lnhibition of LY294002 in retinal neovascularization via down-regulation the Pl3K/AKT-VEGF pathway in vivo and in vitro
Yu Di, Xiao-Long Chen
Department of Ophthalmology, Shengjing Affiliated Hospital,China Medical University, Shenyang 110004, Liaoning Province, China
INTRODUCTION
The phosphatidylinositol 3-kinase/serine-threonine kinase(PI3K/AKT) is a signal transduction pathway activated by several membrane receptors such as growth factors, and plays a critical role in cellular energy metabolism, cytoplasmic movement and cellular cycle progression[1]. The PI3K/AKT signaling pathway involves numerous pathological and physiological processes and mediates angiogenesis in many diseases[2-3].
Recent researches demonstrated that the PI3K/AKT pathway activates vascular endothelial growth factor (VEGF) and advances neoplasm blood vessel[4]. Our recent study revealed that PI3K/AKT is closely involved in the retinopathy of prematurity (ROP)[5-6]. There is a strong possibility that the anti-angiogenic effect of LY294002 (a specific inhibitor of PI3K) is related to downregulation of PI3K/AKT, but the exact mechanism of its anti-angiogenic activity is still unclear.
In this study, LY294002 was applied to the oxygen-induced retinopathy (OIR) model and human umbilical vein endothelial cells (HUVECs) to observe the inhibitory effect on retinal neovascularization (RNV), and could, therefore, be valuable for clinical anti-angiogenic therapy.
MATERIALS AND METHODS
AnimalsFrom the Shengjing Hospital of China Medical University, we purchased C57BL/6J timed-pregnant dams. Mouse pups and their mothers were housed at room temperature(21℃) and a 12-h light/dark cycle. The usage of animals were adherence to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Oxygen Induced Retinopathy in MiceIn order to produce OIR, mouse were put in 75%±2% oxygen (from P7 to P12)and returned to normal air on P12. Mice were randomly divided into three groups: normoxia-control, OIR-control and LY294002 treatment groups (n=60/group). Mice in the normoxia-control group were raised in the normal air. Mice in the OIR-control group were injected intraperitoneally with 0.1 mL of phosphate-buffered solution (PBS) once a day from P6 to P9. Mice in the LY294002 treatment group were injected intraperitoneally with 0.1 mL of LY294002 once a day from P6 to P9. Three groups of mice were killed on P17.
Drug PreparationLY2940029 was purchased from Cell Signaling Technology (Sigma, St. Louis, MI, USA), and diluted in dimethyl sulfoxide (DMSO) to create an inventory solution.LY294002 solution (the final concentration) was 40 μmol/L.
Quantitative Assessment of Retinal Neovascularization by ADPase StainingIn view of the higher intensity of adenosine diphosphatase (ADPase) staining and the characteristics of neovascularization, the ADPase-stained flat-mounted retina was used to measure the cluster area, as previously published[7].Each and every retina was separated into twelve equal sections using the Olympus B201 optical microscope (Olympus Corporation, Tokyo, Japan) to count the clock hour scores of neovascularization.
Quantitative Assessment of Retinal Neovascularization by Counting Vascular Cell NucleiBriefly, the eyes were enucleated, formalin-fixed, paraffin embedded and evaluated by conventional histological assessment using hematoxylin and eosin staining. Vascular cell nuclei were counted under a light microscope[8]. Two independent reviewers reviewed the histological features.
ImmunohistochemistryImmunohistochemical analysis was performed with a “Strept Avidin Biotin Complex”immunohistochemistry kit (Boster Bioengineering Co.,Wuhan, China). PBS was used as a negative control, and diaminobenzidine (DAB) was used as the chromogen. Images were observed under a light microscope.
Cell CultureHUVECs (Kirkland, WA, USA) were cultured in Dulbecco’s minimum essential medium (DMEM) with 10% fetal bovine serum (FBS) in atmosphere containing 5%CO2. Cells were then divided into the normoxia-control (20%O2, 5% CO2, 75% N2) and hypoxia groups (1% O2, 5% CO2,94% N2). The hypoxia group was further subdivided into the hypoxia-control group and the hypoxia-LY294002 group.The hypoxia-LY294002 group were treated with LY294002(40 μmol/L) for 30min before being placed in an incubator.Subsequent experiments were carried out after 24h of exposure to hypoxia.
Western Blot AnalysisThe protein from each sample was extracted with lysis buffer. Proteins were isolated and transferred to polyvinylidene fluoride (PVDF) membranes (Merck Millipore, Billerica, MA, USA). Membranes were incubated for the night at 4℃ with phosphorylated (p)-PI3K, p-AKT1/2/3 and VEGF (1:2000, Santa Cruz Biotech nology Inc., USA).Thereafter, mem branes were incubated with horseradishperoxidase-conjugated secondary antibodies (1:2000,Zhongshan Jinqiao Biotechnology Co. Ltd., China) for 1h. For statistical analysis, the protein bands were quantified using Image J software.
