Tranilast inhibits TGF-β-induced collagen gel contraction mediated by human corneal fibroblasts
Ye Liu1, Xiao-Jing Zhao2, Xiao-Shuo Zheng2, Hui Zheng2, Lei Liu3, Ling-Bin Meng4, Qin Li2, Yang Liu2
1Department of Pathology, the Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai 519000, Guangdong Province,China
2Department of Ophthalmology, the Fifth Affiliated Hospital,Sun Yat-sen University, Zhuhai 519000, Guangdong Province,China
3Department of Ophthalmology, the First Hospital of Jilin University, Jilin 130021, Jilin Province, China
4Department of Internal Medicine, Florida Hospital, Orlando,Florida 32803, USA
INTRODUCTION
The cornea is a transparent tissue. Corneal scarring that results from trauma, mechanical injury, infection, or surgery is a leading cause of blindness worldwide, affecting a total of 7 to 10 million people[1]. Corneal transplantation remains the standard strategy for corneal blindness therapy.However, the clinical reach of corneal transplantation is limited by the supply of donor corneas, and its effectiveness can be compromised by postsurgical complications such as graft failure and rejection[2]. There are currently no effective topical treatments for corneal scarring, and the development of new therapies is thus a clinical priority.
More than 90% of the total cornea is composed of stroma. The current theories regard the corneal stroma as being primarily responsible for the transparency of the cornea. Stromal injury may lead to wound healing. This healing is essentially a series reactions at the cellular and biochemical levels that are characterized by inactive fibroblast activation, migration, and proliferation, as well as extracellular matrix (ECM) synthesis and contraction[3]. However, corneal scarring, a severe complication of corneal injury, could be caused by inordinate stroma contraction at the wound site. Previous studies showed that transforming growth factor beta (TGF-β) significantly affects corneal fibrosis and scarring[4-5], with TGF-β signal transduction thus being a promising target of developing anti-fibrotic therapies. The disruption of TGF-β signaling by the targeted delivery of the inhibitory protein Smad7 with a recombinant adeno-associated virus injected into the corneal stroma has been found to attenuate the incidence of corneal haze after photorefractive keratectomy (PRK) in a rabbit model of corneal injury[6]. Treatment with cationic nanoparticles complexed with a combination of three small interfering RNAs targeting TGF-β and its receptor mRNAs also inhibited the occurrence of corneal haze in an in vitro model of rabbit cornea culture[7].
Tranilast, or N-(3’,4’-dimethoxycinnamoyl)-anthranilic acid, is used as an anti-allergic medicine that is widely applied for the administration in allergic diseases (e.g. allergic conjunctivitis,bronchial asthma, and hypertrophic scars)[8]. It also exerts an anti-fibrotic effect by inhibiting TGF-β functions. Tranilast attenuated the development of diastolic dysfunction and histopathological characteristics of experimental diabetic cardiomyopathy by inhibiting TGF-β-induced protein kinase phosphorylation[9]. The topical application of tranilast was also shown to attenuate the development of corneal haze after PRK[10]and to prevent postoperative adhesion after strabismus surgery[11]in rabbit models by suppressing TGF-β expression.Given that corneal fibroblast-mediated collagen contraction is involved in corneal scarring and the loss of corneal transparency, using an in vitro model, we investigated if tranilast has effects on human corneal fibroblast (HCF)-mediated collagen gel contraction in stimulation with TGF-β.Moreover, we tested the effects of tranilast on myosin light chain (MLC) and paxillin phosphorylation, the secretion of tissue inhibitors of metalloproteinases (TIMPs) and matrix metalloproteinases (MMPs), as well as the development of focal adhesions and actin stress fibers in these cells.
MATERIALS AND METHODS
MaterialsThe materials and abbreviations of the companies that supplied the materials are listed as follows. Invitrogen-Gibco (Rockville, MD, USA): fetal bovine serum (FBS),trypsin-EDTA, and 10× Eagle’s minimum essential medium(MEM). Nacalai Tesque (Kyoto, Japan): bovine serum albumin(BSA). Nitta Gelatin (Osaka, Japan): reconstitution buffer and acid solubilized native porcine type I collagen. Sigma-Aldrich (St. Louis, MO, USA): anti-β-actin antibody, tranilast,collagenase, and dispase. Millipore (Bedford, MA, USA): antiphosphotyrosine antibody. BD Biosciences (San Jose, CA,USA): anti-paxillin and anti-MLC antibodies. Cell Signaling(Beverly, MA, USA): anti-phosphorylated paxillin (phospho-Tyr118) and anti-phosphorylated MLC antibodies. Molecular Probes (Eugene, OR, USA): TOTO-3 iodide (642/640), Alexa Fluor 488-conjugated anti-mouse immunoglobulin G antibody,and phalloidin (Alexa Fluor 568-conjugated). R&D Systems(Minneapolis, MN): recombinant human TGF-β1, anti-MMPs and anti-TIMPs antibodies. Amersham Pharmacia Biotech(Uppsala, Sweden): enhanced chemiluminescence (ECL) kit and nitrocellulose membranes. Endotoxin was minimized for all reagents and media for cell culture.
