In vitro inhibition of proliferation, migration and epithelialmesenchymal transition of human lens epithelial cells by fasudil
Jing-Zhi Shao, Ying Qi, Shan-Shan Du, Wen-Wen Du, Fu-Zhen Li, Feng-Yan Zhang
Department of Ophthalmology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province,China
INTRODUCTION
Posterior capsular opacification (PCO) is a common complication of cataract surgery, induced by the epithelial-mesenchymal transition (EMT) of residual epithelial cells located around the equatorial regions of the lens capsule[1].Numerous strategies have been attempted to suppress EMT,such as the use of anti-inflammatory drugs and antimetabolite agents, varying the design and materials of intraocular lens(IOL)[2-5]. However, to date no successful preventative or therapeutic modalities have been developed.
It has been demonstrated that transforming growth factor-β2(TGF-β2) plays an important role in the development of PCO[6]. Previous studies have shown that Rho/Rho-associated protein kinase (Rock) activation is necessary for TGF-βinduced EMT in human lens epithelial cells (HLECs)[7].
Fasudil, which is an inhibitor of Rho-kinase, has been widely used to prevent the development of fibrosis in many tissues[8-14].
Miyamoto et al[12]found that fasudil inhibited tumor growth in head and neck squamous cell carcinoma. Zhou et al[14]also proved that fasudil suppressed liver fibrosis in diabetic nonalcoholic steatohepatitis. These studies have demonstrated the utility of fasudil for the treatment of diseases caused by a process of fibrosis.
In the present study, we investigated the effects of fasudil on the proliferation, migration and EMT of HLECs (line SRA01/04)in order to establish the potential value of fasudil as a treatment for PCO.
MATERIALS AND METHODS
Cell CultureSRA01/04, which was transformed via SV40 T-antigen[15], was obtained from Jennio Biotech Company(Guangzhou, China). Cells were cultured in Roswell Park Memorial Institute 1640 culture (RPMI-1640, Hyclone, GE LifeSciences, Logan, Utah, USA) supplemented with 10%fetal bovine serum (FBS) incubated at 37℃ in a humidified atmosphere containing 5% CO2.
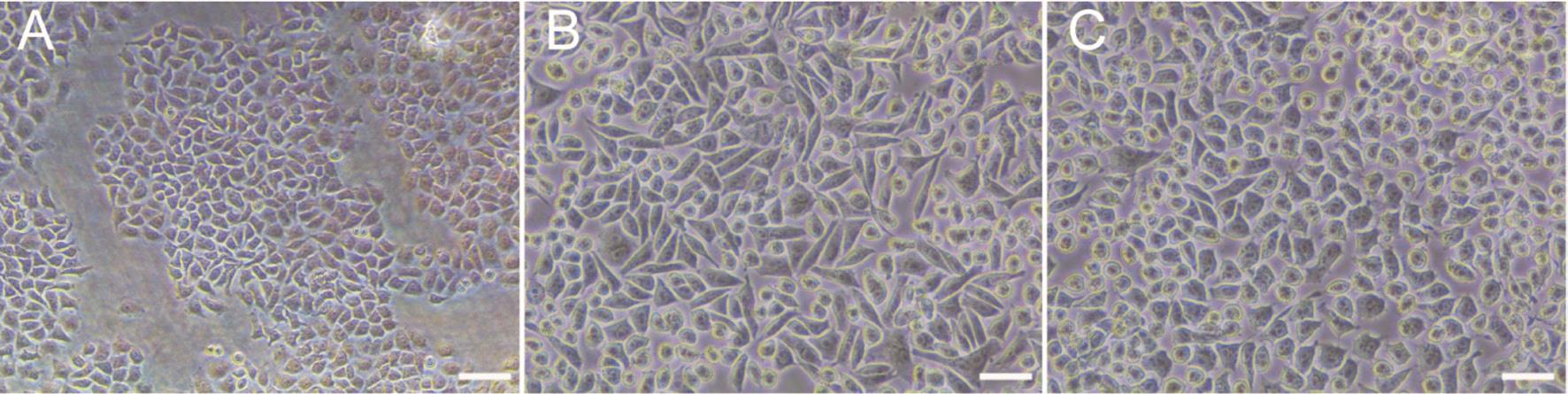
Figure 1 Cells treated with fasudil showed reversal of the cellular morphology induced by TGF-β2 A: Cellular morphology of untreated control samples displayed polygonal or cobblestone-like morphology; B: Cells treated with 10 ng/mL TGF-β2 showed a spindle-shaped cellular morphology; C: HLECs treated with 10 ng/mL TGF-β2 and subsequently treated with 10 μmol/mL fasudil displayed polygonal or cobblestonelike. Scale bar=50 μm.
