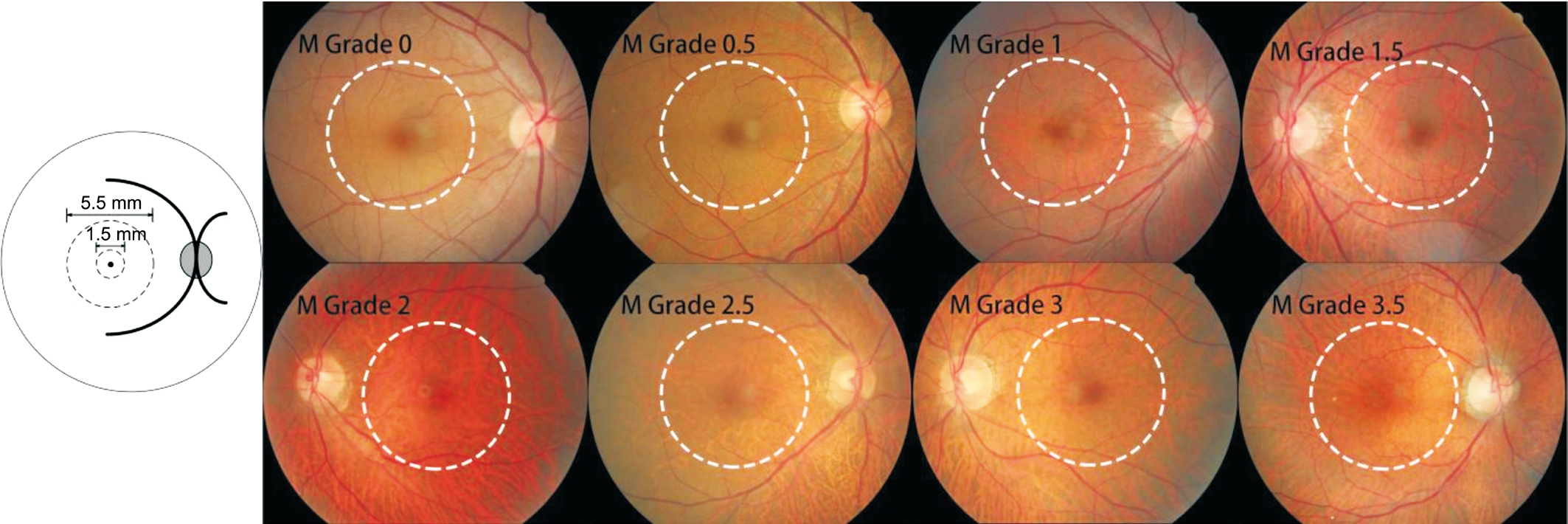
Figure 1 Assessment of fundus tessellation in the macular region.
Yan-Ni Yan1, Ya-Xing Wang2, Yan Yang3, Liang Xu2, Jie Xu2, Qian Wang1, Jing-Yan Yang1, Wen-Jia Zhou1,Wen-Bin Wei1, Jost B. Jonas2,4
1Beijing Tongren Eye Center, Beijing Key Laboratory of Intraocular Tumor Diagnosis and Treatment, Beijing Ophthalmology & Visual Sciences Key Lab, Beijing Tongren Hospital, Capital Medical University, Beijing 100730, China
2Beijing Institute of Ophthalmology, Beijing Tongren Eye Center, Beijing Tongren Hospital, Capital Medical University,Beijing Ophthalmology & Visual Science Key Lab, Beijing 100730, China
3Beijing Aier-Intech Eye Hospital, Beijing 100730, China
4Department of Ophthalmology, Medical Faculty Mannheim of the Ruprecht-Karls-University, Seegartenklinik Heidelberg 68167, Germany
In a previous cross-sectional population-based study, a higher degree of fundus tessellation was strongly associated with thinner subfoveal choroidal thickness[1]. It indicated that the degree of fundus tessellation might be taken as for subfoveal choroidal thickness, if measurements of choroidal thickness are not available. Retinal vein occlusion (RVO) may be associated with abnormal choroidal vasculature. So far there is no studies investigating longitudinal changes of choroid in RVOs. Since the technique to measure subfoveal choroidal thickness has been available only for few years so far, it has not yet been possible to longitudinally assess long-term changes in subfoveal choroidal thickness. We therefore choose the degree of fundus tessellation to reflect subfoveal choroidal thickness, assessed changes in the degree of fundus tessellation over a 10-year period in patients with RVO.

