the surgical eyes for the 1stand 2ndweeks, though without reaching statistical significance (Figure 4).
Higher Dark Time Intraocular PressureThe dark time recorded IOP was significantly higher than light time recorded IOP from the second week to the third week after microbeads injection.Therefore, IOP diurnal fluctuation was significantly higher in the microbead-injected eyes. The fluctuation levels of IOP in the surgical eyes were 7.21±0.398 mm Hg (n=5), 9.50±1.017 mm Hg(n=5) and 10.66±0.894 mm Hg (n=5) from the first week to the third week after injection. Comparatively, they were significantly lower in the control eyes, which were 4.69±0.323 mm Hg (n=5), 2.84±1.122 mm Hg (n=5) and 4.98±0.603 mm Hg (n=5) respectively (Figure 5).
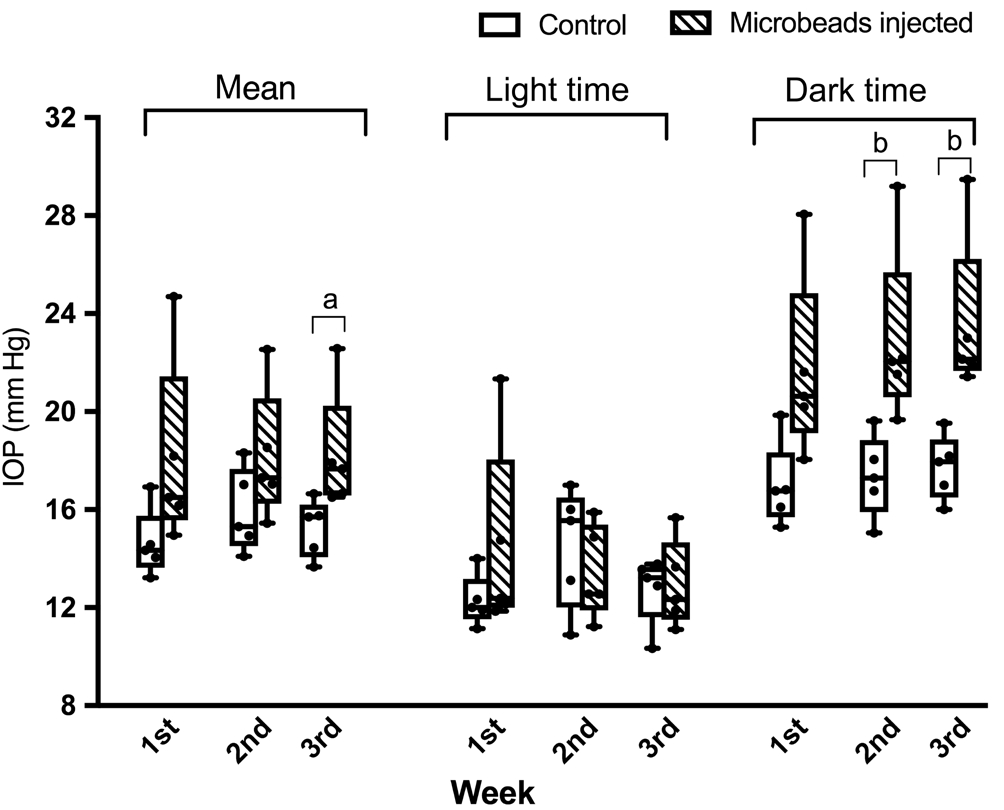
Figure 4 The mean IOP was significantly elevated in the third week after microbeads injection There were no differences of light time IOP between two groups in all three weeks. But there were significantly higher IOP than the control eyes both two and three weeks after microbeads injection.aP<0.05,bP<0.01.
No Significant Retinal Ganglion Cells Loss After Microbeads InjectionAfter microbeads injection for 3wk,the RGC density was compared between groups. There was no significant difference in RGC density between the surgical eyes and control eyes. The density of RGC in the surgical group was 2759±157.1/mm2and 3061±157.2/mm2(n=4) in the control eyes (Figure 6), therefore showing a trend towards a lower density in the surgical group without reaching statistical significance.
