Lacrimal gland tumors in Turkey: types, frequency, and outcomes
Yağmur Seda Yeşiltaş1, Ahmet Kaan Gündüz1, Esra Erden2, Carol L. Shields3
1Department of Ophthalmology, Ankara University Faculty of Medicine, Ankara 06620, Turkey
2Department of Pathology, Ankara University Faculty of Medicine, Ankara 06230, Turkey
3Ocular Oncology Service, Wills Eye Hospital, Thomas Jefferson University, Philadelphia, PA 19107, USA
INTRODUCTION
Lacrimal gland tumors (LGTs) are uncommon with an estimated incidence of ˂1/1 000 000 per year[1].LGTs represent 3% to 18% of all orbital tumors[2-5]. There may be differences in the distribution of LGTs in different countries depending on geographical region, whether clinical or histopathological diagnosis is used, characteristics of the reporting institution, and patient referral patterns[1,5-6]. Lesions of the lacrimal gland can be divided into epithelial and nonepithelial lesions. Approximately 55%-80% of the LGTs are of non-epithelial origin and 20%-45% are of epithelial origin[2-5,7].
Non-epithelial masses include non-granulomatous and granulomatous inflammation and lymphoproliferative lesions.Benign epithelial tumors constitute about half of epithelial tumors. Malignant epithelial tumors comprise the other half of epithelial tumors. Benign epithelial tumors include lacrimal ductal cyst (dacryops) and pleomorphic adenoma (PA).Malignant epithelial tumors include adenoid cystic carcinoma(ACC), carcinoma ex pleomorphic adenoma (Ca-ex-PA), de novo adenocarcinoma, and other rare entities. Of the malignant epithelial tumors, ACC is the most common accounting for about 66% of the cases, followed by Ca-ex-PA (18%), de novo adenocarcinoma (9%), mucoepidermoid carcinoma (3%), and other less common lesions[5]. In this study, we aimed to review the clinical, radiological, and treatment features of LGTs from an oncology center in Turkey.
SUBJECTS AND METHODS
A retrospective review of 99 eyes of 92 cases with the diagnosis of LGT managed on the Ocular Oncology Service at Ankara University Faculty of Medicine in Turkey between January 1999 and March 2017 was made. Two cases which had the histopathologic diagnosis of normal lacrimal gland tissue during the study interval were excluded. The study was approved by the Institutional Review Board. A signed consent form was obtained and kept on file for all patients whose clinical and radiologic photographs were used in this study.
Table 1 Demographics and histopathologic/clinical diagnoses in 92 cases with LGTs n (%)
LGTs: Lacrimal gland tumors.
No. of lesions No. of cases Gender (M/F) Mean age (range, y)Non-epithelial tumors 64 (64.6) 58 (63.0) 23/35 38.5 (7-80)Inflammatory lesions (dacryoadenitis) 56 (56.6) 50 (54.3) 19/31 34.9 (7-68)Non-granulomatous inflammation 46 (46.5) 43 (46.7) 18/25 35.2 (10-68)Granulomatous inflammation 10 (10.1) 7 (7.6) 1/6 32.6 (7-68)Non-necrotizing granulamotous inflammation 7 (7.1) 5 (5.4) 0/5 32.0 (7-68)Necrotizing granulomatous inflammation 3 (3.0) 2 (2.2) 1/1 34.0 (23-44)Lymphoproliferative lesions 8 (8.1) 8 (8.7) 5/3 61.1 (30-80)Lymphoma 5 (5.0) 5 (5.4) 3/2 68.8 (45-80)Benign reactive lymphoid hyperplasia 3 (3.0) 3 (3.3) 2/1 48.3 (30-72)Epithelial tumors 32 (32.3) 31 (33.7) 10/21 42.2 (13-72)Benign tumors 17 (17.2) 16 (17.4) 6/10 40.6 (16-70)Dacryops 3 (3.0) 2 (2.2) 1/1 28.5 (17, 40)Pleomorphic adenoma 14 (14.1) 14 (15.2) 5/9 42.3 (16-70)Malignant tumors 15 (15.1) 15 (16.3) 4/11 43.8 (13-72)Adenoid cystic carcinoma 12 (12.1) 12 (13.0) 3/9 39.2 (13-72)Carcinoma ex pleomorphic adenoma 2 (2.0) 2 (2.1) 0/2 59.5 (53, 66)Adenocarcinoma 1 (1.0) 1 (1.1) 1/0 69 Others 3 (3.0) 3 (3.3) 1/2 58.3 (41-74)Cavernous hemangioma 1 (1.0) 1 (1.1) 0/1 60 Dermoid cyst 1 (1.0) 1 (1.1) 1/0 74 Leukemic infiltration (acute myeloid) 1 (1.0) 1 (1.1) 0/1 41 Total 99 92 34/58 40.3 (7-80)LGTs
A careful history was taken in each case. Every patient underwent complete ophthalmic examination and imaging[computed tomography (CT) or magnetic resonance imaging(MRI)]. The diagnosis was usually made after orbital biopsy and histopathologic examination but in a few cases the diagnosis was reached based on clinical and radiological findings. Complete blood count, peripheral blood smear,chest-abdominal CT, neck ultrasonography, and lately 18F-fluorodeoxyglucose positron emission tomography were used to assess the presence of systemic involvement in the cases with lacrimal gland carcinoma and ocular adnexal lymphoma (OAL). The medical evaluation was made by the medical oncologist and follow-up tests were repeated as necessary.
RESULTS
The study included 92 cases with a mean age of 40.3 (range:7-80)y. Seven cases had bilateral lesions. Fifty-eight (63.1%)were females and 34 (36.9%) were males. Demographic data and histopathologic/clinical diagnoses are listed in Table 1.The most frequent signs and symptoms were eyelid swelling[49 (60.5%) cases] (Figure 1A), proptosis [45 (55.5%) cases],and presence of an eyelid mass or lump [16 (19.7%) cases](Figure 2A, Table 2).