Human Corneal Fibroblasts Isolation and CultureHCFs were obtained from the remaining tissue after the corneal transplantation surgeries. The present study was performed according to the principles of the Declaration of Helsinki for research involving human subjects. Cell isolation and culture were performed as described[12]. In brief, after mechanically removing the endothelial layer of the cornea, the remaining tissue was incubated with 2 mg/mL of dispase in MEM at 37℃ for 1h. The single-cell suspension of HCFs was achieved by mechanically removing the epithelial layer and treating with 2 mg/mL of collagenase in MEM at 37℃. The cells were maintained in MEM added with 10% FBS and were incubated at 37℃ under 5% (vol/vol) CO2. Three individual donors’ cells were employed. Three to seven passages were used for the present study.
Collagen Gel Contraction AssayCollagen gel was prepared as described elsewhere[12]. Reconstitution buffer, 3 mg/mL of type I collagen, 10×MEM, and 1.1×106cells/mL of HCF suspension were combined at the ratio of 1:7:1:2 (vol/vol/vol/vol)on ice. The mixture was equally (0.5 mL) added into a 24-well culture plate that was pre-coated with 1% BSA. A microspatula was used to free the collagen gels and solidified mixture from the sides of the wells. TGF-β or tranilast in 0.5 mL serum-free MEM was added onto the top of each gel. A ruler was used daily to measure the diameter of the gels, and gel contraction was calculated at every time point by subtracting the baseline diameter. Each measurement was triplicated and averaged.
Immunoblot AnalysisFollowing a previous protocol,immunoblot analysis was performed[13]. For paxillin and MLC,ice-cold lysis buffer was used to lyse collagen gel/HCFs culture, and 10 μg protein of the lysates were subjected to SDS-polyacrylamide gel electrophoresis (PAGE). For TIMP-1, TIMP-2,MMP-1, and MMP-3, culture supernatants were fractionated by SDS-PAGE. The proteins were separated and transferred onto a nitrocellulose membrane. The following blocking was performed in 5% nonfat milk at room temperature for one hour. The specific primary antibodies (1:1000 dilution) were incubated at 4℃ for overnight. Then, the membrane was washed extensively with Tris-buffered saline with Tween-20.Horseradish peroxidase-conjugated secondary antibodies were incubated at room temperature for one hour and detected by ECL reagents. The intensity of the immunoreactive bands was measured by ImageJ (NIH, Bethesda, MD, USA).
Gelatin ZymographyAccording to the previous study,gelatin zymography for culture supernatants was performed[14].A mixture of culture supernatant (8 μL) and nonreducing SDS sample buffer (4 μL) were applied to SDS-PAGE (0.1%gelatin) at 4℃. The gel then went through the following process: primary incubation in 2.5% Triton X-100 at room temperature for 1h, secondary incubation in a reaction buffer of 1% Triton X-100, Tris-HCl (50 mmol/L), and CaCl2(5 mmol/L)for 18h at 37℃, and final staining with 0.5% Coomassie brilliant blue.
Fluorescence MicroscopyAccording to a described protocol,F-actin staining and phosphotyrosine of HCFs cultured in collagen gel were performed[13]. Fixation of HCFs in collagen gels were performed by applying 1% paraformaldehyde in phosphate-buffered saline (PBS) for half an hour at room temperature, allowed to dry, and then permeabilized by 1% Triton X-100 in PBS for another half hour. After 1%BSA blocking, the cells were incubated for 1h with antiphosphotyrosine (1:200 dilution) antibody at room temperature and then incubated with Alexa Fluor 488-conjugated secondary antibody (1:1000 dilution) for a half hour. Finally,the cells were incubated with 1:200 diluted phalloidin (Alexa Fluor 568-conjugated) for a half hour for F-actin staining and incubated with 1:3000 diluted TOTO-3 iodide for 10min for nuclei staining. A confocal scanning laser microscope(Axiovert200M; Carl Zeiss, Japan) was used for imaging.
Statistical AnalysisQuantitative data are expressed as means±standard deviation (SD). The experiments were performed at least three times (triplicated for each independent experiment). Significant differences were determined using Dunnett’s multiple comparison test or the Tukey-Kramer test. A significant level of P value of 0.05 was used for all statistical tests.
RESULTS
Effect of Tranilast on Human Corneal Fibroblastsmediated Collagen Gel Contraction Induced by TGF-βTo investigate if tranilast can affect collagen contraction mediated by HCFs, we employed a three-dimensional collagen gel for cell culture with or without 1 ng/mL of TGF-β as well as several concentrations of tranilast for various durations. In our 3-day incubation with tranilast at a range of 30 to 300 μmol/L,the inhibition of TGF-β-stimulated gel contraction by tranilast was dose-dependent. We observed a statistically significant effect that started from 100 μmol/L and maximized at 300 μmol/L(Figure 1). This effect of 300 μmol/L tranilast was also duration-dependent and became statistically significant at day 2 (Figure 2).
Effects of Tranilast on TGF-β-induced Stress Fibers and Focal Adhesion Formation in Human Corneal FibroblastsThe 3-day cell culture in collagen gels with no TGF-β contained no stress fibers and exerted a dendritic morphology and few phosphotyrosine-specific fluorescence signals (Figure 3A-3C). Cells cultured with TGF-β exhibited actin stress fibers accompanied with phosphotyrosine immunofluorescence in a punctate pattern. The merged fluorescence signal of F-actin and phosphotyrosine implied that stress fiber formation may correlate with phosphotyrosine expression (Figure 3E-3G).However, compared with cells only stimulated by TGF-β,the group exposed to TGF-β and treated with 300 μmol/L of tranilast showed a relatively normal morphology with less stress fiber formation and focal adhesions (Figure 3I-3K). The negative control of normal mouse IgG staining confirmed the specificity of phosphotyrosine staining (Figure 3D, 3H).