MTT AssaySRA01/04 cells were seeded in 96-well plates at the density of 2×103cells per well. Samples were exposed to different concentrations of fasudil (10, 20, 40, 60 or 100 μmol/mL)for 24, 48 or 72h, incubated as described above. After 20 μL 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide(MTT, 5 mg/mL, Solarbio, Beijing, China) was added into each well and incubated for 4h at 37℃. The mixed solution was discarded and replaced with 150 μL dimethylsulfoxide(DMSO, Beijing, China) to dissolve the formazan precipitates,and samples were agitated in an orbital shaker for 10min at room temperature. The absorbance at 490 nm of each well was tested using an iMark™ microplate absorbance reader (Bio-Rad,Hercules, USA).
Cell Migration AssayScarification testing was performed to measure the migration ability of the cells: the SRA01/04 cells were seeded in 24-well plate with 1×104cells per well. After applying 1% FBS medium to synchronize the cell cycle for 24h, a straight line was scratched across the middle of each well with a yellow pipette tip, and then rinsed with phosphate buffered saline (PBS) to remove the suspended cells. Samples were then cultured either with or without 10 μmol/mL fasudil for another 24, 48 or 72h, then, the scratch gaps were viewed with a light microscope and photographed.
Western Blot AssayCells collected for analyzing were dispersed in RIPA total protein extraction lysis buffer (CWBIO,Beijing, China) supplemented with phenylmethanesulfonyl fluoride (PMSF, CWBIO, Beijing, China). Cells were cooled on ice for 30min, followed by centrifuging at 14 000 g, 4℃for 10min. The supernatant was separated via sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) in Tris-glycine gel and transferred to polyvinylidene fluoride(PVDF) membranes (Millipore, Billerica, USA). After blocking with 5% nonfat-dried milk for 1h at room temperature, the PVDF membranes were stained with primary antibodies, followed by probing with horseradish peroxidase (HRP)-labeled secondary antibody. BiodlightTMECL chemiluminescence HRP substrate(Bioworld, Dublin, OH, USA) was used to detect the binding of antibodies with Fluorchem RTM(ProteinSimple, San Jose, CA,USA). The intensities of the resulting bands were quantified by AlphaviewTMSA software (provided by Proteinsimple).
Table 1 SRA01/04 cell growth incubated at different times with different concentrations of fasudil
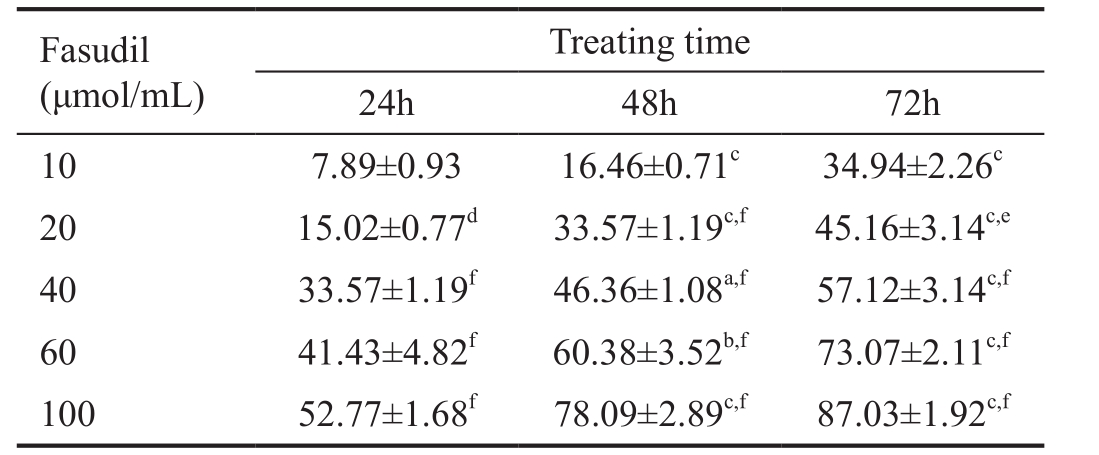
aP˂0.05,bP˂0.01,cP˂0.001 were based upon results on corresponding concentrations;dP˂0.05,eP˂0.01,fP˂0.001 were based upon results at corresponding treatment times.