Figure 1 Assessment of fundus tessellation in the macular region.
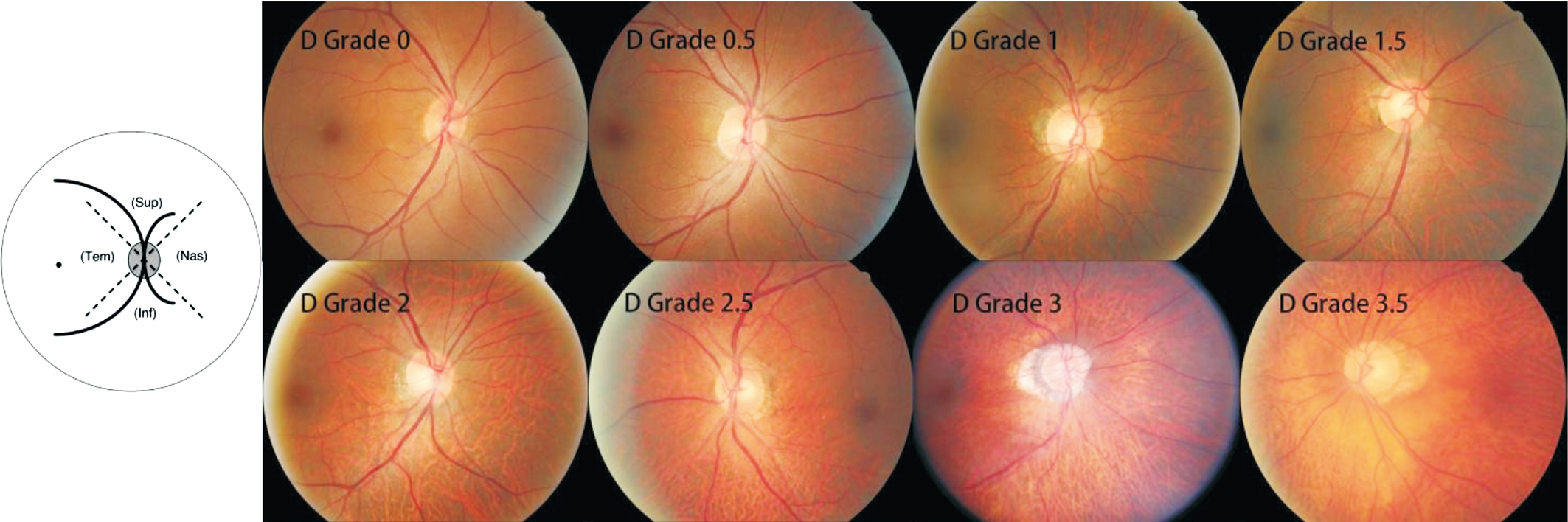
Figure 2 Assessment of fundus tessellation in the peripapillary region Inf: Inferior; Nas: Nasal; Sup: Superior; Tem: Temporal.
The Beijing Eye Study is a population-based longitudinal cohort study in Beijing which started in 2001 at baseline.The Medical Ethics Committee of Beijing Tongren Hospital approved the study, and informed consent to participate in the study was obtained from all participants. The study was performed in accordance with the tenets of the Declaration of Helsinki. In 2001, 4403 individuals ful filling the inclusion criterion of an age of 40y or more were included. The average age of the participants was 56.2±10.6y (median, 54y; range,40-83y), of whom 1973 (44.8%) were from rural region and 2430 (55.2%) were from urban region, 2505 (56.9%) were women and 1898 (43.1%) were men. In 2006 and 2011, the study was repeated by inviting all participants from Beijing Eye Study 2001. Totally 3468 individuals (response rate, 78.8%)with a mean age of 64.6±9.8y (median, 64y; range, 50-93y)participated in the Beijing Eye Study 2011.
Data CollectedAll study participants underwent a detailed physical and ophthalmic examination, and a questionnaire about their habits and customs, education level, incoming, and known systemic diseases.