The diagnosis was made histopathologically in 91 (91.9%)tumors and on a clinical and radiological basis in 8 (8.1%)tumors. The surgical technique used in obtaining biopsy from 91 tumors included anterior orbitotomy using upper lid skin crease approach in 87 tumors, orbitotomy via Kronlein approach in 2 tumors, anterior orbitotomy via conjunctival approach in 1 tumor, and exenteration (based on the prior histopathologic diagnosis of adenocarcinoma) in 1 tumor.During surgery, incisional biopsy was performed in 39 (42.8%)lesions, total excisional biopsy in 32 (35.2%), and subtotal excisional biopsy in 19 (20.9%).
Final diagnoses were idiopathic orbital inflammation(pseudotumor) in 46 (46.5%) lesions, PA in 14 (14.1%)(Figure 2D), ACC in 12 (12.1%) (Figure 4C), granulomatous inflammation in 10 (10.1%), lymphoma in 5 (5.0%)(Figure 3B), benign reactive lymphoid hyperplasia (BRLH)in 3 (3.0%), dacryops in 3 (3.0%), Ca-ex-PA in 2 (2.0%),adenocarcinoma in 1 (1.0%), dermoid cyst in 1 (1.0%),cavernous hemangioma in 1 (1.0%), and leukemic infiltration in 1 (1.0%). Non-epithelial tumors comprised 64.6% (n=64) of all LGTs, epithelial tumors 32.3% (n=32), dermoid cyst 1.0%(n=1), cavernous hemangioma 1.0% (n=1), and leukemic infiltration 1.0% (n=1). There were in total 78 (78.8%) benign and 21 (21.2%) malignant tumors. Malignant tumors included 15 cases of malignant epithelial tumors, 5 cases of lymphomas and 1 case of leukemic infiltration.Of 7 bilateral lesions, 6 were diagnosed as dacryoadenitis and 1 as dacryops. Six dacryoadenitis and 2 dacryops lesions did not undergo biopsy. Additional treatments for residual/progressive disease and recurrences included systemic corticosteroid treatment in 56 (56.6%) lesions, external beam radiotherapy (EBRT) in 23 (23.2%), further eye-sparing tumor resection in 7 (7.1%), exenteration in 6 (6.1%),immunosuppresive/immunomodulatory treatment in 5 (5.1%),and systemic chemotherapy in 3 (3.0%). The preoperative CT and MRI findings were correlated with the histopathology results. Imaging data from the cases with dacryoadenitis and lymphoproliferative lesions demonstrated enlarged lacrimal gland with a molded configuration and ill-defined margins(Figures 1B, 3A). Inflammatory and lymphoproliferative lesions were usually hypointense or isointense on both T1 and T2-weighted images (Figures 1B, 3A) and demonstrated contrast enhancement on MRI.
Table 2 Distribution of LGTs according to age, duration of symptoms, and signs/symptoms n (%)

LGTs: Lacrimal gland tumors; PA: Pleomorphic adenoma.aBased on 81 cases because of lack of data in the remaining 11 cases.