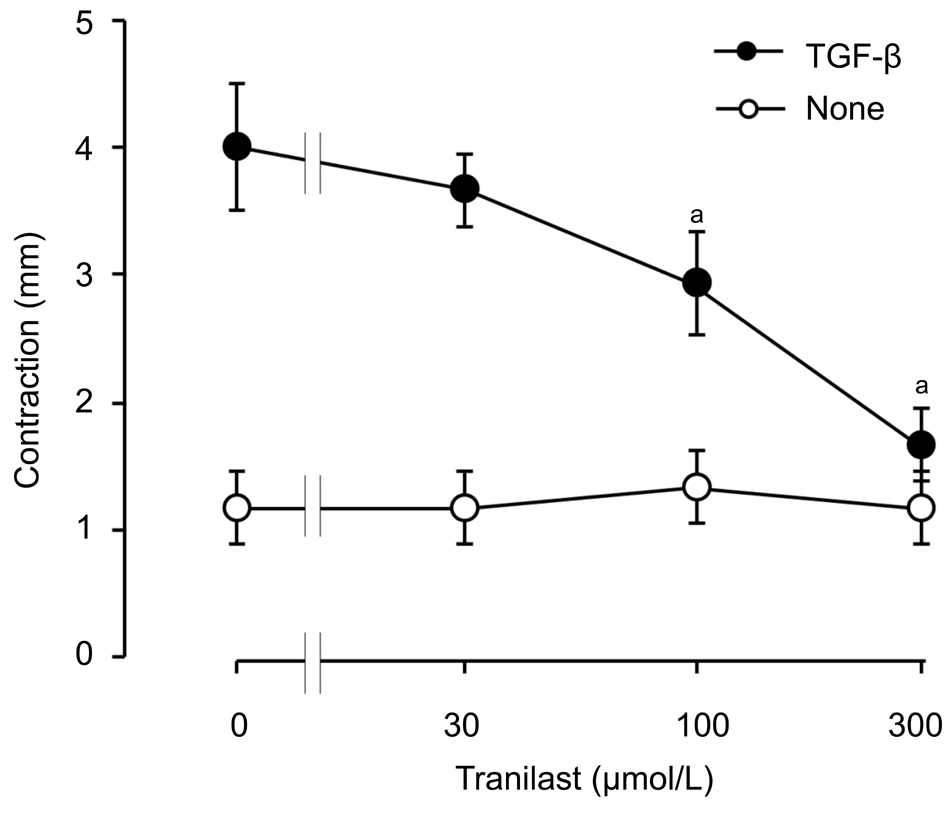
Figure 1 Concentration-dependent inhibitory effect of tranilast on HCF-mediated collagen gel contraction induced by TGF-β A 3-day cell culture was performed in collagen gels without or with 1 ng/mL of TGF-β and with the labeled concentrations of tranilast.The severity of gel contraction was measured. Data are presented as means±SD and analyzed by Tukey-Kramer test.aP˂0.05.
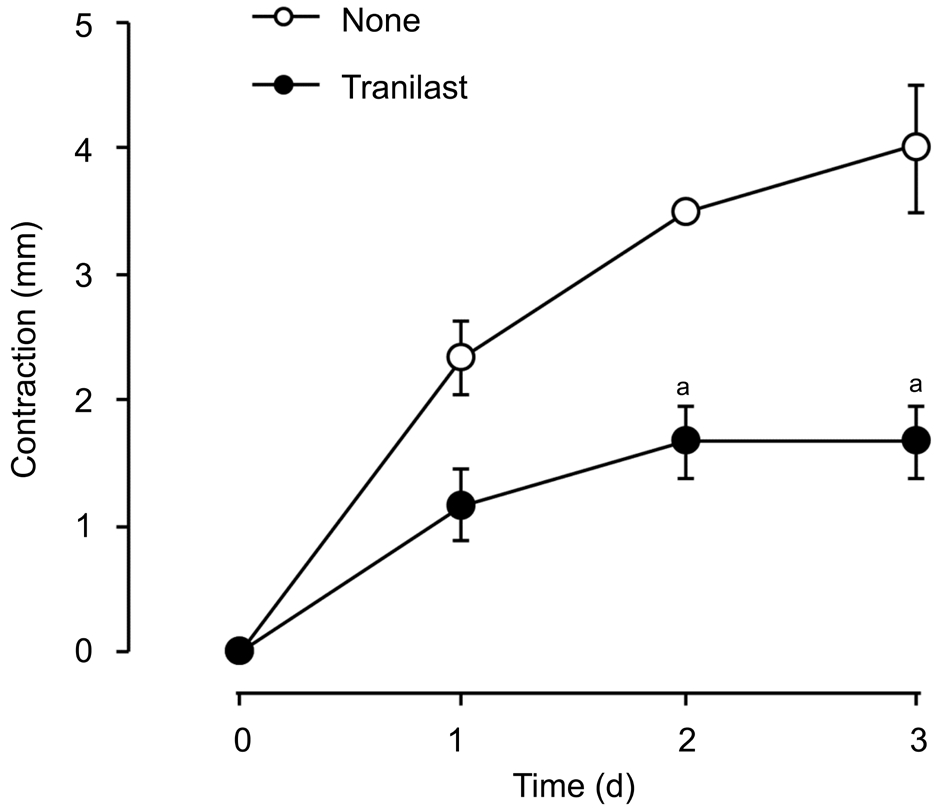
Figure 2 The longitudinal effects of tranilast on the HCF-mediated contraction of collagen gel induced by TGF-β Cell incubation was carried out in collagen gels with 1 ng/mL TGF-β with or without 300 μmol/L tranilast, before gel contraction was determined. Means±SD are calculated. Data were analyzed by the Tukey-Kramer test.aP˂0.05.