Ophthalmic examination included best corrected visual acuity[BCVA, Snellen charts (manufacturer: Precision Vision; IL 60181, USA)], intraocular pressure (IOP; CT-60 computerized tonometer, Topcon Ltd., Japan), slit lamp anterior segment photography (Neitz Instruments Co., Tokyo, Japan), biomicroscopy of the anterior segment (Lensstar 900 Optical Biometer,HaagStreit, Koeniz 3098, Switzerland), color fundus photographs(Type CR6-45NM, Canon Inc., USA) and optical coherence tomography (OCT; Spectralis, Heidelberg Engineering Co.,Heidelberg, Germany). IOP was measured three times and the average value was taken. The pupil was dilated before performing color fundus photographs and OCT. The 45°color fundus photographs centered on the macula and optic disc were performed. By using OCT with enhanced depth imaging modality the macular and optic disc were scanned. We measured the retinal nerve fiber layer thickness and subfoveal choroid thickness by using the Heidelberg Eye Explorer software. For the participants, only OCT images of the right eye were taken.
Assessment of Fundus Tessellation ProgressionUsing the color fundus photographs, fundus tessellation was graded as described recently[1], and was graded between grade 0 and grade 3.5 (Figures 1 and 2). The assessment of fundus tessellation was performed by a trained examiner (Yan YN),and supervised by 2 experienced ophthalmologists (WangYX, Jonas JB). The reproducibility of the technique has been reported in our previous study. Progression of fundus tessellation was calculated by fundus tessellation degree of 2011 minus degree of 2001.
Table 1 Demographic parameters, ocular parameters in groups of patients with RVO and the control group in the Beijing Eye Study 2011 mean±SD
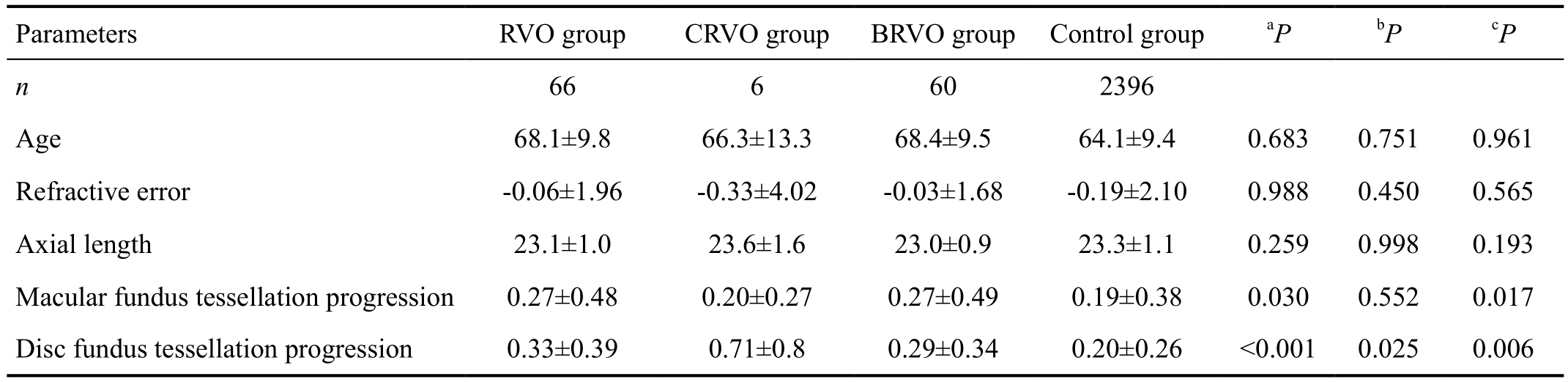
aStatistical significance of the difference between the total RVO group and the control group;bStatistical significance of the difference between the CRVO group and the control group;cStatistical significance of the difference between the BRVO group and the control group.
For the statistical analysis, we used a commercially available software package (SPSS for Windows, version 22.0, IBM-SPSS,Chicago, IL, USA). Corelation between the degree of fundus tessellation progression and other parameters were firstly analyzed using univariate linear regression. Then multivariate linear regression were performed. We assessed standardized regression coefficients beta, regression coefficients B, and 95%CI. All P values were 2-sided and P value less than 0.05 was considered statistically significant.
Assessment of Retinal Vein OcclusionThe assessment for RVO was described in detail in our previous studies[2].