Real-time Reverse Transcription-polymerase Chain ReactionExtraction of RNA from the HUVECs and retinas using TRIzol reagent (Invitrogen, Carlsbad, CA, USA).cDNA synthesis was performed according to the RNA polymerase chain reaction (PCR) protocol (PrimeScriptTM RT Reagent Kit-Perfect Real Time, Takara Bio, Otsu, Japan).β-actin was used as a normalizing control. The primer sequences were PI3K: 5’-GGCTTGGACCGAATGCT-3’(forward) and 5’-TTGTTGAAGGCTGTGGC -3’ (reverse);AKT: 5’-AGCAAACAGGCTCAC AGGT T-3’ (forward)and 5’-TAAGTCCTCCCCATCTCCCT-3’ (reverse);VEGF: 5’-CCCGACAGGGAAGACAAT-3’ (forward)and 5’-TCTGGAAGTGAGCCAACG -3’ (reverse); and β-actin: 5’-GAGAGGGAAATCGTGCGTGA-3’ (forward)and 5’-GCCTAGAAGCATTTGCGGTG-3’ (reverse). PCR reactions were performed in a volume of 25 µL using SYBR Green PCR Master Mix (Premix Ex TaqTM-Perfect Real Time,Takara Bio, Otsu, Japan) with a 7300 Real-Time PCR System(Applied Biosystems, Foster City, CA, USA).
Statistical AnalysisSignificant differences were determined using the independent-sample t-test. All data were represented by the mean±standard deviation (SD). P˂0.05 was significant significance.
RESULTS
Inhibition of LY294002 in Oxygen-induced Retinopathy ModelTo ascertain if LY294002 significantly alters the development of new blood vessels in the eye, we examined the morphology and pathology of the retina. Neovascular plexus and non-perfusion area in the OIR-control group(6.20±1.21) was more than in the normoxia-control group(0.91±0.10) (t=5.762, P˂0.05). Compared to the OIR-control group (6.20±1.21), the retinas from the LY294002 treatment group had less severe neovascular plexus and nonperfusion area, vascular tortuosity and less irregular expansion(1.41±0.52, t=5.363, P˂0.05; Figure 1). This result showed that LY294002 inhibits the formation of new blood vessels, but does not reduce the number of existing vessels systemically.As shown in Figure 2, the nuclei of preretinal neovascular cells breaking through the internal limiting membrane (ILM)in the OIR-control group (22.25±1.82) was more than in the normoxia-control group (8.91±1.12, t=5.131, P˂0.05).Moreover, LY294002 treatment group (10.50±1.58) were significantly less than those in the retinas from the OIR-control group (22.25±1.82, t=4.182, P˂0.05), verifying the antineovascularization effects of LY294002 on the retina.
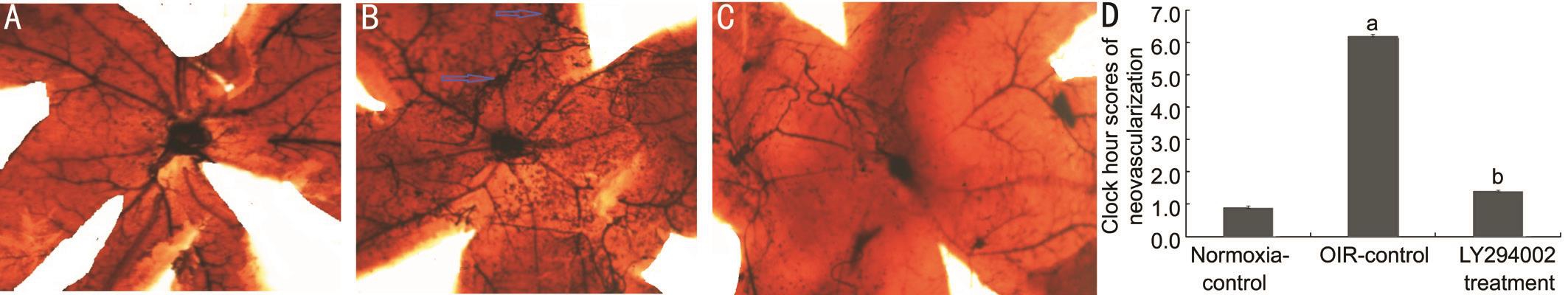
Figure 1 Typical retinal angiogram from the eyes of groups Images are typical retinal angiogram of the retina of the normoxia-control group (A), the OIR-control group (B), and the LY294002 treatment group (C). Statistical analysis results were shown in D. Arrows indicate neovascularization (magnification: 100×, n=15).aP˂0.05 vs normoxia-control group;bP˂0.05 vs OIR-control group.