In the present study, treated rat eyes showed gradual IOP elevation in dark time from the second week after surgery as well as greater diurnal IOP fluctuation from the second week compared to control eyes. In addition, the light time IOP levels in both injected eyes and control eyes were similar and they both had higher dark time IOP throughout the observation period. However, at three weeks after microbeads injection,there was no significant RGC loss in this sustained ocular hypertensive model.
Previous reported microbeads induced ocular hypertensive models all applied multiple injections. Weber and Zelenak[13]injected 8 to 12 times per week before an initial sustained IOP elevation in rhesus monkeys. Sappington et al[14]performed an additional injection 2wk after the first injection to achieve 8wk IOP increase. However, IOP induced in these studies showed great variability and the inflammation caused by multiple injections should be taken into account to the axonal damage.Regarding our modified approach, the injection of fluorescent microbeads was followed by the same volume of dispersive viscoelastic solution. The use of dispersive viscoelastic solution was aimed at preventing excessive postoperative IOP surge from blockage of the trabecular meshwork by more cohesive viscoelastic and also to reduce the leakage of microbeads after needle withdrawn. Hence our method can reduce the IOP variability and prevent the need of further injection.Furthermore, the advantage of using fluorescent microbeads is that they can be tracked after injection in live animals.
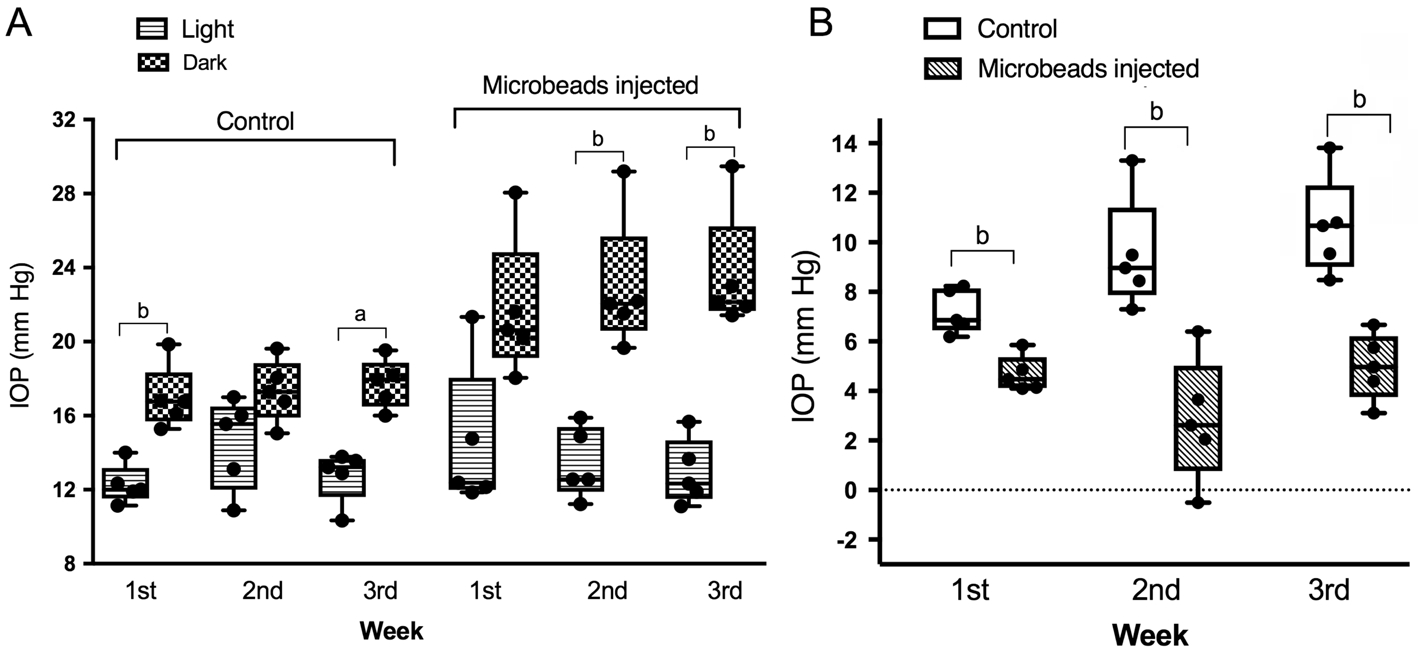
Figure 5 The dark time IOP levels were higher than the light time in both groups A: Comparison of light and dark time IOP in both groups;B: Fluctuation levels of IOP in two groups. The fluctuation between day and night was much higher in the microbead injected eyes in all three weeks.aP<0.05,bP<0.01.