Of the 3468 subjects included into the study, assessment of RVO and fundus tessellation progression were available for 2462 subjects (71.0%) with a mean age of 64.3±9.5y(median, 63y; range, 50-91y), a mean refractive error of-0.15±2.00 diopters (D; median, 0.25 D; range, -22.00 D to 7.50 D), and a mean axial length of 23.2±1.1 mm (median,23.1 mm; range, 18.96-30.88 mm). Altogether, 66 patients in the study population fulfilled the diagnosis of RVO. Of the 66 participants, 59 participants with unilateral branch retinal vein occlusion (BRVO), 5 participants with unilateral central retinal vein occlusion (CRVO), 1 participants with bilateral BRVO, and 1 participant with BRVO in one eye and CRVO in another eye. The RVO group as a whole and differentiated into the CRVO and BRVO group did not differ significantly in age,refractive error, and axial length (Table 1). Mean degree of peripapillary fundus tessellation progression were significantly higher in the whole RVO group (0.33±0.39, P<0.001), CRVO group (0.71±0.8, P=0.025) and BRVO group (0.29±0.34,P=0.006) than the control group (0.20±0.26; Table 1).Mean degree of macular fundus tessellation progression were significantly higher in the whole RVO group (0.27±0.48, P=0.03),and BRVO group (0.27±0.49, P=0.017) than the control group(0.19±0.38). It did not differ significantly (P=0.552) between CRVO group (0.20±0.27) and control group (0.19±0.38).
The mean peripapillary fundus tessellation progression was significantly (P=0.025) higher in the contralateral unaffected eyes of RVO patients (0.30±0.37) than in the control group(0.19±0.25). While the peripapillary fundus tessellation progression did not differ significantly (P=0.678) between RVO eyes (0.32±0.38) and the contralateral unaffected eyes(0.30±0.37) in patients with unilateral RVOs.
In univariate analysis, higher peripapillary fundus tessellation progression was associated significantly with the systemic parameters of older age (P<0.001), male gender (P<0.001),longer smoking duration (P=0.001) and more pack-years of cigarettes (P=0.036), lower body mass index (BMI; P=0.042),decrease of BMI (P<0.001) in 5y, presence of hypertension(P<0.001), diabetes mellitus (P<0.001), and dyslipidemia(P<0.001), higher systolic blood pressure (P<0.001), lower diastolic blood pressures (P=0.024), increase of systolic blood pressures in 10y (P=0.028), higher blood concentration of creatinine (P<0.001), decrease of blood concentration of cholesterol (P=0.002) and low-density lipoproteins (LDL;P<0.001) in 5y, and with the ocular parameters of lower BCVA of distance (P<0.001), decrease of BCVA (P<0.001)in 10y, more myopic refractive error (P=0.009), more myopic change of refractive error (P<0.001) in 10y, decrease of IOP (P=0.009) in 10y, thicker lens thickness (P<0.001),longer anterior corneal curvature (P<0.001), longer axial length (P<0.001), presence of tilted disc (P=0.003), larger cup/disc diameter ratio (P=0.005), larger parapapillary beta zone (P<0.001), thicker fovea thickness (P=0.001), thinner global retinal nerve fiber layer thickness (P<0.001), thinner peripapillary choroidal thickness (PPCT; P<0.001). There was a statistically marginal association between higher degree of fundus tessellation progression and lower IOP (P=0.050). A higher degree of peripapillary fundus tessellation progression was not significantly associated with the systemic parameters of level of education (P=0.107), change of diastolic blood pressures in 5y (P=0.60), blood concentration of glycosylatedhemoglobin (P=0.921), cholesterol (P=0.080), glucose(P=0.311), LDL (P=0.096), high-density lipoproteins (HDL;P=0.785) and triglyceride (P=0.110), change of blood concentration of glucose (P=0.489), HDL (P=0.771) and triglyceride (P=0.769) in 5y, and with the ocular parameters central corneal thickness (P=0.185), area of optic disc(P=0.294), area of parapapillary alpha zone (P=0.097),horizontal (P=0.107) and vertical (P=0.597) bruch membrane opening (BMO) length (P=0.026),and vertical/horizontal BMO length ratio (P=0.176).