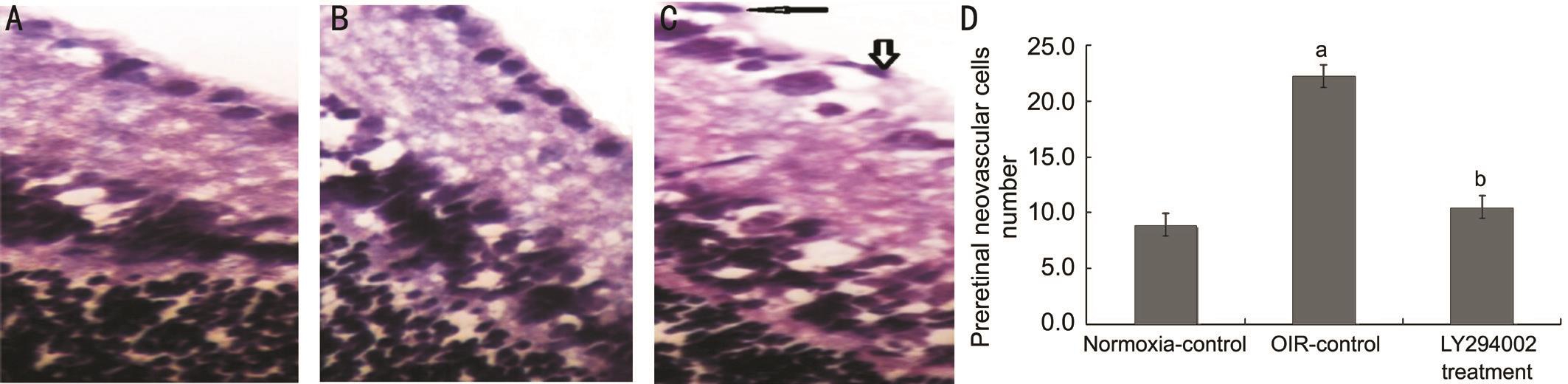
Figure 2 Preretinal neovascular cells breaking through the ILM of P17 mice were counted Images are representative retinal sections from the normoxia-control (A), OIR-control (B), and LY294002 treatment group (C). Statistical analysis results were shown in D. Arrows indicate preretinal vascular endothelial cells (Magnification: ×400, n=150).aP˂0.05 vs normoxia-control group;bP˂0.05 vs OIR-control group.
Expression of p-PI3K, p-AKT and Vascular Endothelial Growth Factor by ImmunohistochemistryLocalization and expression levels of p-PI3K, p-AKT and VEGF in three groups were observed by immunohistochemistry (Figure 3).Immunohistochemical staining of retina slices showed that expression levels of p-PI3K, p-AKT and VEGF were weakly detected only in ganglion cell layer (GCL) and inner plexiform layer (IPL) of the normoxia-control group, whereas they were strongly detected in GCL, IPL, inner nuclear layer (INL), outer nuclear layer (ONL) and neovascularization breaking through the ILM in the OIR-control group. However, the expression of p-PI3K, p-AKT and VEGF in the LY294002 treatment group was lower in the GCL, IPL, INL and ONL compared with the OIR-control group. These results demon strated that hypoxia activated the PI3K/AKT-VEGF signaling pathway.
Inhibition of LY294002 Through PI3K/AKT-VEGF Signaling PathwayFor purpose of detecting the effect of LY294002, we performed Western blot analysis and realtime reverse transcription-polymerase chain reaction (RTPCR) for quantitative detection. Western blotting showed that p-PI3K, p-AKT, and VEGF protein levels in the OIR-control group were increased compared with the normoxiacontrol group (all P˂0.05), whereas they were reduced by 46.12%, 34.34%, and 43.69% in the LY294002 treatment group, respectively, contrasted with the OIR-control group (all P˂0.05; Figure 4). Real-time RT-PCR showed similar results in retina samples: PI3K, AKT, and VEGF mRNA levels in the LY294002 treatment group were reduced by 44.44%, 49.78%,and 34.49%, respectively, compared with the OIR-control group (all P˂0.05; Figure 5). These results demon strated that LY294002 inhibited RNV by inhibiting the expression of PI3K, AKT and VEGF in the OIR model.
HUVECs Apoptosis Induction by LY294002 under Hypoxic Conditions Through PI3K/AKT-VEGF Signaling PathwayOur previous data showed that the early apoptotic rate was decreased, but that the late apoptotic rate was significantly increased in the hypoxia-LY294002 group compared to the hypoxia-control group, which indicated that LY294002 exerted pro-apoptotic effects on HUVECs[9]. Transfection with LY294002 decreased the expression levels of all three proteins (p-PI3K, p-AKT, and VEGF). Compared with the hypoxia-control group, protein levels of p-PI3K, p-AKT, and VEGF in the hypoxia-LY294002 group were decreased by 50.51%, 59.56% and 46.92%, respectively (all P˂0.05; Figure 6). Real-time RT-PCR showed that PI3K, AKT, and VEGF mRNA levels in the hypoxia-LY294002 group were reduced by 43.60%, 40.61%, and 53.59%, respectively, compared with the hypoxia-control group (all P˂0.05; Figure 7). These results indicated that LY294002 not only inhibited the expression of PI3K, AKT and VEGF, but also inhibited apoptosis of HUVECs in vitro.
DISCUSSION
PI3K is considered to act as second messengers[10-11], which is commonly observed in human tumors[12]. The pathway of PI3K/AKT is closely correlated to tumor progression and resistance to chemotherapy[9]. Dysregulation of this pathway is frequently observed in a variety of tumors, including breast,ovarian and other carcinomas[13-14].