LGTs Dacryops PA Carcinoma Cavernous hemangioma Inflammatory lesions(dacryoadenitis)Lymphoproliferative lesions Dermoid cyst Leukemia Total No. of tumors Age (y)˂20 12 (24.0) - 1 (50.0) 1 (7.1) 2 (13.3) - - - 16 (17.4)20-40 19 (38.0) 1 (12.5) 1 (50.0) 6 (42.9) 4 (26.7) - - - 31 (33.7)41-60 15 (30.0) 2 (25.0) - 4 (28.6) 6 (40.0) 1 (100.0) - 1 (100.0) 29 (31.5)61-80 4 (8.0) 5 (62.5) - 3 (21.4) 3 (20.0) - 1 (100.0) - 16 (17.4)Duration of symptomsa˂6mo 23 (52.2) 4 (50.0) - 3 (27.3) 8 (61.5) 1 (100.0) 1 (100.0) 1 (100.0) 41 (49.3)6-12mo 15 (34.1) 4 (50.0) 1 (50.0) 6 (54.5) 3 (23.1) - - - 29 (37.3)˃12mo 6 (13.6) - 1 (50.0) 2 (18.2) 2 (15.4) - - - 11 (13.3)Signs and symptoms Eyelid swelling 32 (72.7) 6 (75.0) 2 (100.0) 3 (27.3) 5 (38.5) - - 1 (100.0) 49 (60.5)Ptosis 4 (9.1) 2 (25.0) - 1 (9.1) - - - - 7 (8.6)Eyelid mass or lump 8 (18.2) 3 (37.5) - 4 (36.4) 1 (7.7) - - - 16 (19.7)Inflammatory symptoms 11 (25.0) - - - - - - - 11 (13.6)Pain 2 (4.5) - - 1 (9.1) 8 (61.5) - - - 10 (12.3)Proptosis 19 (43.2) 3 (37.5) - 9 (81.8) 11 (84.6) 1 (100.0) 1 (100.0) - 45 (55.5)
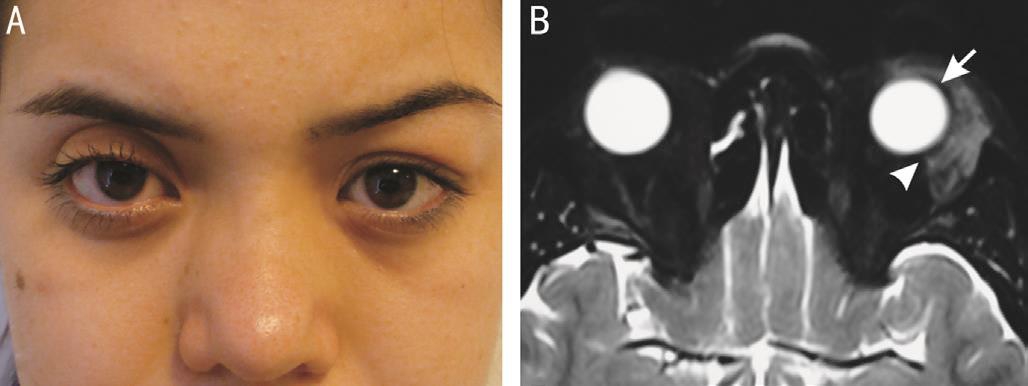
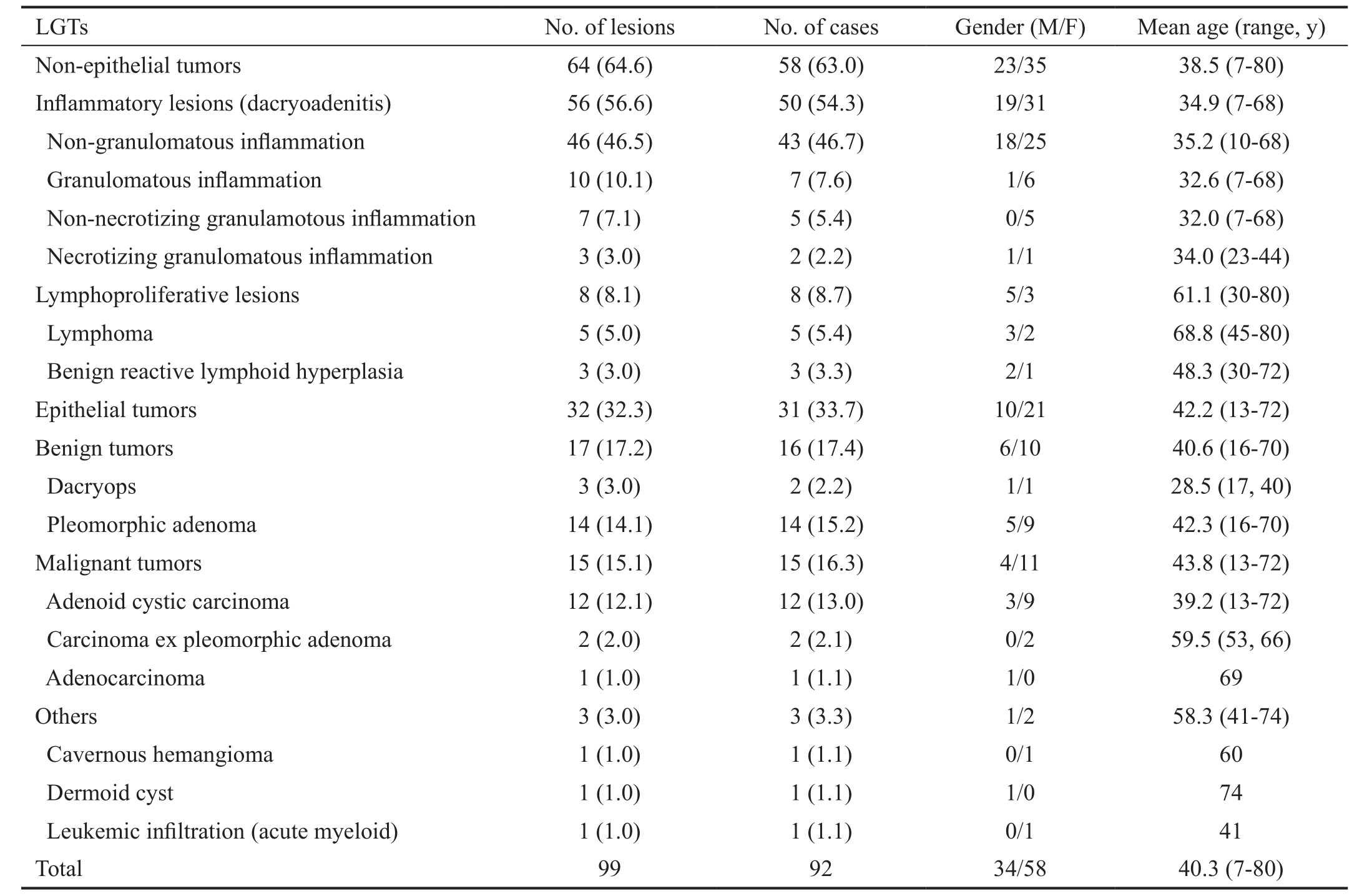
Figure 1 Non-granulomatous dacryoadenitis A: A 14 year-old girl presented with indolent left eyelid edema of 2mo duration; B: T2-weighted axial MRI demonstrating dacryoadenitis hypointense with respect to the extraocular muscles and cerebral gray matter. The tumor displays a molded configuration with ill-defined margins (arrowhead)conforming to the shape of the globe (arrow).

Figure 2 Lacrimal gland PA A: A 70 year-old woman complained of 8mo history of painless mass in the left upper eyelid causing the left eye to be displaced downwards and medially; B: Coronal orbital CT shows displacement and indentation of the globe (arrowhead)by the round-to-oval well-circumscribed mass and expansion of the lacrimal gland fossa (arrows), with no evidence of destructive erosion of neighbouring bone; C: Gross photograph of the totally excised PA with an intact pseudocapsule; D: Histopathologic examination shows PA with an epithelial component composed of tubular and ductal structures surrounded by hyaline-rich stroma (hematoxylin-eosin).
The cases with benign epithelial tumors demonstrated a round-to-oval well-circumscribed orbital mass (Figure 2B).Three of 14 cases with PA showed bone thinning (expansion)(Figure 2B). The cases with carcinomas demonstrated wellcircumscribed round-to-oval orbital mass without bone destruction in 7/15 cases (Figure 4A) and ill-defined tumors with bone destruction in 5/15 cases. In 3 cases with carcinoma,data on tumor shape was lacking. Epithelial tumors usually demonstrated isointensity on T1-weighted images (Figure 4A), hyperintensity on T2-weighted images and demonstrated contrast enhancement on MRI.