Treating time 24h 48h 72h 10 7.89±0.93 16.46±0.71c34.94±2.26c20 15.02±0.77d33.57±1.19c,f45.16±3.14c,e40 33.57±1.19f46.36±1.08a,f57.12±3.14c,f60 41.43±4.82f60.38±3.52b,f73.07±2.11c,f100 52.77±1.68f78.09±2.89c,f87.03±1.92c,fFasudil(μmol/mL)
Statistical AnalysisAll data were presented as mean±SD from three independent experiments. Statistical bilateral significance between two and multiple groups was calculated using Student’s t-test or ANOVA. P values ˂0.05 were defined as significant.
RESULTS
Prevention of the Proliferation in SRA01/04 Cells by FasudilProliferation of SRA01/04 cells was inhibited by fasudil in dose-dependent and time-dependent manners (Table 1).The IC50 for 72h of fasudil on SRA01/04 proliferation was 22.37 μmol/mL.
Reverse of TGF-β2 Induced Morphological Characteristics in SRA01/04 Cells by FasudilWhen SRA01/04 cells were exposed to TGF-β2 and differentially supplemented with or without fasudil for 3d, microscopic examination of SRA01/04 cell morphology revealed a cobblestone-like appearance(Figure 1A). In contrast, cells cultured with TGF-β2 exhibited an elongated, fibroblast-like shape (Figure 1B). The morphological changes induced by TGF-β2 were noticeably suppressed when treated with fasudil (Figure 1C).
Inhibition of the Migration in SRA01/04 Cells by FasudilScarification testing demonstrated that the migration of SRA01/04 cells was significantly reduced after the treatment of fasudil for 24-72h (Figure 2). Under control conditions, the scrape wounds were nearly re-epithelialized at 48h and at 72h re-epithelialization of the previously cell-free areas was largely complete. In contrast, samples treated with fasudil continued to show large gaps at 72h.
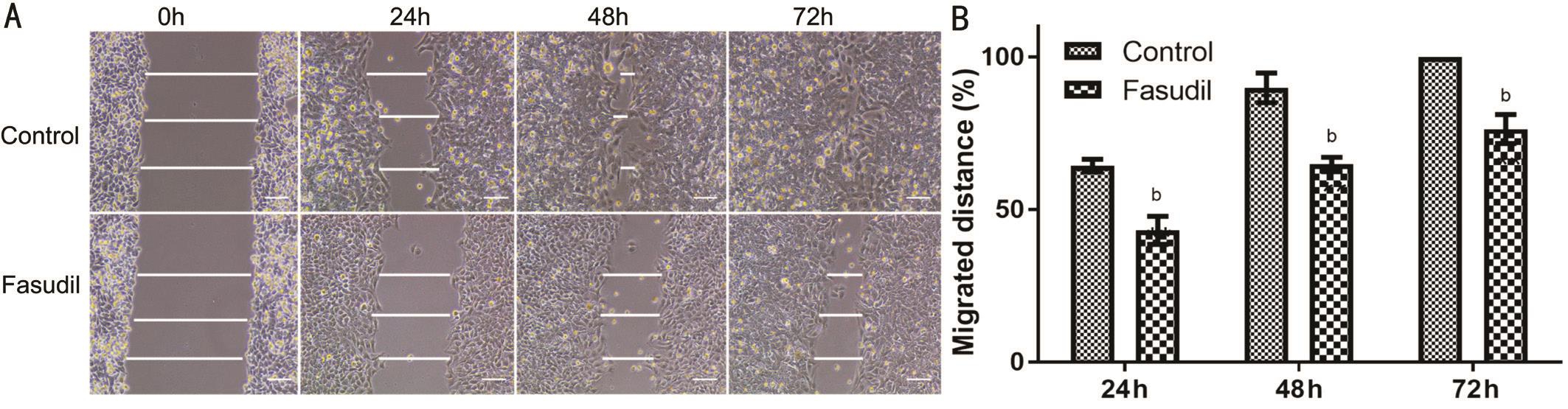
Figure 2 Inhibition of SRA01/04 cell migration is shown (monolayer wound assay) Wounded monolayers (2×105cells/100 μL) were incubated with serum-deprived culture with or without fasudil. Wound closure was followed at 0, 24, 48 and 72h after scratching. A: Different time intervals of fasudil inhibited the migration of SRA01/04 cells as compared to cells without fasudil; B: Data from at least three independent experiments were expressed as the mean±SD.bP˂0.01 vs untreated cells. Scale bar=100 μm.