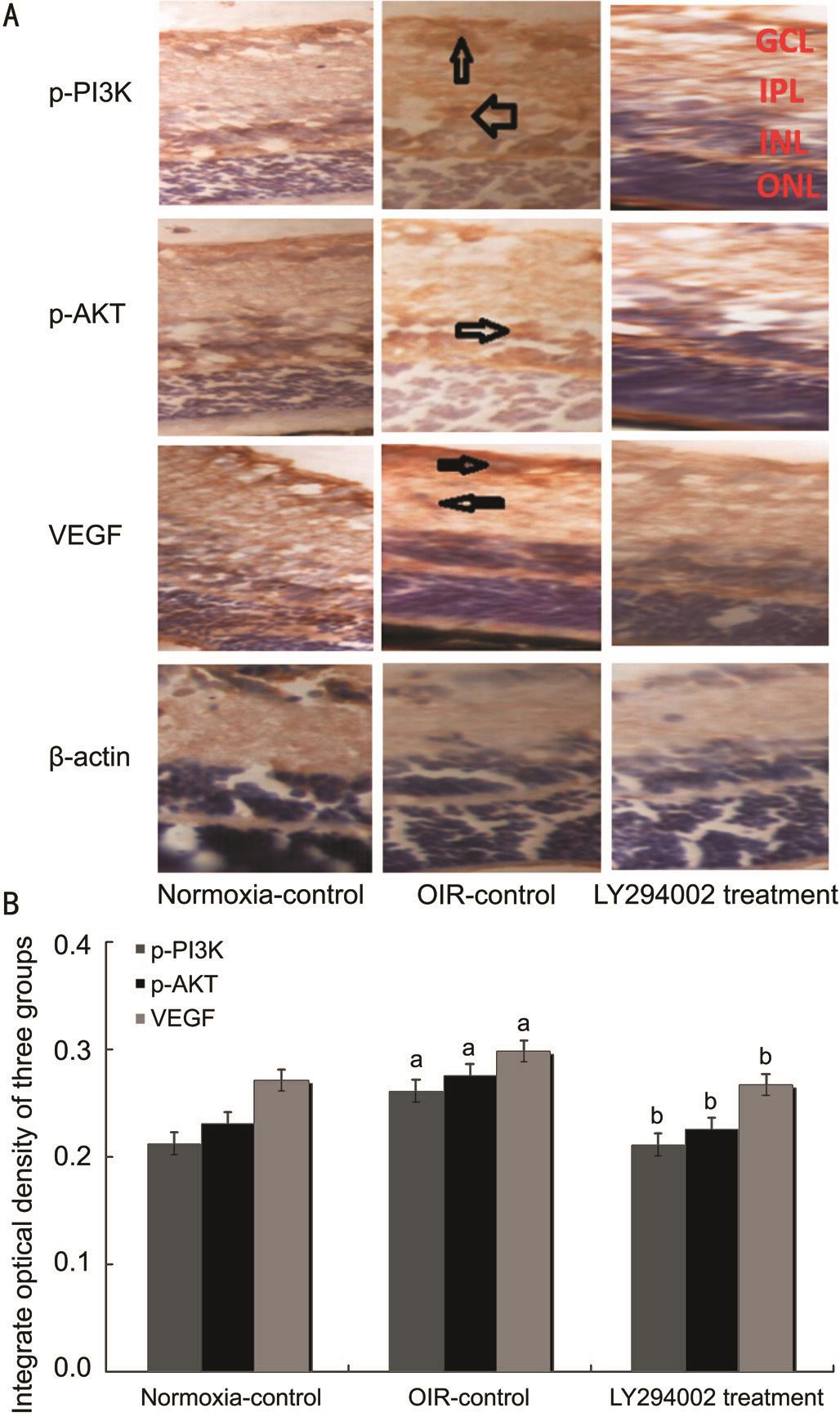
Figure 3 Hypoxia upregulated PI3K/AKT-VEGF expression in the OIR model A: Protein expression of p-PI3K, p-AKT and VEGF were determined by immunohistochemistry (magnification: 400×);B: The integrate optical density (IOD) of p-PI3K, p-AKT and VEGF in three groups.aP˂0.05 vs normoxia-control group;bP˂0.05 vs OIR-control group.
Previous research studies have revealed that PI3K/AKT is an effective angiogenesis factor. The effective angiogenesis characteristics of PI3K/AKT were confirmed in a rabbit cornea model[15]and in a mouse ischemic retinal model[16]. In addition,PI3K/AKT can also regulate the expression of genes related to angiogenesis and matrix remolding, including VEGF, matrix metalloproteinase (MMP)-2, MMP-9[9,17]. Thus, PI3K/AKT can directly and indirectly induce angiogenesis.