At a mean follow-up of 54.8 (range: 1-205)mo, 81 cases were alive, 6 cases died from metastasis (5 with ACC and 1 with Caex-PA) and 5 cases died from nontumor-related causes (2 with dacryoadenitis, 1 with adenocarcinoma, 1 with lymphoma, and 1 with cavernous hemangioma).
Analysis of Histopathological Subgroups Inflammatory lesions (dacryoadenitis, 50 cases, 56 lesions)Forty-four (88.0%) cases with dacryoadenitis had unilateral involvement. Bilateral lacrimal gland involvement was noted in only 6 (12.0%) cases. All but one of the cases underwent anterior orbitotomy for tissue diagnosis. An incisional biopsy was performed in 37 (74.0%) lesions, subtotal excisional biopsy in 8 (16%) lesions, and dacryoadenectomy in 5 (10.0%)lesions as the initial procedure.
Forty-six (82.1%) lesions had non-granulomatous and 10 (17.9%) lesions had granulomatous inflammation. Two cases with dacryoadenitis were of the sclerosing type. Seven of 10 granulomatous lesions had non-necrotizing and 3 had necrotizing inflammation. Ten (17.8%) lesions were classified as acute dacryoadenitis (˂1mo duration of symptoms) and 46(82.2%) lesions as chronic dacryoadenitis (˃1mo duration of symptoms) (Figure 1A). Systemic features associated with non-granulomatous inflammation included systemic vasculitis(1 case) and dermatomyositis (1 case). Of the granulomatous inflammation cases, 1 had sarcoidosis and 1 had Wegener granulomatosis.
Systemic corticosteroid therapy was administered in all cases after diagnosis was made. Other additional treatments for progressive disease and recurrences included further tumor resection/debulking of the lacrimal gland lesion (4 cases),azathioprine (3 cases), cyclosporine+azathioprine (1 case),cyclosporine (1 case), and EBRT [3 cases (4 eyes)]. Four cases(8.0%) with orbital inflammatory lesions had to be kept on continuous low dose immunosuppressive treatment because of frequent recurrences.
Lymphoproliferative lesions (8 cases, 8 lesions)Of 5 lymphoma cases, 4 had extranodal marginal zone lymphoma (ENMZL)whereas histopathologic typing could not be made in 1 case.Systemic involvement was not detected in any case. Five cases underwent orbital EBRT (4 cases with lymphoma and 1 case with BRLH). The EBRT dose was 40 Gy for lymphomas and 30 Gy for BRLH. The remaining lymphoma case had total excisional biopsy of the orbital lesion. No additional treatment was given in the remaining 3 cases. No recurrence was observed in any case.
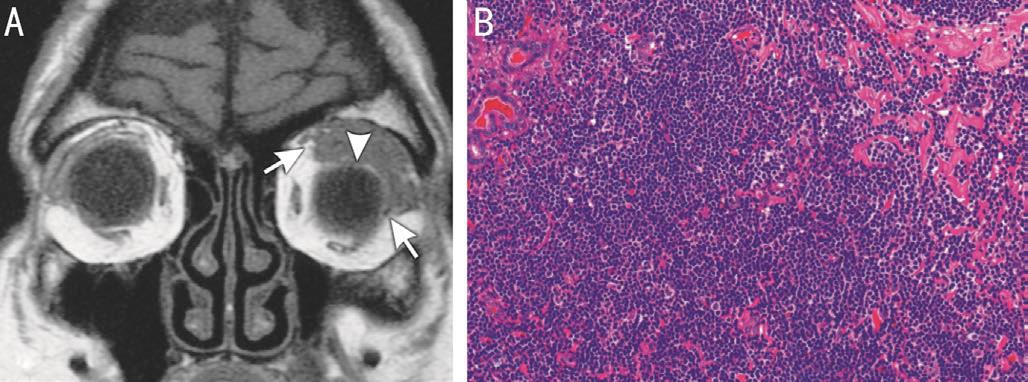
Figure 3 Lacrimal gland lymphoma in a 75 year-old man A:T1-weighted coronal MRI shows that the lacrimal gland mass has a molded appearance (arrowhead) with ill-defined margins (arrows) and is isointense with respect to the extraocular muscles and cerebral gray matter; B: Histopathologic examination shows a diffuse pattern of atypical lymphoid cells infiltrating normal glandular tissue consistent with the diagnosis of lymphoma (hematoxylin-eosin).
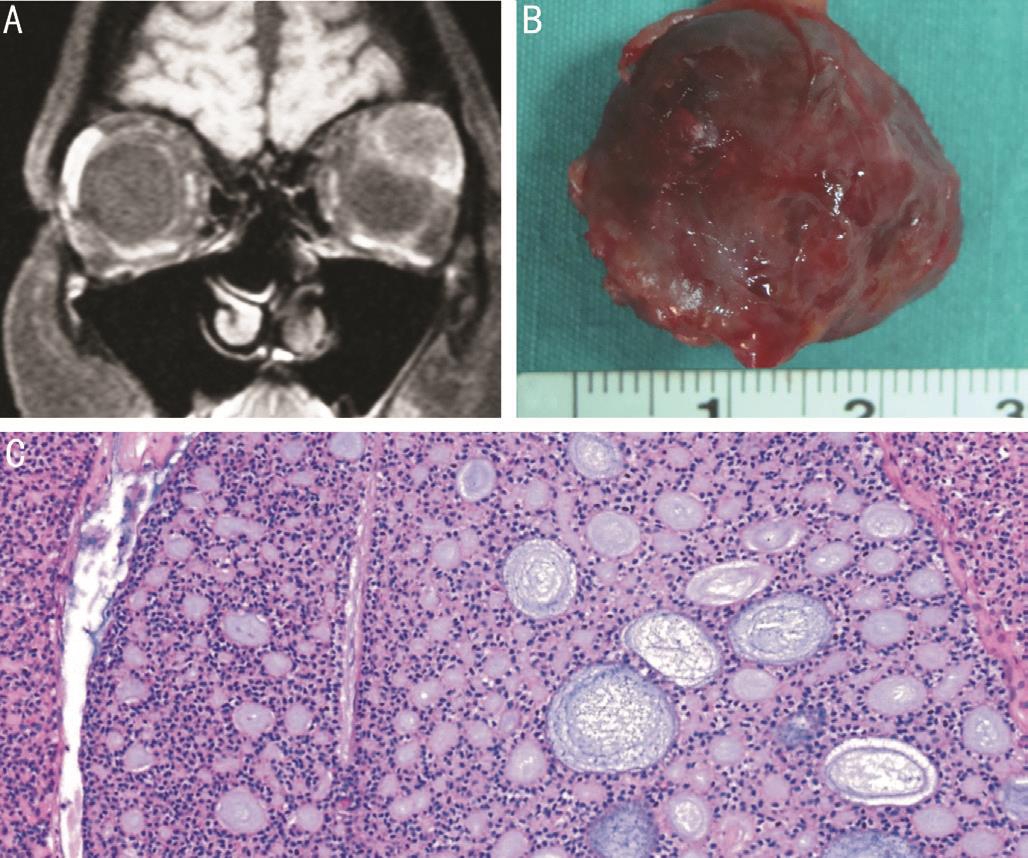
Figure 4 Lacrimal gland ACC in a 32 year-old woman A:Contrast-enhanced T1-weighted coronal image shows heterogeneously enhancing mass with round-to-oval appearance; B: Gross photograph of the completely excised ACC shows a well-circumscribed mass with irregular margins; C: Histopathologic examination shows that nests and columns of tumor cells are arranged around gland-like spaces filled with basophilic and eosinophilic material, forming a “Swiss cheese”pattern consistent with a cribriform ACC (hematoxylin-eosin).