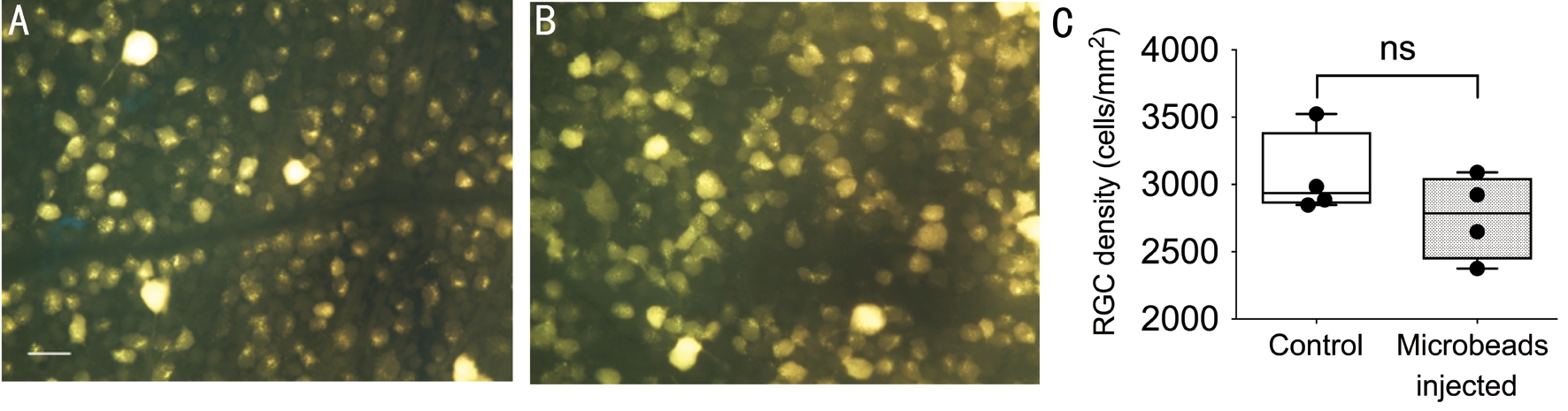
Figure 6 No significant difference of RGCs density between two groups A, B: Representatives of fluorogold labeled RGC in the control and microbeads injected eyes; C: Averaged RGC density in both groups. ns: No significance; Scale bar: 25 μm.
In human clinical studies, a large diurnal fluctuation in IOP was reported to be associated with more rapid visual field loss and poorer visual outcomes in both angle closure and open angle glaucoma patients[18-19]. Currently methods of documenting IOP fluctuation are being recommended, for high risk glaucoma patients, including the use of phasing techniques and 24-hour IOP measurement devices like the Sensimed Trigger fish[20]. In rodents, the higher dark phase IOP has already been reported in the literature[21]and can be further exacerbated with the use of laser photocoagulation of the trabecular meshwork[12].It is said to be caused by circadian rhythm and sympathetic innervation differences between light and dark phase[22]. As far as we know, this is the first time that the both light and dark time IOP levels were investigated in a microbead-induced sustained ocular hypertension model. It first revealed that the diurnal IOP fluctuation existed in normal DA rat eyes.
After microbeads injection, although there was no difference in daytime IOP, the night time IOP was higher in surgical eyes from two weeks onwards after injection compared to control eyes. Upon the mechanism of circadian rhythm and sympathetic nerve innervation, pupil dilation during the dark,may also exacerbate the obstruction of trabecular meshwork by microbeads, and subsequently elevates the IOP. This also leads to greater diurnal fluctuation in the surgical eyes.