Effects of Tranilast on Paxillin and Myosin Light Chain Phosphorylation in Human Corneal FibroblastsThe phosphorylation of paxillin (Figure 4) and MLC (Figure 5)in HCFs can be induced by TGF-β. The phosphorylation was concentration-dependently inhibited by tranilast. The 300 μmol/L of tranilast also inhibited the total paxillin expression (Figure 4)without exerting an effect on the total expression of MLC(Figure 5).
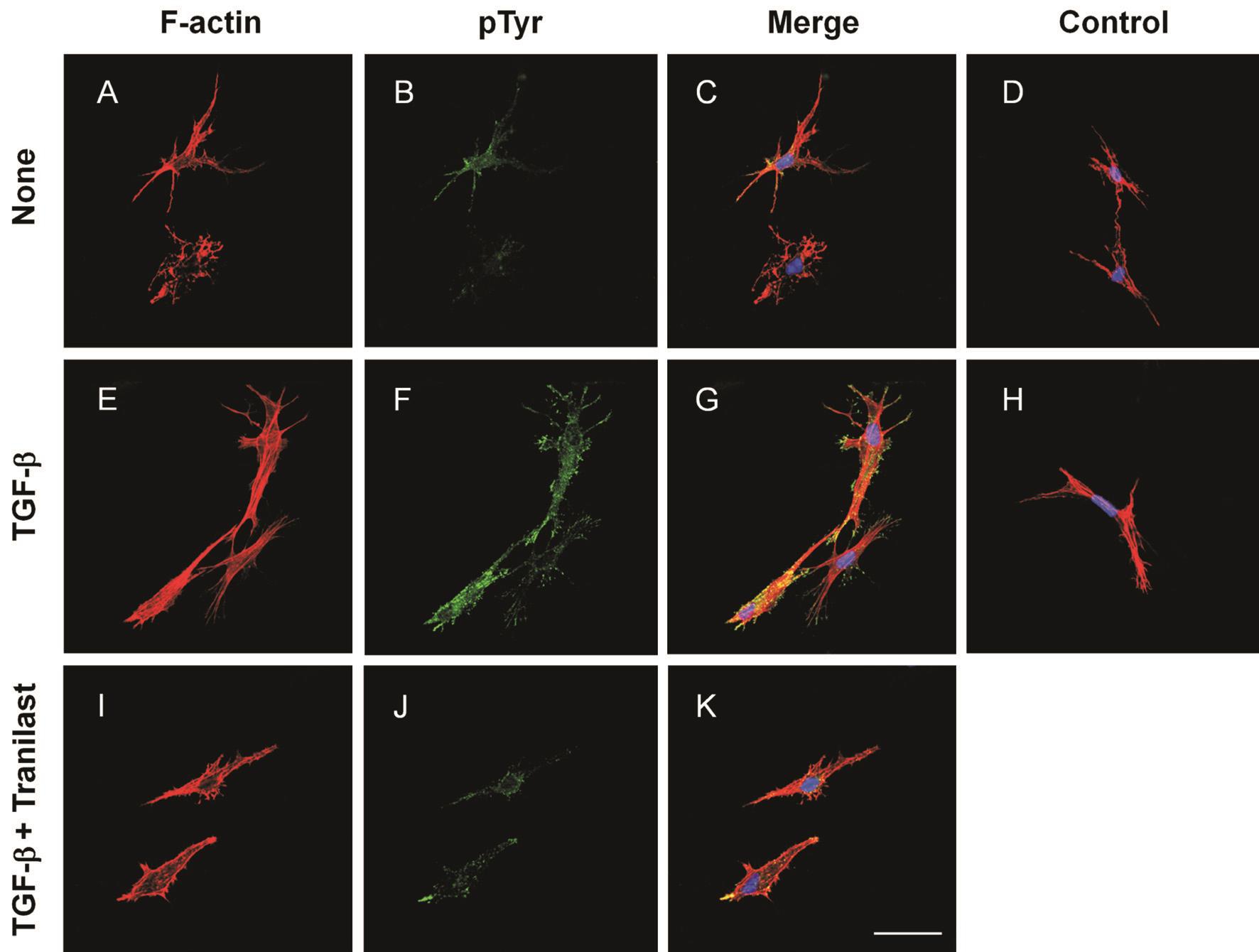
Figure 3 Inhibitory effect of tranilast on stress fiber and focal adhesion formation containing phosphotyrosine in HCFs Fluoresce micrographs of cells seeded in collagen gels over 3-day experiments without an addition (A-D), with TGF-β (1 ng/mL) alone (E-H), or with 1 ng/mL TGF-β and 300 μmol/L tranilast (I-K), with tranilast treatment to the cells 6h before the application of TGF-β. Phalloidin (Alexa Fluor 568-labeled) staining for the cells (F-actin, red) (A, E, I), with anti-phosphotyrosine (pTyr) antibody and secondary antibody (Alexa Fluor 488-conjugated) (green) (B, F, J). Merged images with TOTO-3 iodide-stained nuclei (blue) (C, G, K). Control merged images in which antiphosphotyrosine antibody was replaced by normal mouse IgG (D, H). Scale bar=20 μm.