Dacryops (2 cases, 3 lesions)One case had unilateral and the other case had bilateral involvement. The unilateral case was treated with total excision because of the eyelid complications of dacryops and the bilateral case was observed.
Pleomorphic adenoma (14 cases, 14 lesions)Of 14 PA cases,13 cases underwent anterior orbitotomy and 1 case Kronlein(bone removing) orbitotomy. Ten cases had total excision(Figure 2C) and 4 had subtotal excision because breach of tumor pseudocapsule was present at surgery. In 3 of 4 cases in whom subtotal excisional biopsy was performed,recurrence occurred at a mean of 95.9 (range: 40-185)mo. All recurrences were treated with further tumor excision because of the infiltrative nature of the recurrent tumor. Two cases had significant residual orbital tumor at the end of follow-up.
Adenoid cystic carcinoma (12 cases, 12 lesions)Total excisional biopsy via anterior orbitotomy was performed in 11 cases and Kronlein orbitotomy in 1 case of ACC (Figure 4B). Eight of 12 cases had the cribriform (Figure 4C) ACC while in 4 cases subtyping was not made. The 8thAmerican Joint Committee on Cancer classifications (AJCC) of 12 cases at initial diagnosis were as follows: T1aN0M0 (1 case),T2aN0M0 (7 cases), T2bN0M0 (1 case), T2cN0M0 (1 case),T3cN0M0 (1 case), and T4bN0M0 (1 case). Perineural invasion was found in 7 cases and microscopic tumor presence at surgical margins was detected in 6 cases at histopathologic examination. EBRT was administered in 6 cases (T1a and T2a), EBRT+chemotherapy in 2 (T2a), exenteration+EBRT in 1 (T2b), and bone removal exenteration+EBRT in 2 (T3c and T4b). The EBRT dose was 55-60 Gy. In one case with T2c ACC, additional treatment could not be performed after surgery due to the patient’s refusal of treatment. Recurrence in 2 cases was treated with exenteration in 1 case (T2a) and EBRT+chemotherapy in the other case (T1a). Eight cases(66.7%) with lesser AJCC stages (≤T2a)±tumor negative margins were treated with eye-sparing therapy (excisional biopsy+EBRT±chemotherapy) initially while 3 cases (25.0%)with more advanced tumors (≥T2b)+positive surgical margins were treated with exenteration+EBRT. Four of 8 cases receiving eye-sparing surgery initially and 2 of 3 cases receiving exenteration developed metastasis. At a mean followup of 55.5 (range: 3-159)mo, 3 of 3 cases ≤21 years of age and 4 of 9 cases ˃21y were alive, 5 had expired.
Carcinoma ex pleomorphic adenoma (2 cases, 2 lesions)After diagnosis was established using anterior orbitotomy, both cases with Ca-ex-PA eventually underwent exenteration and EBRT (55-60 Gy). The time to development of Ca-ex-PA after incomplete excision of PA was 20 and 23y respectively in our 2 cases.
Adenocarcinoma (1 case, 1 lesion)This case was diagnosed with adenocarcinoma at a remote destination and managed with exenteration and EBRT (55 Gy) at our institution.
Cavernous hemangioma (1 case, 1 lesion)Total excisional biopsy via anterior orbitotomy was performed in the case with cavernous hemangioma.
Dermoid cyst (1 case, 1 lesion)The case with dermoid cyst was managed with total excision via anterior orbitotomy.
Leukemic infiltration (1 case, 1 lesion)The case with leukemia was a 41 year-old woman who had previously diagnosed acute myeloid leukemia and underwent incisional biopsy via anterior orbitotomy for the lacrimal gland lesion that proved to be leukemic infiltration.
DISCUSSION
LGTs usually occur unilaterally and reflect a variety of underlying etiologic factors. A minority of cases demonstrate bilateral involvement at presentation, constituting a different clinical spectrum compared to unilateral presentations[8]. Tang et al[8]studied 97 patients with bilateral lacrimal gland disease and found that the most common diagnoses were idiopathic dacryoadenitis (30%), sarcoidosis (20%), lacrimal gland prolapse (15%), and lymphoma (11%). In our study, bilateral lacrimal gland involvement was found in 7 cases. Six of 7 cases had dacryoadenitis and 1 had dacryops.