Since the loss of RGCs is the fundamental hallmark of glaucomatous pathology, the fluorogold labeled RGCs were counted and compared three weeks after microbeads injection.Unfortunately, the RGCs density in surgical eyes did not suffer a significant loss compared to the control eyes, although there was a difference in density of 302 cells/mm2. This may be due to the small sample size or shorter investigation duration. For glaucoma research using animal models, RGCs survival is the key element to compare effectiveness of neuroprotective strategies. A longer duration of observation may be necessary to induce a significant change in RGC density. Alternatively,for our model other parameters like the soma size of the RGCs and the axon density in the optic nerve can also be investigated.
We introduced our modified technique microbead occlusion,which result in a greater fluctuation of IOP in surgical eyes compared to controls from the second week after injection and a moderate elevation of nocturnal IOP from the second week onwards. This is the first time that the nocturnal IOP and diurnal fluctuation were taken into consideration as measurement parameters in microbead occlusion-induced sustained ocular hypertension models. This finding would be of interest to investigators looking for a feasible and inexpensive model for diurnal IOP fluctuations and its relation to optic nerve damage.
Conflicts of Interest: Fu L,None;Lai JSM,None;Lo ACY,None;Shih KC,None.
REFERENCES
1 Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020.Br J Ophthalmol2006;90(3):262-267.
2 Maier PC, Funk J, Schwarzer G, Antes G, Falck-Ytter YT. Treatment of ocular hypertension and open angle glaucoma: meta-analysis of randomised controlled trials.BMJ2005;331(7509):134.
3 Caprioli J, Varma R. Intraocular pressure: modulation as treatment for glaucoma.Am J Ophthalmol2011;152(3):340-344.e2.
4 Asrani S, Zeimer R, Wilensky J, Gieser D, Vitale S, Lindenmuth K.Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma.J Glaucoma2000;9(2):134-142.
5 Caprioli J, Coleman AL. Intraocular pressurefluctuation a risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study.Ophthalmology2008;115(7):1123-1129.e3.
6 Moon Y, Kwon J, Jeong DW, Lee JY, Lee JR, Han S, Kook MS.Circadian patterns of intraocular pressure fluctuation among normaltension glaucoma optic disc phenotypes.PLoS One2016;11(12):e0168030.
7 Sanchez-Parra L, Pardhan S, Buckley RJ, Parker M, Bourne RR.Diurnal intraocular pressure and the relationship with swept-source OCT-derived anterior chamber dimensions in angle closure: The IMPACT study.Invest Ophthalmol Vis Sci2015;56(5):2943-2949.
8 Bourne RRA, Zhekov I, Pardhan S. Temporal ocular coherence tomography-measured changes in anterior chamber angle and diurnal intraocular pressure after laser iridoplasty: IMPACT study.Br J Ophthalmol2017;101(7):886-891.
9 Bengtsson B, Leske MC, Hyman L, Heijl A; Early Manifest Glaucoma Trial Group. Fluctuation of intraocular pressure and glaucoma progression in the early manifest glaucoma trial.Ophthalmology2007;114(2):205-209.
10 Kóthy P, Tóth M, Holló G. In fluence of selective laser trabeculoplasty on 24-hour diurnal intraocular pressurefluctuation in primary open-angle glaucoma: a pilot study.Ophthalmic Surg Lasers Imaging2010;41(3):342-347.
11 Varma R, Hwang LJ, Grunden JW, Bean GW, Sultan MB. Assessing the efficacy of latanoprost vs timolol usingan alternate efficacy parameter: the intervisit intraocular pressure range.Am J Ophthalmol2009;148(2):221-226.
12 Kwong JM, Vo N, Quan A, Nam M, Kyung H, Yu F, Piri N, Caprioli J. The dark phase intraocular pressure elevation and retinal ganglion cell degeneration in a rat model of experimental glaucoma.Exp Eye Res2013;112:21-28.
13 Weber AJ, Zelenak D. Experimental glaucoma in the primate induced by latex microspheres.J Neurosci Methods2001;111(1):39-48.
14 Sappington RM, Carlson BJ, Crish SD, Calkins DJ. The microbead occlusion model: a paradigm for induced ocular hypertension in rats and mice.Invest Ophthalmol Vis Sci2010;51(1):207-216.
15 Ito YA, Belforte N, Cueva Vargas JL, Di Polo A. A magnetic microbead occlusion model to induce ocular hypertension-dependent glaucoma in mice.J Vis Exp2016;(109):e53731.