Table 2 Associations of peripapillary fundus tessellation progression with ocular and systemic parameters (Multivariate Analysis; PPCT was dropped, presence of RVO was added)
Table 3 Associations between the presence of RVO and systemic and ocular parameters in the Beijing Eye Study 2011
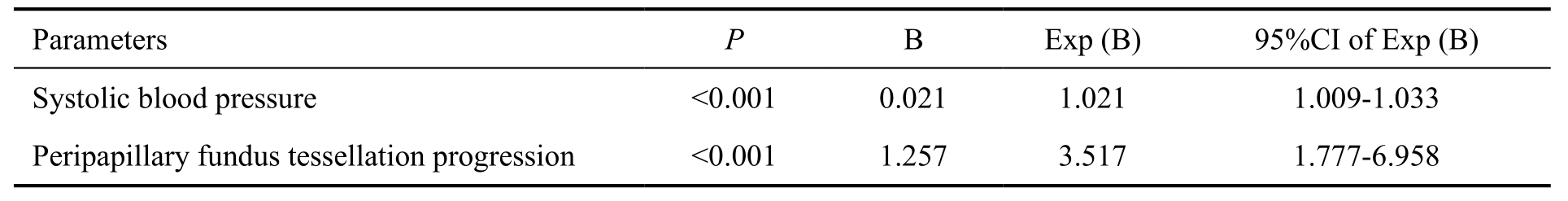
In multivariate analysis, peripapillary fundus tessellation progression increased with thinner PPCT (P<0.001), older age (P<0.001), decrease of BCVA (P=0.002) and worse BCVA (P=0.030). If PPCT was dropped, higher degree of peripapillary fundus tessellation progression was significantly associated with older age (P<0.001), prevalence of tilted disc (P=0.001), decrease of BCVA (P=0.001), longer axial length (P=0.007). After adjustment for these parameters (age,tilted disc, change of BCVA, axial length) and after adding parameters of presence of RVO to the list of independent parameters in the multivariate analysis, progression of peripapillary fundus tessellation was associated with the presence of RVO (P=0.004; regression coefficient B, 0.094; 95%CI,0.029, 0.158; standardized coefficient β, 0.056) (Table 2).
In multivariate analysis, fundus tessellation progression in the macular region increased with thinner subfoveal choroidal thickness (SFCT) (P<0.001), older age (P<0.001), thinner central corneal thickness (P=0.007), shorter axial length(P=0.004), worse BCVA (P=0.011) and higher level of education (P=0.040). SFCT was dropped because the strong association with degree of fundus tessellation progression.In that model, higher degree of macular fundus tessellation progression was significantly associated with older age(P<0.001), worse BCVA (P<0.001), higher level of education(P=0.004), decrease of BCVA (P=0.003), higher IOP(P=0.006) and lower diastolic blood pressure (P=0.020).After adjustment for these parameters (age, BCVA, level of education, change of BCVA, IOP and diastolic blood pressure) and after adding parameters of presence of RVO to the list of independent parameters in the multivariate analysis,progression of macular fundus tessellation in macular region was not associated significantly with the presence of RVO(P=0.660).
In a second part, a logistic regression analysis was performed to assess associations between RVO with systemic and ocular parameters. We found that presence of RVO was associated significantly with longer smoking duration (P=0.002;regression coefficient B: 0.052; OR: 1.054; 95%CI: 1.020,1.089), higher systolic blood pressure (P=0.012; regression coefficient B: 0.043; OR: 1.044; 95%CI: 1.010, 1.080),shorter anterior corneal curvature (P=0.024; regression coefficient B: -3.877; OR: 0.021; 95%CI: 0.001, 0.604). We then performed a binary regression analysis that included in its list of independent variables all parameters that were associated with RVO in the univariate analysis and then added degree of fundus tessellation progression to the list of independent parameters. Presence of RVO was associated with more peripapillary fundus tessellation progression (P<0.001;regression coefficient B: 1.257; OR: 3.517; 95%CI: 1.777,6.958; Table 3), while it is not associated with macular fundus tessellation progression (P=0.147).
In our longitudinal population-based study, peripapillary fundus tessellation progression was associated significantly with the presence of RVO (P=0.004) after adjustment for age, presence of tilted disc, change of BCVA in 10y and axial length, while macular fundus tessellation progression was not associated with the presence of RVO. As a corollary, presence of RVO was associated significantly (P<0.001) with higher peripapillary fundus tessellation progression after adjusting for smoking duration, systolic blood pressure and anterior corneal curvature, while it is not associated with macular fundus tessellation progression. Peripapillary fundus tessellation progression in both RVO eyes and contralateral unaffected eyes of RVO patients were higher than that in the control group. While peripapillary fundus tessellation progression did not differ significantly between RVO eyes and the contralateral unaffected eyes in patients with unilateral RVOs.