The most frequent LGT was idiopathic orbital inflammation(IOI) in our series accounting for 46 (46.5%) tumors followed by PA 14 (14.1%), ACC 12 (12.1%), granulomatous inflammation 10 (10.1%), lymphoma 5 (5.0%), BRLH 3 (3.0%), dacryops 3(3.0%), Ca-ex-PA 2 (2.0%), adenocarcinoma 1 (1.0%), dermoid cyst 1 (1.0%), cavernous hemangioma 1 (1.0%), and leukemic infiltration 1 (1.0%). Our findings with respect to the frequency of orbital lesions are in accordance with other previous major publications[2,4-5].
LGTs may be found in patients of all ages, but are primarily encountered in middle-aged adults. Our series reflects the same tendency (Tables 1, 2). In our series, the cases with dacryoadenitis presented at a mean age of 35y, those with PA at a mean age of 42y and carcinomas at a mean age of 44y. There is a bimodal age distribution for ACC and ACC can also occur in younger patients. There were 3 cases of ACC aged less than 21y in our series. On the other hand, lymphomas affected more elderly patients with a mean age of 69.
Patient history and clinical features are important in differentiating LGTs from each other (Table 2)[9]. Cases with acute dacryoadenitis usually presented with inflammatory signs including pain,eyelid edema, and conjunctival hyperemia. On the other hand,chronic dacryoadenitis cases presented with salient features including an upper eyelid mass and mild pain/discomfort.In our series, there were only 2 patients with dacryoadenitis presenting with pain. This low frequency may be attributable to the retrospective nature of our study and mild pain being an easily overlooked/missed symptom. Lymphoproliferative lesions were not usually associated with inflammatory signs.In our series, cases with PAs usually presented with ˃6mo duration of symptoms and no pain. Lacrimal gland carcinoma cases, on the other hand, presented with ˂6mo duration of symptoms and pain (Table 2). Orbital imaging (CT and MRI)is necessary to assist in the differentiation of LGTs[10]. Cases with dacryoadenitis and lymphoproliferative lesions usually presented with ill-defined oblong masses that molded to the shape of the globe and bone on CT and MRI. PAs usually displayed well-defined round-to-oval configuration sometimes with bone expansion. While early-caught carcinomas also had similar imaging findings to PAs, more aggressive or long-standing carcinomas displayed an ill-defined molded configuration to the lateral orbital bone similar to inflammatory and lymphoproliferative lesions. However, unlike inflammatory and lymphoproliferative lesions, carcinomas may cause bone destruction.
Inflammatory lesions can be divided into two groups including non-granulomatous and granulomatous. In our study, nongranulomatous lesions were present in approximately 80%of cases, and granulomatous lesions in the remaining 20% of cases, consistent with the reports of Shields et al[7]and von Holstein et al[1]. Non-granulomatous orbital inflammation consists mostly of IOI where no identifiable cause for inflammation can be found but there may be a few cases associated with vasculitis, infections, and other causes.
Recently, taking a generous biopsy or debulking the lacrimal gland has been suggested as a means to improve the success rate of subsequent corticosteroid treatment[11]. In our series, 13 cases had subtotal/total excision of the lacrimal gland in line with these observations. High dose systemic corticosteroids(1 mg/kg·d oral prednisolone) with a slow taper in the range of 5-10 mg per week, is the generally accepted standard treatment for dacryoadenitis[12]. However, response rate to corticosteroids is variable and ranges from 55% to 79%[13].Methotrexate, azathioprine, cyclophosphamide, cyclosporine,rituximab, infliximab, and EBRT (20-30 Gy) may be added to the treatment in unresponsive or recurrent disease[14-15].In our study, 8 of 50 (16.0%) cases with lacrimal gland inflammatory lesions required additional treatments besides oral corticosteroids.
Orbital lymphoproliferative lesions are the other common cause of non-epithelial tumors of the lacrimal gland. The distribution of lymphoma subtypes in our study confirms that ENMZL is the most frequent lymphoma subtype of the lacrimal gland (4 of 5 cases). In our study, all cases with ENMZL, except for the case who died from nontumorrelated cause, were alive at last follow-up, suggesting a good prognosis for ENMZL. If OAL is limited to the orbital and adnexal structures, incisional/excisional biopsy followed by EBRT is considered the treatment of choice. Furthermore,Mohammad and Kroosh[16]reported that small lacrimal gland lymphomas can be sufficiently treated by surgical excision alone and local control of disease is 100% at 5y. This was the case in one of our patients who was treated with surgical excision alone. EBRT is effective in 97% of OAL in terms of local tumor control. While conventional doses of 30-40 Gy produce excellent local control, there has been recent evidence that 2 Gy×2 (boom-boom treatment) may be sufficient to control the orbital disease with a high response rate of 96%[17].PA is the most common benign tumor of the lacrimal gland,constituting 65% of all epithelial tumors. It usually occurs in patients between 30-40 years of age, but has been reported in children as young as 6 years old[18]. The youngest patient in our series was 16 years old. Complete excision of the tumor within its pseudocapsule via orbitotomy is recommended for treatment. Recurrence may occur if satellite nodules are not excised or the primary lesion is incompletely excised.Furthermore, it has been suggested that there is a significant risk of malignant transformation (about 10%) associated with long-standing PA that has never been excised or incompletely resected recurrent PA[19].
There may a spectrum of lesions including dacryoadenitis,lymphoma, and ACC simulating PA radiologically[20]. Recently,preoperative incisional biopsy and subsequent excision of lacrimal gland and biopsy track has been recommended as a more appropriate management option for PAs if there is doubt about diagnosis[20-21]. There is currently no robust evidence suggesting that prior incisional biopsy increases the risk of recurrence in PA.