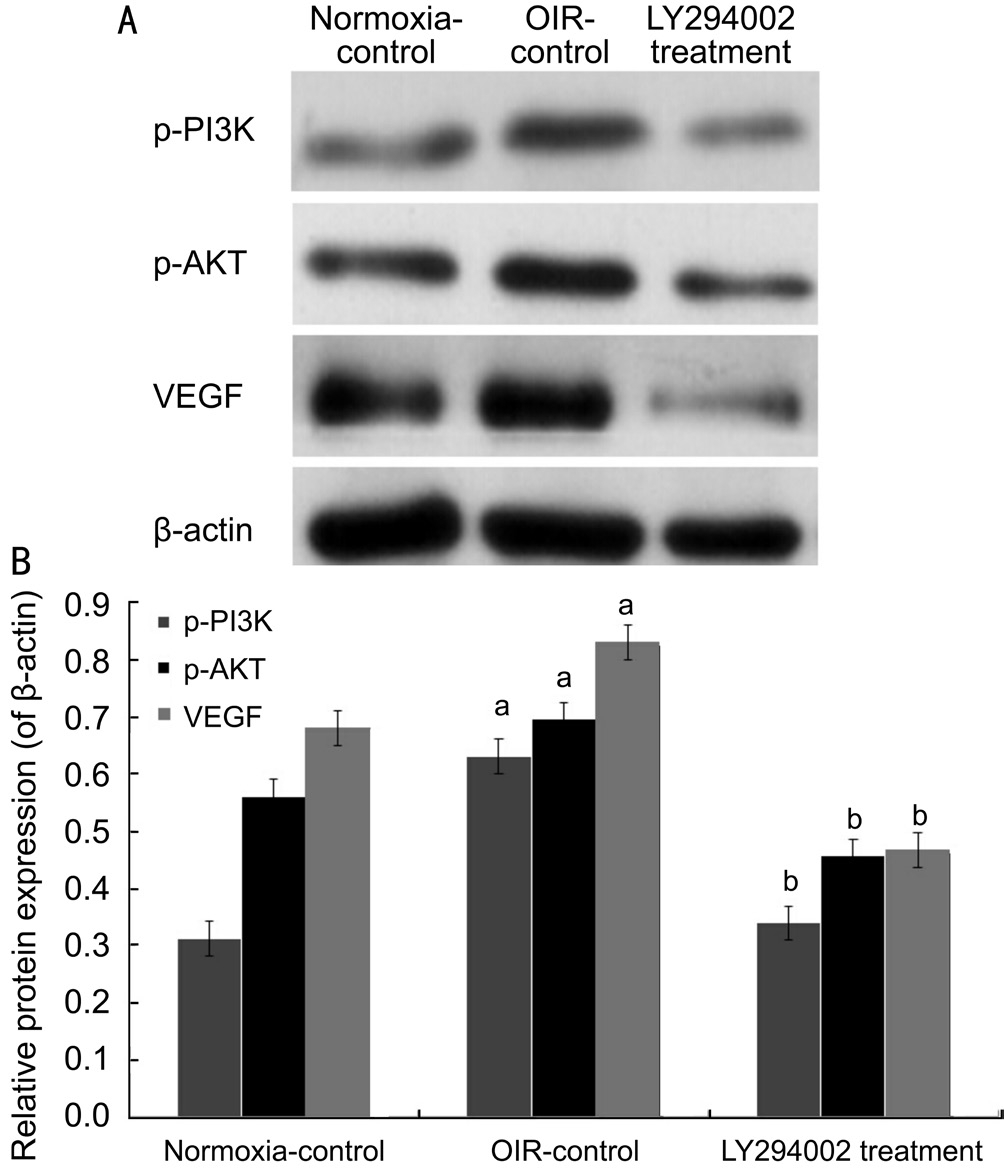
Figure 4 LY294002 inhibited RNV by inhibiting protein expression of PI3K/AKT-VEGF signaling pathway in the OIR mice model A: Western blot assay for protein expression of p-PI3K,p-AKT and VEGF; B: Statistical analysis.aP˂0.05 vs normoxiacontrol group;bP˂0.05 vs OIR-control group.
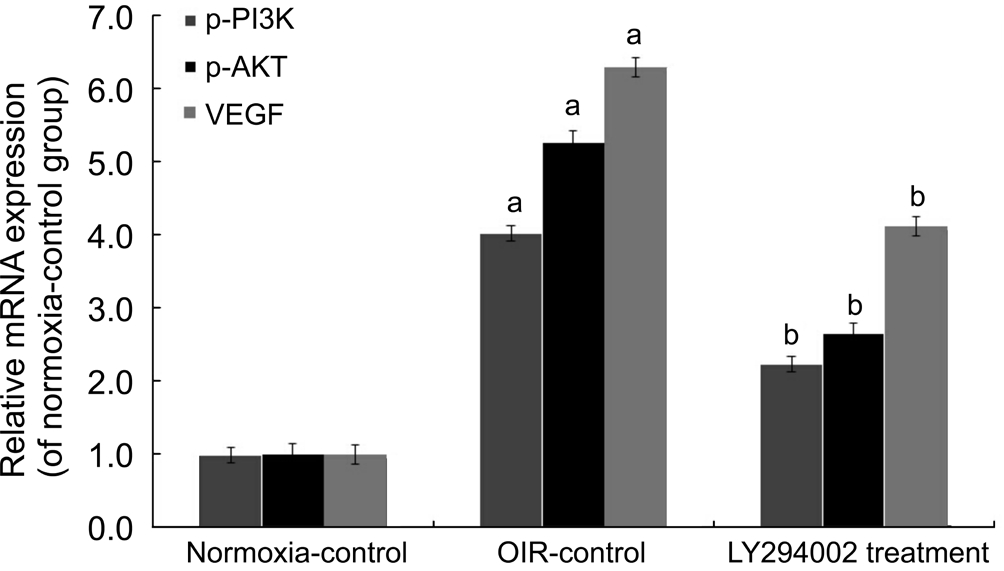
Figure 5 LY294002 inhibited RNV by inhibiting mRNA expression of PI3K/AKT-VEGF signaling pathway in the OIR mice model mRNA expression levels of PI3K, AKT and VEGF were determined by real-time RT-PCR.aP˂0.05 vs normoxia-control group;bP˂0.05 vs OIR-control group.
LY294002, a PI3K inhibitor, easily passes through the cell membrane and inhibits mitogen-activated protein kinase(MAPK) activity by competitively inhibiting the ATP binding site in the PI3K subunit, but does not inhibit the expression of proteinkinase C (PKC), proteinkinase A (PKA), or other phosphate kinases. Several studies have shown that LY294002 can significantly inhibit the growth of lung carcinoma[18],gastric carcinoma[19], ovarian carcinoma[20]and the like.However, the role of LY294002-induced RNV inhibition has not been frequently investigated. Here we report evidence that LY294002 as a small molecule inhibitor is effective and safe in inhibiting ocular angiogenesis in vivo and in vitro.