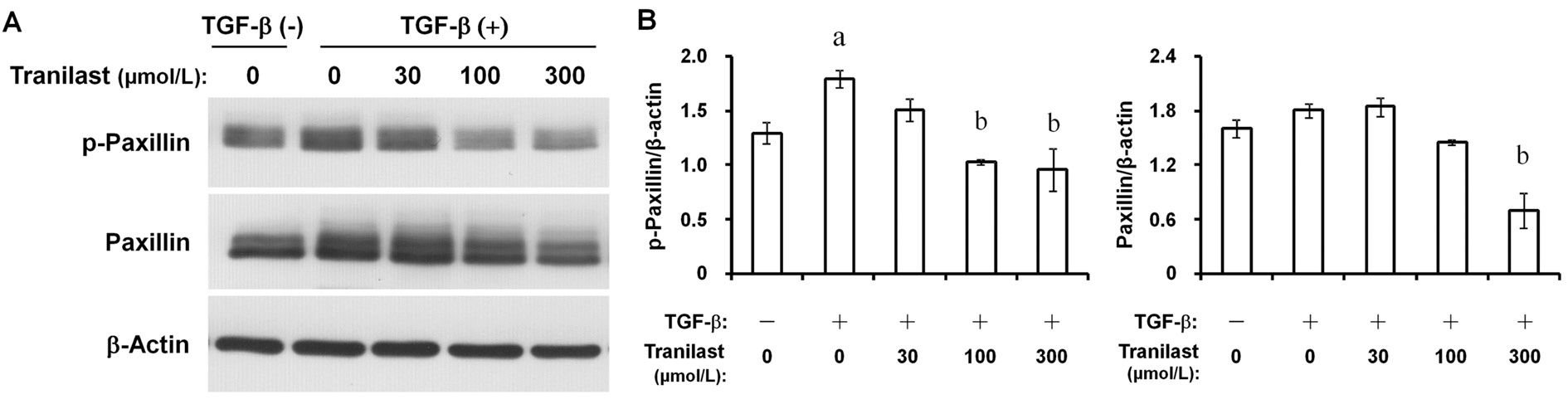
Figure 4 Inhibition by tranilast of TGF-β-induced paxillin phosphorylation in HCFs A: Multiple doses of tranilast were applied to cells seeded in collagen gels for 6h. Cells were then incubated with or without 1 ng/mL TGF-β for 3d. Cell lysates were incubated with antiphosphorylated (p-) antibody or paxillin for immunoblot analysis. β-actin was set as the internal control; B: Quantitation of the ratio of p-paxillin or total paxillin to β-actin for the cells treated as above. Data are presented as means±SD. Dunnett’s test was employed.aP˂0.05 compared with the corresponding value of cells incubated without an addition.bP˂0.05 compared with the corresponding value of the cells incubated with TGF-β only.
Effects of Tranilast on the Secretion of MMPs and TIMPs by Human Corneal FibroblastsImmunoblot analysis revealed that TGF-β increased the release of MMP-1 and MMP-3 in culture. The secretion of these proteins was sensitively and dose-dependently inhibited by tranilast. In supernatant gelatin zymography, the amounts of the pro and active forms of MMP-2 were significantly increased by TGF-β. However,the effect was suppressed by tranilast at 300 μmol/L. TGF-β minimally stimulated the release of TIMP-2 from HCFs, which was not inhibited by tranilast (Figure 6).
DISCUSSION
The current study revealed that tranilast inhibited the HCFsmediated collagen gel contraction induced by TGF-β in a doseand duration-dependent manner. Tranilast also attenuated TGF-β-stimulated paxillin and MLC phosphorylation, as well as focal adhesion and stress fiber formation. In addition, tranilast inhibited the secretion of MMP-1, MMP-2, as well as MMP-3 by HCFs stimulated by TGF-β.

Figure 5 Inhibitory effect of tranilast on MLC phosphorylation in HCFs induced by TGF-β A: Multiple doses of tranilast were applied to cells seeded in collagen gels for 6h, followed by a 3-day incubation with or without 1 ng/mL TGF-β. Cell lysates were incubated with antiphosphorylated (p-) antibody or MLC for immunoblot analysis. β-actin was set as the internal control. B: Quantitation of the ratio of p-MLC or total MLC to β-actin for cells with the same treatment. Data are presented as means±SD. Dunnett’s test was used for the data analyses.aP˂0.05 compared with the corresponding value of cells incubated without the addition.bP˂0.05 compared with the corresponding value of cells incubated with TGF-β only.