ACC is the most common malignant epithelial tumor of the lacrimal gland. Histopathologic types include cribriform,tubular, and solid (basaloid) pattern. The prognosis of ACC is associated with age, negative surgical margin, grade of perineural invasion, tumor histopathology, and tumor size[22].Tellado et al[23]showed that patients younger than 19y tended to have low-grade tumors of non-basaloid type and therefore the rates of local control and survival were better than in adults. In our series, 3 of 3 cases ≤21 years of age were alive compared with 4 of 9 cases ˃21 years of age, reflecting the same tendency with the previously published literature.Perineural invasion is present in approximately 85% of cases with ACC. Perineural invasion demonstrates strong correlation with local tumor recurrence and skull base invasion.Histopathologically, the cribriform type has the best prognosis whereas the basaloid type has the worst prognosis[24].
Depending on the AJCC stage of ACC, an eye-sparing treatment approach may be attempted[25]. In our series, 8 cases with lesser AJCC stages (≤T2a) ± tumor negative margins were treated with eye-sparing surgery initially compared to 3 more advanced cases (≥T2b) with positive surgical margins treated with exenteration. EBRT was used in all cases. Regardless of the treatment approach, ACC is a locally invasive tumor and metastasis occurs frequently, with significant resultant mortality. In advanced tumors with extensive spread to soft tissues and bone marrow, exenteration±orbitectomy and EBRT are still the mainstays of treatment, although this kind of mutilating surgery may not positively affect long-term survival.
Ca-ex-PA is the second most common primary epithelial malignancy of the lacrimal gland. It may occur de novo or may originate from malignant transformation of a primary or recurrent PA with sudden rapid enlargement. The carcinomatous component of Ca-ex-PA may show a variety of morphologies; however, adenocarcinoma, mucoepidermoid carcinoma, and ACC are more common[26]. In our study, there were 2 cases of adenocarcinoma ex PA which developed 20 and 23y after incomplete excision of the primary PAs.
Lacrimal gland adenocarcinoma is a rare malignancy and usually occurs in an older population compared to ACC[27].Adenocarcinoma has a propensity to metastasize earlier than ACC and is associated with a shorter patient survival than ACC. In lacrimal gland adenocarcinomas, exenteration and EBRT are the recommended methods for treatment[27-28].
In summary, LGTs represent a wide variety of conditions.Fortunately, 79% are benign and can be managed with surgical excision or corticosteroids. The remaining 21% of tumors that are malignant comprise ACC, lymphoma, Ca-ex-PA,and adenocarcinoma. Some of the malignant tumors can be challenging to manage with a significant mortality rate.
ACKNOWLEDGEMENTS
Authors’ contributions:Concept: Gündüz AK; Design:Gündüz AK, Yeşiltaş YS; Supervision: Gündüz AK, Shields CL; Resource: Gündüz AK, Yeşiltaş YS; Materials: Gündüz AK, Yeşiltaş YS, Erden E; Data collection and/or processing:Gündüz AK, Yeşiltaş YS; Analysis and/or interpretation:Gündüz AK, Yeşiltaş YS; Literature search: Gündüz AK,Yeşiltaş YS; Writing: Gündüz AK, Yeşiltaş YS; Critical reviews: Gündüz AK, Shields CL.
Conflicts of Interest:Yeşiltaş YS, None; Gündüz AK, None;Erden E, None; Shields CL, None.
REFERENCES
1 von Holstein SL, Therkildsen MH, Prause JU, Stenman G, Siersma VD,Heegaard S. Lacrimal gland lesions in Denmark between 1974 and 2007.Acta Ophthalmol 2013;91(4):349-354.
2 Günalp I, Günduz K. Biopsy-proven orbital lesions in Turkey: a survey of 1092 cases over 30 years. Orbit 1994;13(2):67-79.
3 Johansen S, Heegaard S, Bogeskov L, Prause JU. Orbital spaceoccupying lesions in Denmark 1974-1997. Acta Ophthalmol Scand 2000;78(5):547-552.
4 Seregard S, Sahlin S. Panorama of orbital space-occupying lesions. The 24-year experience of a referral centre. Acta Ophthalmol Scand 1999;77(1):91-98.5 Shields JA, Shields CL, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: the 2002 Montgomery Lecture, part 1. Ophthalmology 2004;111(5):997-1008.
6 Lacrimal Gland Tumor Study Group. An epidemiological survey of lacrimal fossa lesions in Japan: number of patients and their sex ratio by pathological diagnosis. Jpn J Ophthalmol 2005;49(5):343-348.
7 Shields CL, Shields JA, Eagle RC, Rathmell JP. Clinicopathologic review of 142 cases of lacrimal gland lesions. Ophthalmology 1989;96(4):431-435.
8 Tang SX, Lim RP, Al-Dahmash S, et al. Bilateral lacrimal gland disease:clinical features of 97 cases. Ophthalmology 2014;121(10):2040-2046.
9 Günalp I, Gunduz K. Epithelial tumors of the lacrimal gland. Orbit 1994;13(3):147-154.
10 Gündüz K, Shields CL, Günalp I, Shields JA. Magnetic resonance imaging of unilateral lacrimal gland lesions. Graefes Arch Clin Exp Ophthalmol 2003;241(11):907-913.
11 Mombaerts I, Cameron JD, Chanlalit W, Garrity JA. Surgical debulking for idiopathic dacryoadenitis: a diagnosis and a cure. Ophthalmology 2014;121(2):603-609.
12 Pakdaman MN, Sepahdari AR, Elkhamary SM. Orbital inflammatory disease: pictorial review and differential diagnosis. World J Radiol 2014;6(4):106-115.
13 Andrew NH, Kearney D, Sladden N, McKelvie P, Wu A, Sun MT, McNab A, Selva D. Idiopathic dacryoadenitis: clinical features, histopathology,and treatment outcomes. Am J Ophthalmol 2016;163:148-153.e1.
14 Wilson MW, Shergy WJ, Haik BG. Infliximab in the treatment of recalcitrant idiopathic orbital inflammation. Ophthal Plast Reconstr Surg 2004;20(5):381-383.
15 Sergott RC, Glaser JS, Charyulu K. Radiotherapy for idiopathic inflammatory orbital pseudotumor. Indications and results. Arch Ophthalmol 1981;99(5):853-856.