Our study found that the neovascularization clock hour scores and the number of preretinal neovascular cell nuclei were clearly lower in the LY294002 treatment group than in the OIR-control group. Intravitreal injection of LY294002 can selectively inhibit intraocular angiogenesis without affecting retinal morphology.
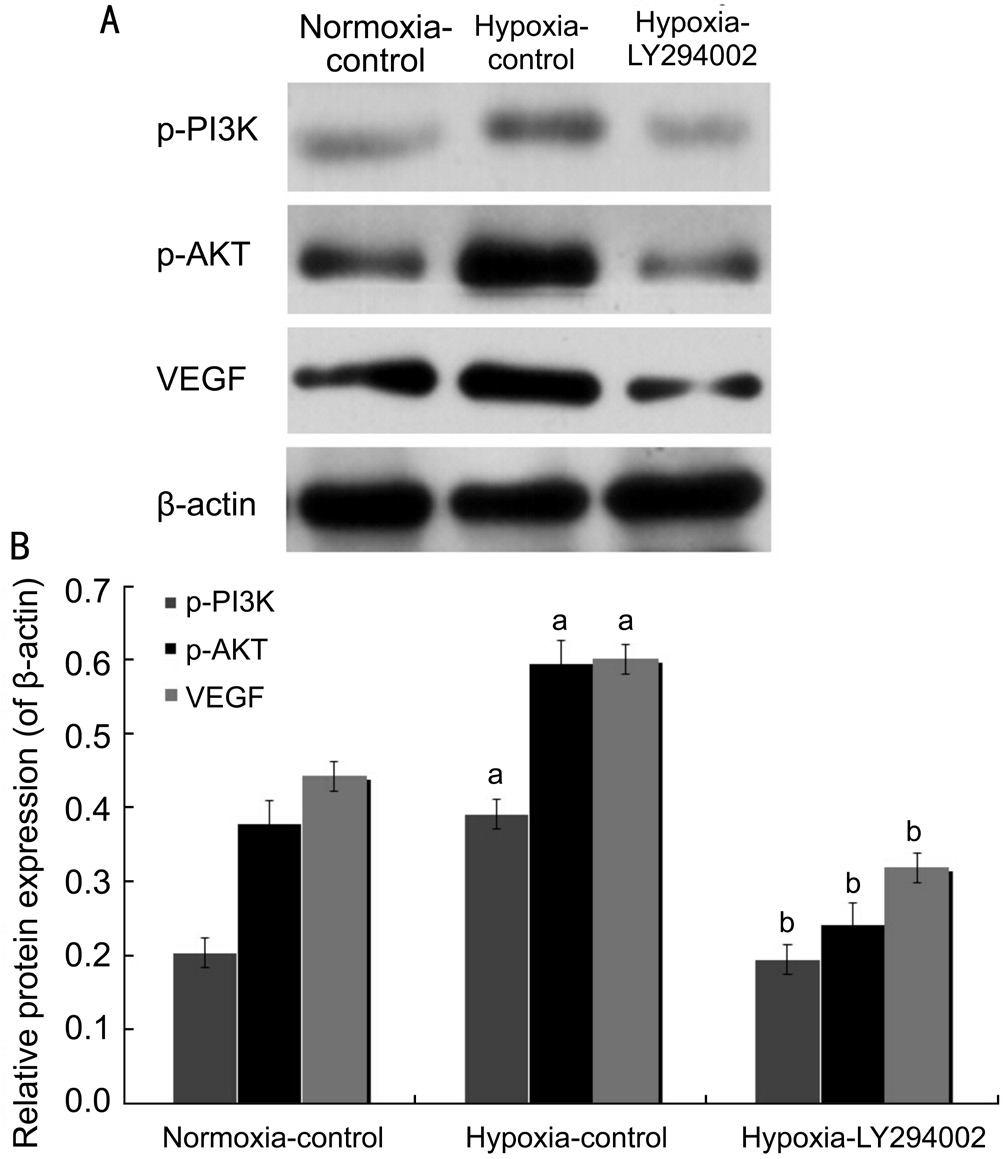
Figure 6 LY294002 inhibited apoptosis of HUVECs by inhibiting protein expression of PI3K/AKT-VEGF signaling pathway in vitro A: Western blot assay for protein expression of p-PI3K, p-AKT and VEGF; B: Statistical analysis.aP˂0.05 vs normoxia-control group;bP˂0.05 vs hypoxia-control group.