In our previous study we found that the degree of fundus tessellation is strongly associated with SFCT[1]. Degree of fundus tessellation might be taken as for SFCT, if measurements of choroidal thickness are not available[1].Investigations revealed that the SFCT of eyes with a recent BRVO was significantly thicker than that of the fellow eye because of increased production of VEGF mediating choroidal vasodilation and increased choriocapillaris permeability, which subsequently induce choroidal swelling[3-9]. Researches about choroidal changes in long-standing RVOs were rare. Our results were consistent with an investigation in which Hae evaluated changes in PPCT in patients with unilateral BRVO over 12mo using OCT with enhanced depth imaging, and found that the PPCT decreased significantly over 12mo in both BRVO-affected (mean PPCT decreased from 213.5±51.7 μm at baseline to 129.6±39.3 μm at 12mo) and nonaffected eyes(mean PPCT decreased from 194.1±39.8 μm at baseline to 156.6±56.2 μm at 12mo; P<0.001, both eyes). In Chinese with an age of 50+ years, with each year increase in age,PPCT decreased only by 2 μm[11]. While in Kang et al’s[10]study the mean SFCT did not change significantly over 12mo in both BRVO-affected eyes and nonaffected contralateral eyes. Similarly, in the current study, we find the 10-year peripapillary fundus tessellation progression was associated with presence of RVO, while the macular fundus tessellation progression was not associated. In our previous populationbased study conducted in the same population, we did not find an association of SFCT or PPCT with presence of RVO[11-12]. These investigations are cross-sectional and choroidal thickness can not reflect changes in choroid over time.
The pathogenesis of RVO may be explained by the vascular theory. A large number of studies confirmed that some systemic diseases, such as hypertension, diabetes mellitus,atherosclerosis and cardiovascular disease are main risk factors of RVO, and systemic hypertension is a particularly important risk factor for RVO[2,13-22]. This suggests RVO may be the performance of these systemic diseases in the eye and the vascular abnormality and consequent vascular insufficiency may be related to pathogenesis of RVO. The importance of these systemic diseases in RVO may be because these diseases cause retinal microvascular changes, such as arterio-venous crossing signs, retinal arterial thinning and sclerosis, at the arteriovenous crossings. As the vascular changes are systemic.When RVO occurs, it indicates that a certain degree of retinal small vessel changes have occurred. So the corresponding choroidal arterial thinning may lead to blood perfusion deficient and thinning of the choroid which may increase visibility of large choroidal vessels. So fundus tessellation,which has a strong relationship with SFCT, may be reflection of choroidal circulation status. And the fundus tessellation progression may reflect decrease of choroidal circulation. In this study, the fundus tessellation progression in the macular region was not associated with presence of RVO. This may imply that the peripapillary region is a more sensitive to vascular insuf ficiency of the choroid than the macular region in patients with RVO. So monitoring of peripapillary fundus tessellation progression may be useful to detect choroidal circulation insuf ficiency.
In this study, peripapillary fundus tessellation progression in both RVO eyes and contralateral unaffected eyes of RVO patients were higher than that in the control group. While the peripapillary fundus tessellation progression did not differ significantly between RVO eyes and the contralateral unaffected eyes in patients with unilateral RVOs. This is inconsistent with Kang et al’s[10]study in which the peripapillary choroidal thickness decreased significantly more in BRVO affected eyes than in nonaffected contralateral eyes. This may be because in Kang et al’s[10]study half of the BRVO-affected eyes underwent intravitreal anti-vascular endothelial growth factor injections in the BRVO-affected eyes for coexisting macular edema which may have impacted the choroidal thickness.Follow-up time in Kang et al’s[10]study was 12mo while it was 10y in the current study. Our results support the vascular theory that systemic vascular abnormalities such as hypertension may lead to bilateral choroidal vascular insuf ficiency and consequent bilateral choroidal thinning in RVO patients. So higher degree of fundus tessellation progression in RVOs may be caused by some systemic factors in RVO patients, not the RVO itself.
There are some limitations of the present study. Firstly, the assessment of the fundus tessellation progression degree was semiquantitative and subjective, thus depended on the experience of the examiner and the progression was not as accurate as objective and quantitative methods. Based on our previous reproducibility study and consistency with other studies, this assessment method is reliable. Secondly,in the current study, fundus fluorescein angiogram was not performed, which is one of the most important tests for the diagnosis of RVO. It may have led to an underestimation of the incidence of RVOs. Thirdly, the overall participation rate in our study was 60.7% of the baseline cohort, or 66.4% of the survivors, which was lower than other 10-year follow-up eye studies. The nonparticipation may have a in fluence on the results of the survey. The reason for the lower participation rate in the current study is because some residents moved away during the follow-up period. Because the reason to move was independent of the general health condition, it may not have a significant influence on the results. Forth, although fundus tessellation was strongly associated with choroidal thickness in the investigation, the association is not of much utility in clinic. It may suggest that clinically, fundus tessellation would have greater utility as a yes/no variable rather than a method for estimating choroidal thickness.