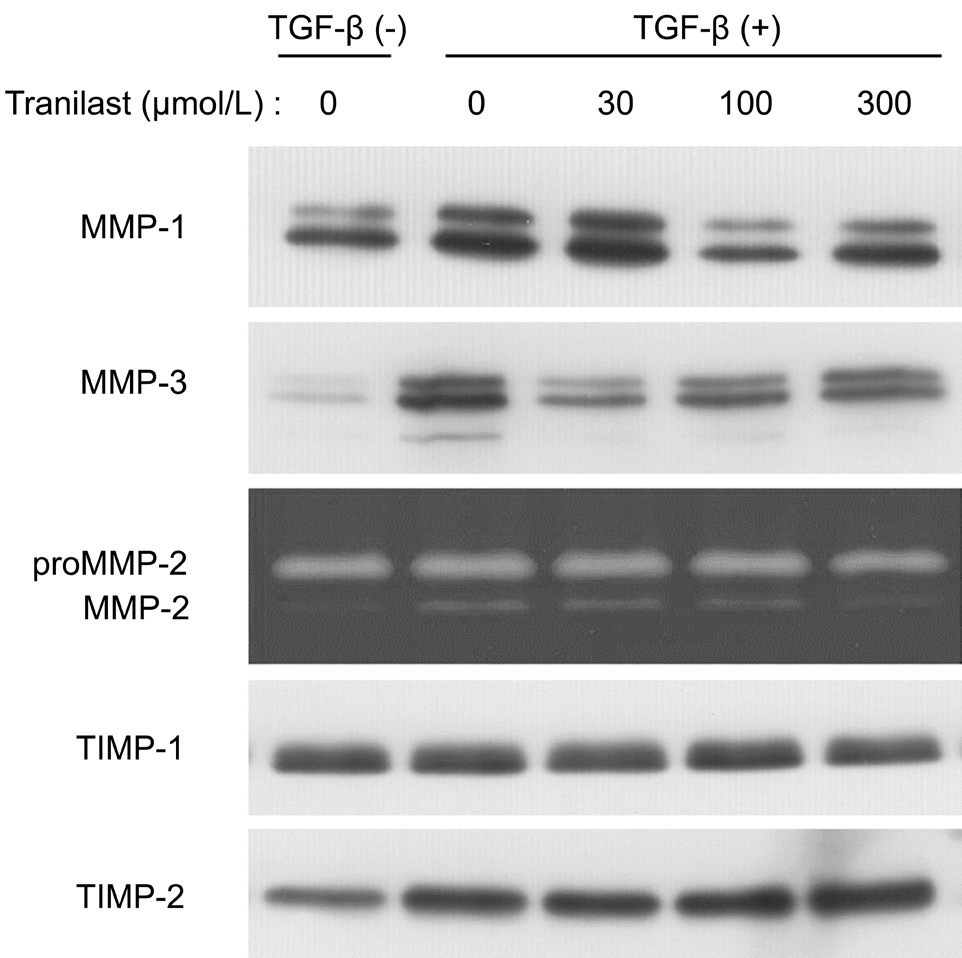
Figure 6 Inhibitory effect of tranilast on the secretion of MMPs and TIMPs by HCFs A 3-day cell incubation was performed in collagen gels with or without 1 ng/mL TGF-β and with the labeled doses of tranilast. The culture supernatants were then used for immunoblot analysis with anti-MMP-1, anti-MMP-3, anti-TIMP-1,or anti-TIMP-2 antibodies and analyzed by gelatin zymography for detecting pro and active forms of MMP-2.
Cornea transparency is largely contributed by the stroma,which consists of ECM (mainly type I collagen) and keratocytes.Corneal fibroblast-mediated ECM contraction is required for wound closure during the normal wound healing process[15].However, excessive contraction may result in the development of corneal scarring and the consequent loss of tissue clarity.Floating three-dimensional collagen gels have been adopted as a model for studies of fibrosis and tissue contractility.TGF-β plays a crucial role in corneal fibrosis. In the current study, we demonstrated that tranilast alleviated the contraction of collagen gel under the stimulation of TGF-β, which was mediated by HCFs with dose- and duration-dependent characteristics. Corneal haze after photorefractive keratectomy can be inhibited by topical tranilast through down-regulating TGF-β1 expression in keratocytes[10]. Additionally, 0.5%tranilast ophthalmic solution instillation was effective in preventing adhesion and fibrosis after strabismus surgery[11].The present results implied that tranilast could also effectively prevent or treat corneal scarring through its inhibitory action on the contractility of corneal fibroblasts.
Cell contractility has been reported to be enhanced by TGF-β stimulation. The mechanisms include focal adhesion activation and actin microfilaments polymerization and reorganization[13].Another study showed that actin stress fibers formation and the activation of adhesion molecules related to integrin-ECM interactions could contribute to the contraction of collagen gel mediated by HCFs[12]. We have now shown that TGF-βstimulated actin stress fibers and focal adhesion formation in HCFs can be inhibited by tranilast. By activating both serinethreonine and tyrosine kinases signaling pathways, TGF-β induces downstream molecule phosphorylation[16]. Paxillin,a focal adhesions component, takes tyrosine phosphorylation under the TGF-β stimulation[17]. The phosphorylation of MLC further enhances stress fiber formation as well as actomyosin contraction[18]. Based on our current data, we demonstrated that tranilast suppressed TGF-β-induced paxillin and MLC phosphorylation in HCFs cultured in collagen gels. Thus, partly due to the inhibition of paxillin and MLC phosphorylation,tranilast alleviated the focal adhesion formation and stress fibers induced by TGF-β. The effects of tranilast could also inhibit the fibrotic tissue contraction in corneal scarring.