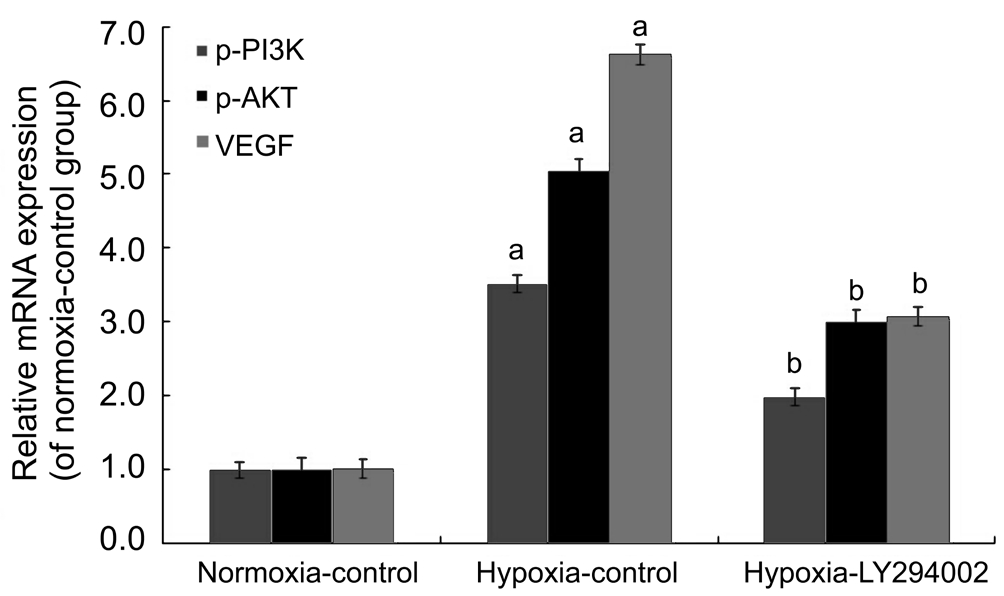
Figure 7 LY294002 inhibited apoptosis of HUVECs by inhibiting mRNA expression of the PI3K/AKT-VEGF signaling pathway in vitro mRNA expression levels of PI3K, AKT and VEGF were determined by real-time RT-PCR.aP˂0.05 vs normoxia-control group;bP˂0.05 vs hypoxia-control group.
Immunohistochemical, Western blot and real-time RT-PCR analyses indicated that protein and mRNA expression levels of PI3K, AKT, and VEGF in RNV were reduced by intravitreal injection of LY294002. To further investigate the effect of LY294002 in OIR, we also examined the expression of the PI3K/AKT-VEGF pathway in HUVECs under hypoxia treated with LY294002. In this experiment, because of the sufficient source, convenient materials and convenient operation of HUVEC, so it was used to simulate the endothelial cell (EC)of retinopathy of prematurity (ROP) or RNV, and HUVEC was the main material for studying EC of RNV in vitro. The results of our previous and this experimental study indicated that LY294002 activated apoptosis of HUVECs under hypoxia conditions by depressing the PI3K/AKT-VEGF signaling pathway.
Our results confirm that depletion of PI3K may play an antiangiogenic role by reducing the production of VEGF in vivo and in vitro. We also discoveryed the anti-angiogenic effect of LY294002 which is due to the reduction in PI3K/AKT-VEGF.Therefore, these results mightily indicate that LY294002 can provide an effective treatment for preventing ROP.
ACKNOWLEDGEMENTS
Foundations:Supported by the National Natural Science Foundation of China (No.81600747); Startup Foundation for Docotors of Liaoning Province (No.201501020).
Conflicts of Interest:Di Y, None; Chen XL, None.
REFERENCES
1 Ornelas IM, Silva TM, Fragel-Madeira L, Ventura AL. Inhibition of PI3K/Akt pathway impairs G2/M transition of cell cycle in late developing progenitors of the avian embryo retina. PLoS One 2013;8(1):e53517.
2 Xi JC, Zang HY, Guo LX, Xue HB, Liu XD, Bai YB, Ma YZ. The PI3K/AKT cell signaling pathway is involved in regulation of osteoporosis. J Recept Signal Transduct Res 2015;35(6):640-645.
3 Song Z, Guo Y, Zhou M, Zhang X. The PI3K/p-Akt signaling pathway participates in calcitriol ameliorating podocyte injury in DN rats. Metabolism 2014;63(10):1324-1333.
4 Xu J, Yi Y, Li L, Zhang W, Wang J. Osteopontin induces vascular endothelial growth factor expression in articular cartilage through PI3K/AKT and ERK1/2 signaling. Mol Med Rep 2015;12(3):4708-4712.
5 Alvarez Y, Astudillo O, Jensen L, Reynolds AL, Waghorne N, Brazil DP, Cao Y, O’Connor JJ, Kennedy BN. Selective inhibition of retinal angiogenesis by targeting PI3 kinase. PLoS One 2009;4(11):e7867.
6 Di Y, Zhang Y, Hui L, Yang H, Yang Y, Wang A, Chen X. Cysteine-rich 61 RNA interference inhibits pathological angiogenesis via phosphatidylinositol 3-kinase/Akt-vascular endothelial growth factor signaling pathway in endothelial cells. Mol Med Rep 2016;14(5):4321-4327.
7 Chikaraishi Y, Shimazawa M, Hara H. New quantitative analysis, using high-resolution images, of oxygen-induced retinal neovascularization in mice. Exp Eye Res 2007;84(3):529-536.
8 Park K, Chen Y, Hu Y, Mayo AS, Kompella UB, Longeras R, Ma JX.Nanoparticle-mediated expression of an angiogenic inhibitor ameliorates ischemia-induced retinal neovascularization and diabetes-induced retinal vascular leakage. Diabetes 2009;58 (8):1902-1913.
9 Di Y, Zhang Y, Nie Q, Chen X. CCN1/Cyr61-PI3K/AKT signaling promotes retinal neovascularization in oxygen-induced retinopathy. Int J Mol Med 2015;36(6):1507-1518.