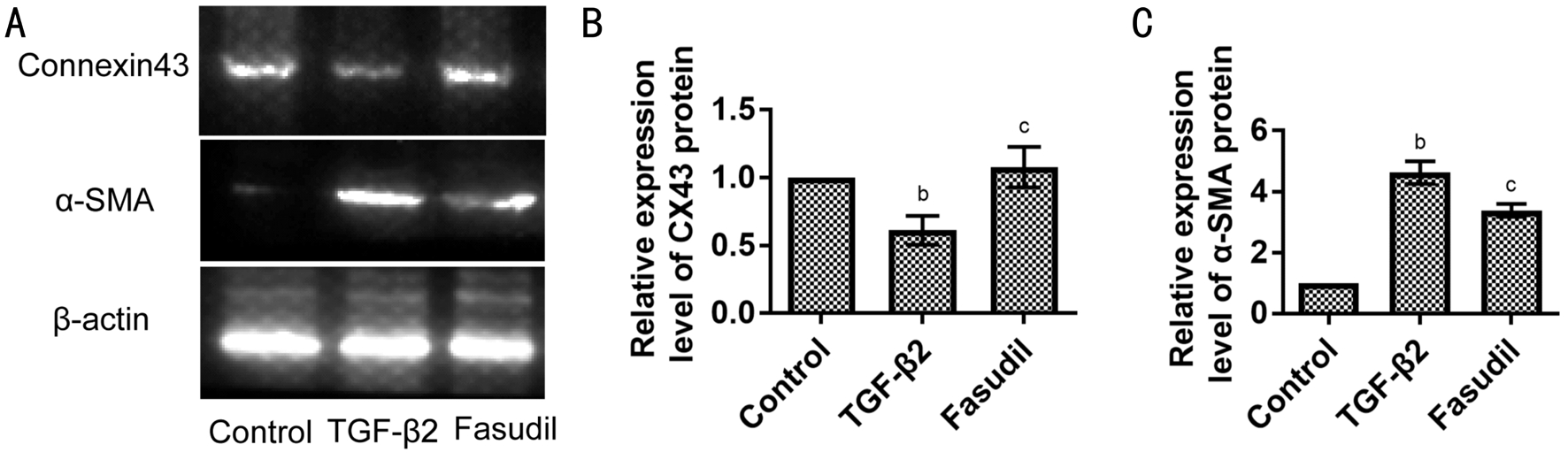
Figure 3 Fasudil increased the expression of Connexin43 protein and decreased the expression of α-SMA protein among EMT induced by TGF-β2 in SRA01/04 cells A: Western blot analysis of Connexin43 and α-SMA protein in SRA01/04 cells. SRA01/04 cells were treated with or without TGF-β2 or co-treated with fasudil for 3d; B: Statistical analysis of the expression level of Connexin43 (CX43) protein is shown;C: Statistical analysis of the expression level of α-SMA protein. Data were expressed as mean±SD.bP˂0.01 vs control group.cP˂0.001 vs TGF-β2 treated group.