16 Bunker S, Holeniewska J, Vijay S, Dahlmann-Noor A, Khaw P, Ng YS, Shima D, Foxton R. Experimental glaucoma induced by ocular injection of magnetic microspheres.J Vis Exp2015;(96).
17 Chiu K, Lau WM, Yeung SC, Chang RC, So KF. Retrograde labeling of retinal ganglion cells by application of fluoro-gold on the surface of superior colliculus.J Vis Exp2008;(16).
18 Baskaran M, Kumar RS, Govindasamy CV, Htoon HM, Wong CY,Perera SA, Wong TT, Aung T. Diurnal intraocular pressure fluctuation and associated risk factors in eyes with angle closure.Ophthalmology2009;116(12):2300-2304.
19 Mokhles P, Schouten JS, Beckers HJ, Azuara-Blanco A, Tuulonen A, Webers CA. A Systematic review of end-of-life visual impairment in open-angle glaucoma: an epidemiological autopsy.J Glaucoma2016;25(7):623-628.
20 De Moraes CG, Jasien JV, Simon-Zoula S, Liebmann JM, Ritch R.Visual field change and 24-hour IOP-Related profile with a contact lens sensor in treated glaucoma patients.Ophthalmology2016;123(4):744-753.
21 Morrison JC, Jia L, Cepurna W, Guo Y, Johnson E. Reliability and sensitivity of the TonoLab rebound tonometer in awake Brown Norway rats.Invest Ophthalmol Vis Sci2009;50(6):2802-2808.
22 Aptel F, Weinreb RN, Chiquet C, Mansouri K. 24-h monitoring devices and nyctohemeral rhythms of intraocular pressure.Prog Retin Eye Res2016;55:108-148.
Correspondence to:Kendrick Co Shih. Department of Ophthalmology, LKS Faculty of Medicine, University of Hong Kong 301B, Cyberport 4, 100 Cyberport Road, Pokfulam,Hong Kong SAR, China. kcshih@hku.hk
Received:2017-10-24 Accepted: 2018-03-16
Abstract ● AlM: To investigate the effect of microbead iridocorneal angle occlusion on intraocular pressure (lOP) diurnal fluctuation in rat eyes.● METHODS: Male Dark Agouti (DA) rats, 8-10 week old, were each given a single intracameral injection of microbeads,followed by injection of dispersive viscoelastic solution.The right eye served as the experimental eye, while the left eye served as the control. lOP was measured twice daily postoperatively for 3wk and compared between groups. At the end of 3wk, the rats were sacrificed and the eyes were harvested for histological analysis and retinal ganglion cell (RGC) counting.● RESULTS: After microbead injection, experimental eyes had significantly higher dark time lOP than controls from the second week to the third week [2ndweek: 22.92±1.631 mm Hg(n=5) vs 17.35±0.751 mm Hg (n=5); 3rdweek: 23.59±1.494 mm Hg vs 17.73±0.592 mm Hg (n=5)], while light time lOP was comparable between groups. The fluctuation levels of lOP in the experimental eyes were 7.21±0.398 mm Hg(n=5), 9.50±1.017 mm Hg (n=5) and 10.66±0.894 mm Hg(n=5) from the first week to the third week after injection.Comparatively, they were significantly lower in the control eyes, which were 4.69±0.323 mm Hg (n=5), 2.84±1.122 mm Hg(n=5) and 4.98±0.603 mm Hg (n=5) respectively. However,at the end of 3wk, the larger fluctuations in lOP in the experimental eyes was not associated with a significant loss of RGCs.● CONCLUSlON: Microbead occlusion exacerbates diurnal lOP fluctuation in rats. This reported model may serve as a method of investigating the pathological effects of lOP fluctuation. A longer observation period, or repeated injections, may be needed to observe a significant change in RGC density.
● KEYWORDS:microbead occlusion; diurnal intraocular pressurefluctuation; glaucoma; rat
DOl:10.18240/ijo.2018.07.07
Citation:Fu L, Lai JSM, Lo ACY, Shih KC. Induction of significant intraocular pressure diurnal fluctuation in rats using a modified technique of microbead occlusion. Int J Ophthalmol 2018;11(7):1114-1119