16 Mohammad AE, Kroosh SS. Treatment of primary lymphoma of the lacrimal gland by surgical excision alone: a 5-year follow-up study. Orbit 2001;20(2):131-140.
17 Fasola CE, Jones JC, Huang DD, Le QT, Hoppe RT, Donaldson SS. Low-dose radiation therapy (2 Gy×2) in the treatment of orbital lymphoma. Int J Radiat Oncol Biol Phys 2013;86(5):930-935.
18 Andrew NH, McNab AA, Selva D. Review of 268 lacrimal gland biopsies in an Australian cohort. Clin Exp Ophthalmol 2015;43(1):5-11.
19 Weis E, Rootman J, Joly TJ, Berean KW, Al-Katan HM, Pasternak S, Bonavolonta G, Strianese D, Saeed P, Feldman KA, Vangveeravong S, Lapointe JS, White VA. Epithelial lacrimal gland tumors: pathologic classification and current understanding. Arch Ophthalmol 2009;127(8):1016-1028.
20 Prabhakaran VC, Cannon PS, McNab A, Davis G, O’Donnell B,Dolman PJ, Ghabrial R, Selva D. Lesions mimicking lacrimal gland pleomorphic adenoma. Br J Ophthalmol 2010;94(11):1509-1512.
21 Lai T, Prabhakaran VC, Malhotra R, Selva D. Pleomorphic adenoma of the lacrimal gland: is there a role for biopsy? Eye (Lond) 2009;23(1):2-6.22 Esmaeli B, Ahmadi MA, Youssef A, Diba R, Amato M, Myers JN, Kies M, El-Naggar A. Outcomes in patients with adenoid cystic carcinoma of the lacrimal gland. Ophthal Plast Reconstr Surg 2004;20(1):22-26.
23 Tellado MV, McLean IW, Specht CS, Varga J. Adenoid cystic carcinomas of the lacrimal gland in childhood and adolescence. Ophthalmology 1997;104(10):1622-1625.
24 Ahmad SM, Esmaeli B, Williams M, Nguyen J, Fay A, Woog J,Selvadurai D, Rootman J, Weis E, Selva D, McNab A, DeAngelis D, Calle A, Lopez A. American Joint Committee on cancer classification predicts outcome of patients with lacrimal gland adenoid cystic carcinoma.Ophthalmology 2009;116(6):1210-1215.
25 Esmaeli B, Yin VT, Hanna EY, Kies MS, William WN Jr, Bell D,Frank SJ. Eye-sparing multidisciplinary approach for the management of lacrimal gland carcinoma. Head Neck 2016;38(8):1258-1262.
26 von Holstein SL, Fehr A, Persson M, Nickelsen M, Therkildsen MH, Prause JU, Heegaard S, Stenman G. Lacrimal gland pleomorphic adenoma and carcinoma ex pleomorphic adenoma: genomic profiles, gene fusions, and clinical characteristics. Ophthalmology 2014;121(5):1125-1133.
27 Heaps RS, Miller NR, Albert DM, Green WR, Vitale S. Primary adenocarcinoma of the lacrimal gland. A retrospective study. Ophthalmology 1993;100(12):1856-1860.
28 von Holstein SL, Coupland SE, Briscoe D, Le Tourneau C, Heegaard S. Epithelial tumours of the lacrimal gland: a clinical, histopathological,surgical and oncological survey. Acta Ophthalmol 2013;91(3):195-206.
Correspondenceto:Ahmet Kaan Gündüz. Department of Ophthalmology, Farilya Business Center 8/50, Ufuk University Avenue, Cukurambar, Ankara 06450, Turkey. drkaangunduz@gmail.com
Received:2017-11-15 Accepted: 2018-04-25
Abstract● AlM: To evaluate the clinical, radiological, and treatment features of lacrimal gland tumors.● METHODS: Retrospective review of 99 eyes of 92 patients with lacrimal gland tumors diagnosed and managed in a single institution between January 1999 and March 2017. Clinical and radiological features, histopathology,treatment methods, and prognosis were evaluated.● RESULTS: The mean patient age was 40.3 (range: 7-80)y.The diagnosis was made histopathologically in 91 (91.9%)tumors and on a clinical and radiological basis in 8 (8.1%)tumors. Final diagnoses included idiopathic orbital inflammation (pseudotumor) in 46 (46.5%) lesions,pleomorphic adenoma in 14 (14.1%), adenoid cystic carcinoma in 12 (12.1%), granulomatous inflammation in 10(10.1%), lymphoma in 5 (5.0%), benign reactive lymphoid hyperplasia in 3 (3.0%), dacryops in 3 (3.0%), carcinoma ex pleomorphic adenoma in 2 (2.0%), adenocarcinoma in 1 (1.0%), dermoid cyst in 1 (1.0%), cavernous hemangioma in 1 (1.0%), and leukemic infiltration in 1 (1.0%). Nonepithelial tumors comprised 64.6% (n=64) of all lacrimal gland tumors, epithelial tumors 32.3% (n=32), dermoid cyst 1% (n=1), cavernous hemangioma 1% (n=1), and leukemic infiltration 1% (n=1). There were in total 78 (78.8%) benign and 21 (21.2%) malignant tumors.● CONCLUSlON: Overall, 65% of lacrimal gland tumors were of non-epithelial origin and 32% of epithelial origin. By histopathology and clinical evaluation, 79% of lacrimal gland tumors were benign. The most common lacrimal gland tumors include idiopathic orbital inflammation (46.5%),epithelial (32.3%), and lymphoproliferative (8.1%) lesions.
● KEYWORDS:orbit; lacrimal gland; tumor; inflammation;epithelial tumor; non-epithelial tumor
DOl:10.18240/ijo.2018.08.08
Citation:Yeşiltaş YS, Gündüz AK, Erden E, Shields CL. Lacrimal gland tumors in Turkey: types, frequency, and outcomes. Int J Ophthalmol 2018;11(8):1296-1302