Inhibition of TGF-β2 Induced EMT in SRA01/04 Cells by Fasudil Through Rho/Rock PathwayTo investigate the effect of fasudil on the TGF-β2-induced EMT, the expression of Connexin43 (the epithelial cell marker) and the expression of α-smooth muscle actin (α-SMA; the mesenchymal cell label) was assessed using Western blot assay. Compared with the control group, TGF-β2 down-regulated the expression of Connexin43 protein and increased the expression of α-SMA protein (Figure 3). Furthermore, fasudil application prevented those changes. When exposed to the combination of TGF-β2 and fasudil, cells expressed more Connexin43 protein and less α-SMA protein than cells only exposed to TGF-β2 (Figure 3).As shown in Figure 4A and 4B, after 20min of exposure to TGF-β2, the phosphorylation Rock/total Rock was significantly increased by 8.72±0.33 fold change (P˂0.001).Downstream myosin light chain (MLC) activation was also assessed, phosphorylation of MLC was enhanced after 20min exposed to TGF-β2 by 3.39±0.18 fold change (P˂0.001).When exposed to fasudil, the phosphorylation state of Rock and MLC protein could not be activated (Figure 4C, 4D). The phosphorylation Rock/total Rock was 0.97±0.06 fold change(P˃0.05) after 20min of exposure to TGF-β2 and fasudil. The phosphorylation MLC/total MLC was 1.16±0.15 fold change(P˃0.05) after 20min of exposure to TGF-β2 and fasudil.
DISCUSSION
PCO is the most frequent complication of cataract surgery[16].EMT is a phenotypic change widely associated with PCO[16].Understanding how EMT affect the process of PCO is critical to furthering our knowledge of the pathophysiology of fibrosis in the lens tissue. Scientists have proved that many pathways,especially the Rho/Rock pathway, play important roles in the process of EMT[7]. Blockage of the Rho/Rock pathway may represent a means to prevent the development of PCO[17]. In this study we demonstrated the inhibitory effect of fasudil on TGF-β2-induced proliferation, migration and EMT of SRA01/04. Fasudil, an inhibitor of Rho-kinase, may be regarded as a potential agent to prevent PCO.
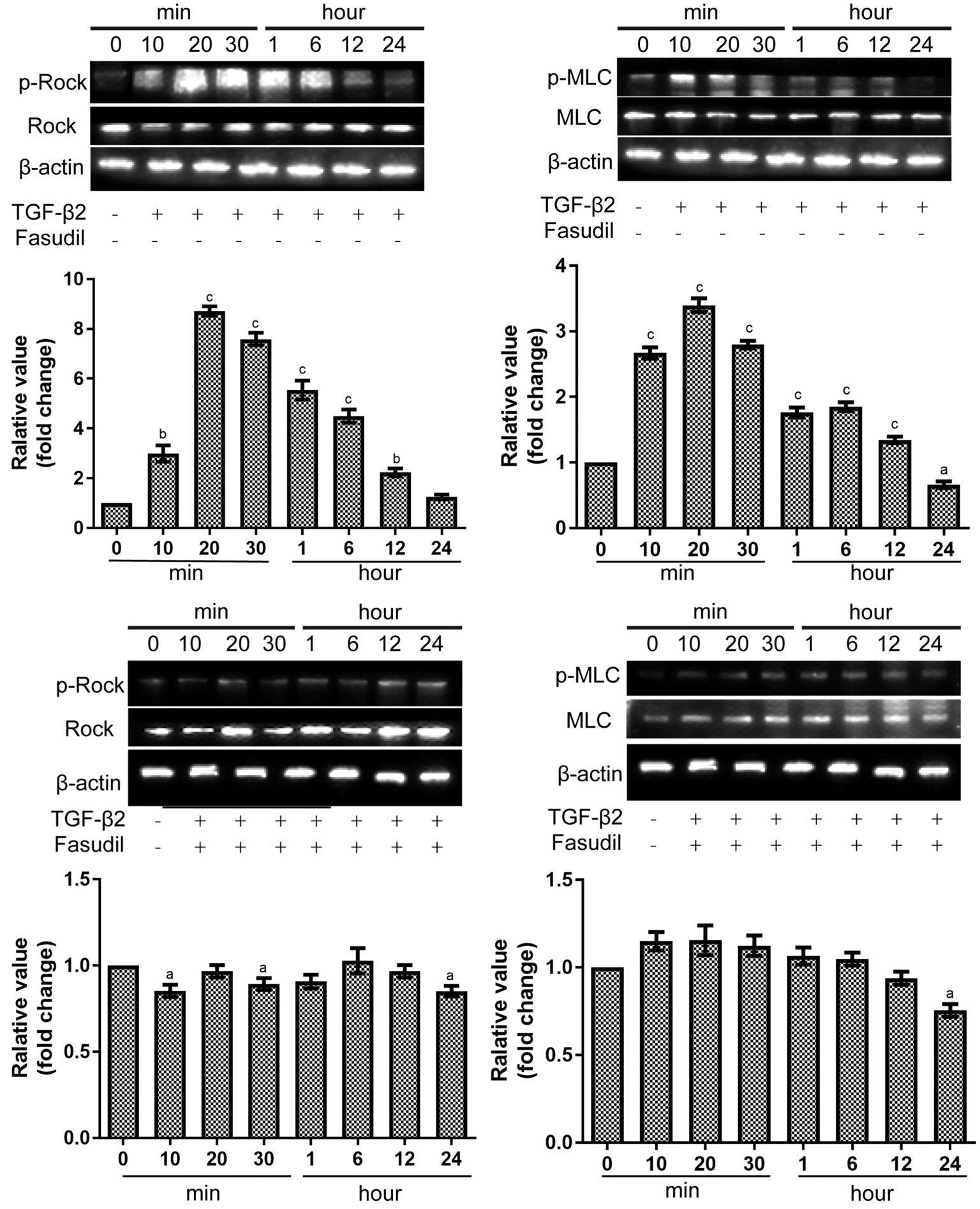
Figure 4 Effects of fasudil on Rho pathway in SRA01/04 cells as measured with Western blot assay The densitometric analysis of the three individual experiments were quantified.aP˂0.05,bP˂0.01,cP˂0.001 vs control samples.