TGF-β induces fibrosis both by eliciting cellular transdifferentiation and modulating MMP activity[19]. MMPs are calcium-dependent zinc-containing endopeptidases. They can target the ECM components to up-regulate the expression of the enzymes that are associated with inordinate scarring at the ocular surface[20].MMPs have been proven to be involved in wound contraction and matrix reorganization. They have exhibited important functions during wound healing[21-22]. An animal study showed that the inhibition of MMPs could reduce matrix contraction and limit subconjunctival scarring[23]. Various studies have also demonstrated that TGF-β up-regulates MMP-1, MMP-2, and MMP-3 expression[14,24-25]. We found that tranilast attenuated the release of these MMPs in HCFs induced by TGF-β, implying that the inhibiting effects of tranilast on TGF-β-induced collagen gel contraction could also be caused by similar mechanisms.
In summary, we have shown that tranilast inhibited the HCF contraction induced by TGF-β by suppressing actin stress fibers formation, focal adhesions formation, and MMPs release. Therefore, tranilast may effectively inhibit corneal scarring by attenuating corneal fibroblast contractility.
ACKNOWLEDGEMENTS
Foundations:Supported by the National Science Foundation of China (No.81770889); the Natural Science Foundation of Guangdong Province (No.2017A030313774); the Research Fund of Jilin Provincial Science and Technology Department to Yang Liu (International Cooperation Item, No.20160414055GH).
Conflicts of Interest:Liu Y, None; Zhao XJ, None; Zheng XS, None; Zheng H, None; Liu L, None; Meng LB, None; Li Q, None; Liu Y, None.
REFERENCES
1 Mariotti SP. Global data on visual impairments 2010: World Health Organization. Available at: http://www.who.int/blindness/.
2 Akanda ZZ, Naeem A, Russell E,Belrose J, Si FF, Hodge WG. Graft rejection rate and graft failure rate of penetrating keratoplasty (PKP) vs lamellar procedures: a systematic review. PLoS One 2015;10(3):e0119934.
3 Garana RM, Petroll WM, Chen WT, Herman IM, Barry P, Andrews P, Cavanagh HD, Jester JV. Radial keratotomy. II. Role of the myofibroblast in corneal wound contraction. Invest Ophthalmol Vis Sci 1992;33(12):3271-3282.
4 Margo JA, Munir WM. Corneal haze following refractive surgery: a review of pathophysiology, incidence, prevention, and treatment. Int Ophthalmol Clin 2016;56(2):111-125.
5 Saika S, Yamanaka O, Sumioka T, Okada Y, Miyamoto T, Shirai K,Kitano A, Tanaka S. Transforming growth factor beta signal transduction:a potential target for maintenance/restoration of transparency of the cornea. Eye Contact Lens 2010;36(5):286-289.
6 Gupta S, Rodier JT, Sharma A, Giuliano EA, Sinha PR, Hesemann NP, Ghosh A, Mohan RR. Targeted AAV5-Smad7 gene therapy inhibits corneal scarring in vivo. PLoS One 2017;12(3):e0172928.
7 Sriram S, Gibson DJ, Robinson P, Pi L, Tuli S, Lewin AS, Schultz G.Assessment of anti-scarring therapies in ex vivo organ cultured rabbit corneas. Exp Eye Res 2014;125:173-182.
8 Darakhshan S, Pour AB. Tranilast: a review of its therapeutic applications.Pharmacol Res 2015;91:15-28.
9 Kelly DJ, Zhang Y, Connelly K, Cox AJ, Martin J, Krum H, Gilbert RE. Tranilast attenuates diastolic dysfunction and structural injury in experimental diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol 2007;293(5):H2860-H2869.
10 Song JS, Jung HR, Kim HM. Effects of topical tranilast on corneal haze after photorefractive keratectomy. J Cataract Refract Surg 2005;31(5):1065-1073.
11 Choi SU, Kim KW, Moon NJ. Effective treatment for prevention of post-operative adhesion after strabismus surgery in experimental rabbit model:0.5% tranilast ophthalmic solution. BMC Ophthalmol 2016;16(1):166.
12 Liu Y, Yanai R, Lu Y, Kimura K, Nishida T. Promotion by fibronectin of collagen gel contraction mediated by human corneal fibroblasts. Exp Eye Res 2006;83(5):1196-1204.
13 Liu Y, Kimura K, Orita T, Teranishi S, Suzuki K, Sonoda KH.Inhibition by all-trans-retinoic acid of transforming growth factor-betainduced collagen gel contraction mediated by human tenon fibroblasts.Invest Ophthalmol Vis Sci 2014;55(7):4199-4205.
14 Liu Y, Kimura K, Orita T, Teranishi S, Suzuki K, Sonoda KH. Alltrans-retinoic acid inhibition of transforming growth factor-β-induced collagen gel contraction mediated by human Tenon fibroblasts: role of matrix metalloproteinases. Br J Ophthalmol 2015;99(4):561-565.