10 Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov 2014;13(2):140-156.
11 Tsvetkov D, Shymanets A, Huang Y, Bucher K, Piekorz R, Hirsch E, Beer-Hammer S, Harteneck C, Gollasch M, Numberq B. Better understanding of phosphoinositide 3-Kinase (PI3K) pathways in vasculature: towards precision therapy targeting angiogenesis and tumor blood supply. Biochemistry (Mosc) 2016;81(7):691-699.
12 Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol 2010;28(6):1075-1083.
13 Zheng D, Zhu G, Liao S, Yi W, Luo G, He J, Pei Z, Li G, Zhou Y.Dysregulation of the PI3K/Akt signaling pathway affects cell cycle and apoptosis of side population cells in nasopharyngeal carcinoma. Oncol Lett 2015;10(1):182-188.
14 Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer 2009;9(8):537-549.
15 Chen J, Shao C, Lu W, Yan C, Yao Q, Zhu M, Chen P, Gu P, Fu Y,Fan X. Adenosine triphosphate-induced rabbit corneal endothelial cell proliferation in vitro via the P2Y2-PI3K/Akt signaling axis. Cells Tissues Organs 2014;199(2-3):131-139.
16 Sharma S, Guru SK, Manda S, Kumar A, Mintoo MJ, Prasad VD,Sharma PR, Mondhe DM, Bharate SB, Bhushan S. A marine sponge alkaloid derivative 4-chloro fascaplysin inhibits tumor growth and VEGF mediated angiogenesis by disrupting PI3K/Akt/mTOR signaling cascade.Chem Biol Interact 2017;275:47-60.
17 Wu YJ, Neoh CA, Tsao CY, Su JH, Li HH. Sinulariolide suppresses human hepatocellular carcinoma cell migration and invasion by inhibiting matrix metalloproteinase-2/-9 through MAPKs and PI3K/Akt signaling pathways. Int J Mol Sci 2015;16(7):16469-16482.
18 Xu Z, Liu D, Fan C, Luan L, Zhang X, Wang E. DIXDC1 increases the invasion and migration ability of non-small-cell lung cancer cells via the PI3K-AKT/AP-1 pathway. Mol Carcinog 2014;53(11):917-925.
19 Xing CG, Zhu BS, Fan XQ, Liu HH, Hou X, Zhao K, Qin ZH. Effects of LY294002 on the invasiveness of human gastric cancer in vivo in nude mice. World J Gastroenterol 2009;15(40):5044-5052.
20 Luo X, Dong Z, Chen Y, Yang L, Lai D. Enrichment of ovarian cancer stem-like cells is associated with epithelial to mesenchymal transition through an miRNA-activated AKT pathway. Cell Prolif 2013;46(4):436-446.
Correspondenceto:Yu Di. Department of Ophthalmology,Shengjing Affiliated Hospital, China Medical University,Shenyang 110004, Liaoning Province, China. diyu81@126.com
Received:2018-01-04 Accepted: 2018-04-08
Abstract● AlM: To investigate the effects of the phosphatidylinositol 3-kinase (Pl3K) inhibitor LY294002 on retinal neovascularization(RNV) in the oxygen-induced retinopathy (OlR) mouse model and human umbilical vein endothelial cells (HUVECs).● METHODS: C57BL/6J mice were randomly divided into normoxia-control, OlR-control and LY294002 treatment groups. LY294002 or phosphate-buffered solution was intraperitoneally injected daily into mouse pups from P6 to P9 in LY294002 treatment group or OlR-control group.Morphological and pathological changes in RNV, as well as expression levels of Pl3K, serine-threonine kinase(AKT) and vascular endothelial growth factor (VEGF) were observed. HUVECs treating with LY294002 were exposed to hypoxia; the expression of Pl3K, AKT and VEGF were examined by Western blot and RT-PCR analyses.● RESULTS: Compared with the OlR-control group, LY294002 significantly inhibit RNV. Adenosine diphosphatase(ADPase) staining and hematoxylin and eosin staining indicated that the clock hour scores of neovascularization and the nuclei of pre-retinal neovascular cells in the LY294002 treatment group were clearly less than those in the OlR-control group (1.41±0.52 vs 6.20±1.21; 10.50±1.58 vs 22.25±1.82, both P<0.05). lntravitreal injection of LY294002 (in the LY294002 treatment group) markedly decreased Pl3K/AKT-VEGF expression compared with the OlR-control group by immunohistochemistry, Western blotting and RT-PCR (all P<0.05). ln HUVECs treated with hypoxia, expression of Pl3K, AKT and VEGF were downregulated in the hypoxia-LY294002 group (all P<0.05).● CONCLUSlON: The Pl3K inhibitor LY294002 can inhibit RNV by downregulating Pl3K, AKT, and VEGF expression in vivo and in vitro. LY294002 may provide an effective method for preventing retinopathy of prematurity (ROP).
● KEYWORDS:vascular endothelial growth factor; phosphatidylinositol 3-kinase; LY294002; retinopathy of prematurity;retinal neovascularization
DOl:10.18240/ijo.2018.08.06
Citation:Di Y, Chen XL. Inhibition of LY294002 in retinal neovascularization via down-regulation the PI3K/AKT-VEGF pathway in vivo and in vitro. Int J Ophthalmol 2018;11(8):1284-1289