Under the TGF-β2 treatment, it was shown that lens epithelial cells undergo EMT to produce myofibroblasts in vitro[18].Connexin43 is the epithelial marker, and α-SMA is the mesenchymal marker. In our model of lens EMT, TGF-β2 down-regulated the Connexin43 protein expression level,in contrast, up-regulated α-SMA protein expression level in SRA01/04 cells. This was consistent with previous studies[19-20].In addition, we demonstrated Rho/ROCK signaling pathway was required for TGF-β2-induced EMT of lens epithelial cells. Phosphorylation-Rho, the correlated protein of Rho/ROCK pathway, was stimulated during the model of EMT.The downstream phosphorylation-MLC was also assessed to be activated in this study. Cho and Yoo[7]found that growth factors activated the Rho in HLEs, and Rho-kinase inhibitor blocked growth factor effects on the actin cytoskeletal organization[21]. Korol et al[18]suggested that TGF-β-induced cytoskeletal reorganization through Rho/ROCK/MRTF-A signaling is critical to EMT of lens epithelial cells. Maddala[21]demonstrated that different growth factors induced actin cytoskeleton reorganization and formation of cell-excetral cellular matrix (ECM) interactions in lens epithelial cells and this response of growth factors appeared to be mediated, at least in part, through the Rho/Rho kinase-mediated signaling pathway. These findings suggested that the Rho/Rock pathway was involved in TGF-β induction of EMT and acted to regulate the EMT of HLEs.
Fasudil, a rho kinase inhibitor, exhibits its inhibitory effects on a host of cell types[22]. Fasudil has been shown to significantly inhibit proliferation of a mouse fibroblast cell line in vitro and in vivo[11], and has demonstrated EMT inhibition effects on renal tubular epithelial cells[9]and hepatic cells[14].Yang et al[23]reported that fasudil inhibited proliferation and metastasis of small cell lung cancer. In the current study we found that fasudil inhibited the proliferation and migration of SRA01/04, and suppressed the EMT in this cell line. That inhibitory property of fasudil prompted us to investigate its underlying mechanism. With the assessment of the correlatedprotein expression of Rho/Rock signaling pathway, Rock and MLC, we found that the phosphorylation state of Rock and MLC protein could be highly activated in the presence of TGF-β2, but did not be activated in the presence of TGF-β2 and fasudil. The Rho/Rock signal transduction pathway plays an important role in the EMT of HLEs. Fasudil efficiently reversed the effects of TGF-β2, and reversed the EMT of HLEs through inhibition of the Rho/Rock pathway.