15 Lyon D, McKay TB, Sarkar-Nag A, Priyadarsini S, Karamichos D.Human keratoconus cell contractility is mediated by transforming growth factor-beta isoforms. J Funct Biomater 2015;6(2):422-438.
16 Kimura K, Orita T, Liu Y, Yang Y, Tokuda K, Kurakazu T, Noda T,Yanai R, Morishige N, Takeda A, Ishibashi T, Sonoda KH. Attenuation of EMT in RPE cells and subretinal fibrosis by an RAR-γ agonist. J Mol Med (Berl) 2015;93(7):749-758.
17 Funderburgh JL, Funderburgh ML, Mann MM, Corpuz L, Roth MR. Proteoglycan expression during transforming growth factor β-induced keratocyte-myofibroblast transdifferentiation. J Biol Chem 2001;276(47):44173-44178.
18 Ye Y, Yang Y, Cai X, Liu L, Wu K, Yu M. Down-regulation of 14-3-3 zeta inhibits TGF-β1-induced actomyosin contraction in human trabecular meshwork cells through RhoA signaling pathway. Invest Ophthalmol Vis Sci 2016;57(2):719-730.
19 Tandon A, Tovey J, Sharma A, Gupta R, Mohan RR. Role of transforming growth factor beta in corneal function, biology and pathology. Curr Mol Med 2010;10(6):565-578.
20 Ollivier FJ, Gilger BC, Barrie KP, Kallberg ME, Plummer CE, O’Reilly S, Gelatt KN, Brooks DE. Proteinases of the cornea and preocular tear film. Vet Ophthalmol 2007;10(4):199-206.
21 Daniels JT, Schultz GS, Blalock TD, Garrett Q, Grotendorst GR,Dean NM, Khaw PT. Mediation of transforming growth factor-beta(1)-stimulated matrix contraction by fibroblasts: a role for connective tissue growth factor in contractile scarring. Am J Pathol 2003;163(5):2043-2052.
22 Porter RA, Brown RA, Eastwood M, Occleston NL, Khaw PT.Ultrastructural changes during contraction of collagen lattices by ocular fibroblasts. Wound Repair Regen 1998;6(2):157-166.
23 Wong TT, Mead AL, Khaw PT. Matrix metalloproteinase inhibition modulates postoperative scarring after experimental glaucoma filtration surgery. Invest Ophthalmol Vis Sci 2003;44(3):1097-1103.
24 Benito MJ, Calder V, Corrales RM, García-Vázquez C, Narayanan S,Herreras JM, Stern ME, Calonge M, Enríquez-de-Salamanca A. Effect of TGF-β on ocular surface epithelial cells. Exp Eye Res 2013;107:88-100.
25 Gronkiewicz KM, Giuliano EA, Sharma A, Mohan RR. Molecular mechanisms of suberoylanilide hydroxamic acid in the inhibition of TGF-β1-mediated canine corneal fibrosis. Vet Ophthalmol 2016;19(6):480-487.
Correspondenceto:Yang Liu. Department of Ophthalmology,the Fifth Affiliated Hospital of Sun Yat-sen University, 52 Road Meihuadong, Zhuhai 519000, Guangdong Province,China. liuyang26@mail.sysu.edu.cn
Received:2018-04-13 Accepted: 2018-05-31
Abstract● AlM: To determine if tranilast affects human corneal fibroblast (HCFs) contraction.● METHODS: HCFs cultured in a three-dimensional type l collagen gel were treated with or without transforming growth factor beta (TGF-β) or tranilast. Gel diameter was measured as an indicator for collagen contraction.lmmunoblot was performed to evaluate myosin light chain(MLC) and paxillin phosphorylation. Confocal microscopy was employed to examine the focal adhesions and actin stress fiber formation. lmmunoblot analysis and gelatin zymography were performed to detect tissue inhibitors of metalloproteinases and matrix metalloproteinases (MMPs)in supernatant.● RESULTS: The inhibitory effect of tranilast on HCFsmediated collagen gel contraction induced by TGF-β was dose-dependent. The significant effect of tranilast was started from 100 μmol/L and maximized at 300 μmol/L. The peak effect of 300 μmol/L tranilast also relied on the duration of treatment, which showed statistical significance from day 2. TGF-β-induced paxillin and MLC phosphorylation, stress fiber formation, focal adhesions, and MMP-1, MMP-2, and MMP-3 secretion in HCFs were also inhibited by tranilast.● CONCLUSlON: Tranilast suppresses the HCFs-cultured collagen gel contraction induced by TGF-β. lt attenuates actin stress fibers formation, focal adhesions, and the secretion of MMPs, with these actions likely contributing to the inhibitory effect on HCF contractility. By attenuating the contractility of corneal fibroblasts, tranilast treatment may inhibit corneal scarring.
● KEYWORDS:tranilast; transforming growth factor beta;corneal fibroblast; corneal wound healing
DOl:10.18240/ijo.2018.08.01
Citation:Liu Y, Zhao XJ, Zheng XS, Zheng H, Liu L, Meng LB, Li Q, Liu Y. Tranilast inhibits TGF-β-induced collagen gel contraction mediated by human corneal fibroblasts. Int J Ophthalmol 2018;11(8):1247-1252