In conclusion, peripapillary fundus tessellation progresses faster in individuals with RVO. This may reflect the thinning and hypoperfusion of choroid in patients with RVO.
Foundations:Supported by the National Natural Science Foundation of China (No.81570891); Beijing Natural Science Foundation of China (No.7151003); Beijing Municipal Administration of Hospitals’ Ascent Plan (No.DFL20150201);the Capital Health Research and Development of Special(No.2016-1-2051).
Conflicts of Interest: Yan YN,None;Wang XY,None;Yang Y,None;Xu L,None;Xu J,None;Wang Q,None;Yang JY,None;Zhou WJ,None;Wei WB,None;Jonas JB,None.
REFERENCES
1 Yan YN, Wang YX, Xu L, Xu J, Wei WB, Jonas JB. Fundus tessellation:prevalence and associated factors: the Beijing Eye Study 2011.Ophthalmology2015;122(9):1873-1880.
2 Zhou JQ, Xu L, Wang S, Wang YX, You QS, Tu Y, Yang H, Jonas JB. The 10-year incidence and risk factors of retinal vein occlusion: the Beijing Eye Study.Ophthalmology2013;120(4):803-808.
3 Okamoto M, Yamashita M, Sakamoto T, Ogata N. Choroidal blood flow and thickness as predictors for response to anti-vascular endothelial growth factor therapy in macular edema secondary to branch retinal vein occlusion.Retina2018;38(3):550-558.
4 Esen E, Sizmaz S, Demircan N. Choroidal thickness changes after intravitreal dexamethasone implant injection for the treatment of macular edema due to retinal vein occlusion.Retina2016;36(12):2297-2303.
5 Rayess N, Rahimy E, Ying GS, Pefkianaki M, Franklin J, Regillo CD, Ho AC, Hsu J. Baseline choroidal thickness as a predictor for treatment outcomes in central retinal vein occlusion.Am J Ophthalmol2016;171:47-52.
6 Arifoglu HB, Duru N, Altunel O, Baskan B, Alabay B, Atas M. Shortterm effects of intravitreal dexamethasone implant (OZURDEX®) on choroidal thickness in patients with naive branch retinal vein occlusion.Arq Bras Oftalmol2016;79(4):243-246.
7 Coban-Karatas M, Altan-Yaycioglu R, Ulas B, Sizmaz S, Canan H,Sariturk C. Choroidal thickness measurements with optical coherence tomography in branch retinal vein occlusion.Int J Ophthalmol2016;9(5):725-729.
8 Chung YK, Shin JA, Park YH. Choroidal volume in branch retinal vein occlusion before and after intravitreal anti-VEGF injection.Retina2015;35(6):1234-1239.
9 Kim KH, Lee DH, Lee JJ, Park SW, Byon IS, Lee JE. Regional choroidal thickness changes in branch retinal vein occlusion with macular edema.Ophthalmologica2015;234(2):109-118.
10 Kang HM, Choi JH, Koh HJ, Lee CS, Lee SC. Significant reduction of peripapillary choroidal thickness in patients with unilateral branch retinal vein occlusion.Retina2018;38(1):72-78.
11 Jiang R, Wang YX, Wei WB, Xu L, Jonas JB. Peripapillary choroidal thickness in adult chinese: the Beijing Eye Study.Invest Ophthalmol Vis Sci2015;56(6):4045-4052.
12 Du KF, Xu L, Shao L, Chen CX, Zhou JQ, Wang YX, You QS, Jonas JB, Wei WB. Subfoveal choroidal thickness in retinal vein occlusion.Ophthalmology2013;120(12):2749-2750.
13 Yang JY, Yang X, Li Y, Xu J, Zhou Y, Wang AX, Gao X, Xu L, Wu SL,Wei WB, Zhao XQ, Jonas JB. Carotid atherosclerosis, cerebrospinal fluid pressure, and retinal vessel diameters: the asymptomatic polyvascular abnormalities in community study.PLoS One2016;11(12): e0166993.
14 Koh V, Cheung CY, Li X, Tian D, Wang JJ, Mitchell P, Cheng CY,Wong TY. Retinal vein occlusion in a multi-ethnic Asian population: the Singapore epidemiology of Eye Disease Study.Ophthalmic Epidemiol2016;23(1):6-13.