In conclusion, fasudil shows its inhibitory effect on the proliferation, migration and EMT of HLEs in vitro. The regulation of TGF-β2/Rho protein expression may be related to the inhibition effect. We conclude that fasudil may be recognized as a potential agent to block the formation of PCO in patients after cataract surgery. Further in vivo studies should be performed to titrate fasudil concentrations and to test delivery modalities, so that this promising PCO therapy can be developed for human cataract surgery patients.
ACKNOWLEDGEMENTS
Foundations:Supported by the National Natural Science Foundation of China (No.U1304812); the Henan Science and Technology Key Project (No.142102310053).
Conflicts of Interest:Shao JZ, None; Qi Y, None; Du SS,None; Du WW, None; Li FZ, None; Zhang FY, None.
REFERENCES
1 Wormstone IM, Wang L, Liu CS. Posterior capsule opacification. Exp Eye Res 2009;88(2):257-269.
2 Wang GQ, Gu HQ, Yuan JQ, Sun HM, Xu YS. F-heparin modified intraocular lenses in Rhesus monkeys. Int J Ophthalmol 2010;3(2):141-144.
3 Pandey SK, Cochener B, Apple DJ, Colin J, Werner L, Bougaran R, Trivedi RH, Macky TA, Izak AM. Intracapsular ring sustained
5-fluorouracil delivery system for the prevention of posterior capsule opacification in rabbits: a histological study. J Cataract Refract Surg 2002;28(1):139-148.
4 Hazra S, Palui H, Vemuganti GK. Comparison of design of intraocular lens versus the material for PCO prevention. Int J Ophthalmol 2012;5(1):59-63.
5 Findl O, Buehl W, Bauer P, Sycha T. Interventions for preventing posterior capsule opacification. Cochrane Database Syst Rev 2010;(2):CD003738.
6 Wormstone IM, Tamiya S, Anderson I, Duncan G. TGF-beta2-induced matrix modification and cell transdifferentiation in the human lens capsular bag. Invest Ophthalmol Vis Sci 2002;43(7):2301-2308.
7 Cho HJ, Yoo J. Rho activation is required for transforming growth factor-beta-induced epithelial-mesenchymal transition in lens epithelial cells. Cell Biol Int 2007;31(10):1225-1230.
8 Deng L, Li G, Li R, Liu Q, He Q, Zhang J. Rho-kinase inhibitor, fasudil,suppresses glioblastoma cell line progression in vitro and in vivo. Cancer Biol Ther 2010;9(11):875-884.
9 Gu L, Gao Q, Ni L, Wang M, Shen F. Fasudil inhibits epithelialmyofibroblast transdifferentiation of human renal tubular epithelial HK-2 cells induced by high glucose. Chem Pharm Bull 2013;61(7):688-694.
10 Guo R, Su Y, Yan J, Sun H, Wu J, Liu W, Xu Y. Fasudil improves short-term echocardiographic parameters of diastolic function in patients with type 2 diabetes with preserved left ventricular ejection fraction: a pilot study. Heart Vessels 2015;30(1):89-97.
11 Jiang C, Huang H, Liu J, Wang Y, Zhao Y, Xu Z. Effects of fasudil on bleomycin-induced pulmonary fibrosis in mice and on the biological behaviors in NIH3T3 mouse fibroblast cell line. Zhonghua Jie He He Hu Xi Za Zhi 2014;37(9):671-676.
12 Miyamoto C, Maehata Y, Motohashi K, Ozawa S, Ikoma T, Hidaka K,Wada-Takahashi S, Takahashi SS, Yoshino F, Yoshida A, Kubota E, Hata R, Lee MC. Fasudil, a Rho kinase inhibitor, suppresses tumor growth by inducing CXCL14/BRAK in head and neck squamous cell carcinoma.Biomed Res 2014;35(6):381-388.
13 Tiftik RN, Baskurt OK, Kul S, Büyükafşar K. The functional significance of the rho/rho-kinase pathway in human erythrocytes. Turk J Haematol 2014;31(2):168-174.
14 Zhou H, Fang C, Zhang L, Deng Y, Wang M, Meng F. Fasudil hydrochloride hydrate, a Rho-kinase inhibitor, ameliorates hepatic fibrosis in rats with type 2 diabetes. Chin Med J 2014;127(2):225-231.