15 Lim LL, Cheung N, Wang JJ, Islam FM, Mitchell P, Saw SM, Aung T,Wong TY. Prevalence and risk factors of retinal vein occlusion in an Asian population.Br J Ophthalmol2008;92(10):1316-1319.
16 Cugati S, Wang JJ, Rochtchina E, Mitchell P. Ten-year incidence of retinal vein occlusion in an older population: the Blue Mountains Eye Study.Arch Ophthalmol2006;124(5):726-732.
17 Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia. The Blue Mountains Eye Study.Arch Ophthalmol1996;114(10):1243-1247.
18 Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study.Trans Am Ophthalmol Soc2000;98:133-141.
19 Hayreh SS, Zimmerman B, McCarthy MJ, Podhajsky P. Systemic diseases associated with various types of retinal vein occlusion.Am J Ophthalmol2001;131(1):61-77.
20 Jaulim A, Ahmed B, Khanam T, Chatziralli IP. Branch retinal vein occlusion: epidemiology, pathogenesis, risk factors, clinical features,diagnosis, and complications. An update of the literature.Retina2013;33(5):901-910.
21 Sperduto RD, Hiller R, Chew E, Seigel D, Blair N, Burton TC, Farber MD, Gragoudas ES, Haller J, Seddon JM, Yannuzzi LA. Risk factors for hemiretinal vein occlusion: comparison with risk factors for central and branch retinal vein occlusion: the eye disease case-control study.Ophthalmology1998;105(5):765-771.
22 Risk factors for branch retinal vein occlusion. The Eye Disease Casecontrol Study Group.Am J Ophthalmol1993;116(3):286-296.
Correspondence to:Wen-Bin Wei. Beijing Tongren Eye Center, Beijing Tongren Hospital, 1 Dong Jiao Min Lane,Beijing 100730, China. weiwenbintr@163.com
Received:2017-11-11 Accepted: 2018-04-25
Abstract ● AlM: To access the 10-year fundus tessellation progression in patients with retinal vein occlusion.● METHODS: The Beijing Eye Study 2001/2011 is a populationbased longitudinal study. The study participants underwent a detailed physical and ophthalmic examination. Degree of fundus tessellation was graded by using fundus photographs of the macula and optic disc. Progression of fundus tessellation was calculated by fundus tessellation degree of 2011 minus degree of 2001. Fundus photographs were used for assessment of retinal vein occlusion.● RESULTS: The Beijing Eye Study included 4403 subjects in 2001, 3468 subjects was repeated in 2011. Assessment of retinal vein obstruction and fundus tessellation progression were available for 2462 subjects (71.0%),with 66 subjects fulfilled the diagnosis of retinal vein occlusion. Of the 66 participants, 59 participants with unilateral branch retinal vein occlusion, 5 participants with unilateral central retinal vein occlusion, 1 participant with bilateral branch retinal vein occlusion, and 1 participant with branch retinal vein occlusion in one eye and central retinal vein occlusion in the other eye. Mean degree of peripapillary fundus tessellation progression were significantly higher in the whole retinal vein occlusion group (0.33±0.39, P<0.001), central retinal vein occlusion group (0.71±0.8, P=0.025) and branch retinal vein occlusion group (0.29±0.34, P=0.006) than the control group (0.20±0.26). After adjustment for age, prevalence of tilted disc, change of best corrected visual acuity, axial length, progression of peripapillary fundus tessellation was associated with the presence of retinal vein occlusion(P=0.004; regression coefficient B, 0.094; 95%Cl, 0.029,0.158; standardized coefficient B, 0.056). As a corollary,after adjusting for smoking duration, systolic blood pressure, anterior corneal curvature, prevalence of RVO was associated with more peripapillary fundus tessellation progression (P<0.001; regression coefficient B: 1.257; OR:3.517; 95%Cl: 1.777, 6.958).● CONCLUSlON: Peripapillary fundus tessellation progresses faster in individuals with retinal vein occlusion. This may reflect the thinning and hypoperfusion of choroid in patients with retinal vein occlusion.
● KEYWORDS:fundus tessellation; retinal vein occlusion;Beijing Eye Study
DOl:10.18240/ijo.2018.07.19
Citation:Yan YN, Wang YX, Yang Y, Xu L, Xu J, Wang Q, Yang JY, Zhou WJ, Wei WB, Jonas JB. 10-year fundus tessellation progression and retinal vein occlusion. Int J Ophthalmol 2018;11(7):1192-1197