15 Ibaraki N, Chen SC, Lin LR, Okamoto H, Pipas JM, Reddy VN.Human lens epithelial cell line. Exp Eye Res 1998;67(5):577-585.
16 Nibourg LM, Gelens E, Kuijer R, Hooymans JM, van Kooten TG,Koopmans SA. Prevention of posterior capsular opacification. Exp Eye Res 2015;136:100-115.
17 de Iongh RU, Wederell E, Lovicu FJ, McAvoy JW. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens:a model for cataract formation. Cells Tissues Organs 2005;179(1-2):43-55.
18 Korol A, Taiyab A, West-Mays JA. RhoA/ROCK signaling regulates TGFβ-induced epithelial-mesenchymal transition of lens epithelial cells through MRTF-A. Mol Med 2016;22.
19 Guo R, Meng Q, Guo H, Xiao L, Yang X, Cui Y, Huang Y. TGF-β2 induces epithelial-mesenchymal transition in cultured human lens epithelial cells through activation of the PI3K/Akt/mTOR signaling pathway. Mol Med Rep 2016;13(2):1105-1110.
20 Zhang G, Kang L, Chen J, Xue Y, Yang M, Qin B, Yang L, Zhang J,Lu H, Guan H. CtBP2 regulates TGFβ2-induced epithelial-mesenchymal transition through Notch signaling pathway in lens epithelial cells. Curr Eye Res 2016;41(8):1057-1063.
21 Maddala R, Reddy VN, Epstein DL, Rao V. Growth factor induced activation of Rho and Rac GTPases and actin cytoskeletal reorganization in human lens epithelial cells. Mol Vis 2003;9:329-336.
22 Feng Y, LoGrasso PV. Rho kinase inhibitors: a patent review (2012-2013).Expert Opin Ther Pat 2014;24(3):295-307.
23 Yang X, Di J, Zhang Y, Zhang S, Lu J, Liu J, Shi W. The Rho-kinase inhibitor inhibits proliferation and metastasis of small cell lung cancer.Biomed Pharmacother 2012;66(3):221-227.
Correspondenceto:Feng-Yan Zhang. Department of Ophthalmology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, Henan Province, China.zhangfengyanx@aliyun.com
Received:2017-02-28 Accepted: 2018-04-09
Abstract● AlM: To study the potential role of fasudil as a treatment for posterior capsular opacification (PCO) of the human crystalline lens.● METHODS: Human lens epithelial cells (HLECs; line SRA01/04) was exposed to transforming growth factor-β2(TGF-β2) to induce the process of epithelial-mesenchymal transition (EMT). Fasudil was applied to the cell samples.lts effect on overall HLECs proliferation and migration was studied, as was its influence on EMT induction by TGF-β2 using cell migration assay, MTT colorimetric assay and Western blot assay.● RESULTS: Fasudil inhibited the proliferation of SRA01/04.lts effect was time- and concentration-dependent. The migration of SRA01/04 cells was significantly reduced 24-72h after fasudil treatment, and the half maximal inhibitory concentration (lC50) was 22.37 μmol/mL at 72h.Reversal of the elongated, fibroblast-like shape changes induced by TGF-β2 in SRA01/04 cells was observed.Fasudil up-regulated the expression of Connexin43 protein and down-regulated the expression of α-SMA protein compared with the cells treated with TGF-β2. Furthermore,when exposed to fasudil, the phosphorylation of Rhoassociated protein kinase (Rock) and myosin light chain(MLC) could not be activated in the cell preparations.● CONCLUSlON: Fasudil suppresses the proliferation and migration of SRA01/04 cells, and inhibits the process of EMT induced by TGF-β2. These results suggest that fasudil may serve as a therapeutic agent for PCO.
● KEYWORDS:fasudil; human lens epithelial cells; TGF-β2;Rho/Rock; epithelial-mesenchymal transition
DOl:10.18240/ijo.2018.08.02
Citation:Shao JZ, Qi Y, Du SS, Du WW, Li FZ, Zhang FY. In vitro inhibition of proliferation, migration and epithelial-mesenchymal transition of human lens epithelial cells by fasudil. Int J Ophthalmol 2018;11(8):1253-1257




