Comparison of anterior segment optical coherence tomography findings in acanthamoeba keratitis and herpetic epithelial keratitis
Young Min Park1,2, Jong Soo Lee3, Ji-Myong Yoo1,4, Jong Moon Park1,2, Seong-Wook Seo1,4, In-Young Chung1,4,Seong Jae Kim1,4
1Department of Ophthalmology, School of Medicine, Gyeongsang National University, Jinju 52727, South Korea
2Gyeongsang National University Changwon Hospital,Samjeongja-ro, Seongsan-gu, Changwon-si, Gyeongsangnamdo 51472, South Korea
3Department of Ophthalmology, School of Medicine, Pusan National University and Medical Research Institute, Pusan National University Hospital, Pusan 49241, South Korea
4Institute of Health Science, Gyeongsang National University,Jinju 52727, South Korea
INTRODUCTION
Although acanthamoeba keratitis (AK) is relatively uncommon, when its diagnosis is delayed or incorrect,it can cause severe ocular damage. Recently, it was found that the main cause of AK is the improper use of contact lenses,including soft contact, hard contact, and orthokeratology lenses[1-2]. The clinical manifestations of AK include severe pain, corneal epithelial defect, corneal haze, and the most characteristic manifestation, radial keratoneuritis[3]. Although AK presents with characteristic signs, it is known to mimic herpetic epithelial keratitis (HEK)[4]. A definitive AK diagnosis is made by confirming its cytopathology using specific staining or culturing, a time-intensive process[4].
High-resolution anterior-segment optical coherence tomography(AS-OCT) allows the non-contact measurement and noninvasive imaging of microscopic structures, including the epithelium, Bowman layer, stroma, Descemet membrane,and endothelium[5-11]. Yamazaki et al[3]reported that AS-OCT provides novel and detailed visual information about radial keratoneuritis in patients with early-stage AK.
The purpose of this study was to investigate the characteristic features of AK that differentiating it from HEK using AS-OCT.
SUBJECTS AND METHODS
This study adheres to the tenets of the Declaration of Helsinki.Written informed consent was obtained from all patients.The medical records of three patients with AK and three with herpetic keratitis were reviewed in this study, such that each group was composed of three eyes. All patients were examined using AS-OCT (Spectralis; Heidelberg Engineering,Heidelberg, Germany) with an anterior segment module that uses raster and single-scan with high-resolution acquisition at a speed of 40 000 A-scans per second, an axial resolution of 3.9 to 7 μm, and a transverse resolution of 14 μm. Slit-lamp biomicroscopy and AS-OCT was performed on all patients during the initial and every subsequent follow-up visit.

Figure 1 AK case 1 A: Radial keratoneuritis observed by slit-lamp biomicroscopy. AS-OCT exhibits reflective bands in the corneal stroma that correspond to the area of radial keratoneuritis (white arrows); B: After AK treatment, radial keratoneuritis disappeared in slit-lamp biomicroscopy, and reflective bands in the corneal stroma disappeared in AS-OCT.
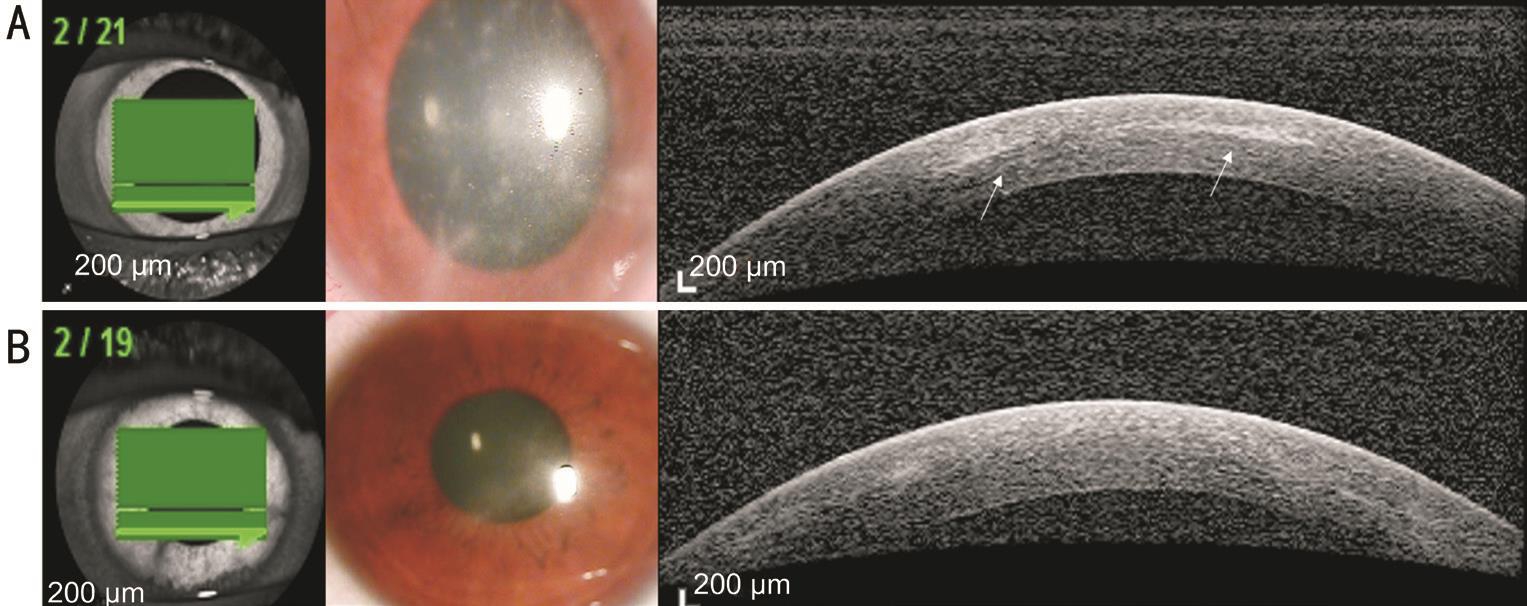
Figure 2 AK case 2 A: Radial keratoneuritis observed by slit-lamp biomicroscopy. AS-OCT exhibits reflective bands in the corneal stroma that correspond to the area of radial keratoneuritis (white arrows); B: After AK treatment, radial keratoneuritis disappeared in slit-lamp biomicroscopy, and reflective bands in the corneal stroma disappeared in AS-OCT.
Table 1 Characteristics, clinical ocular histories and treatments of AK patients
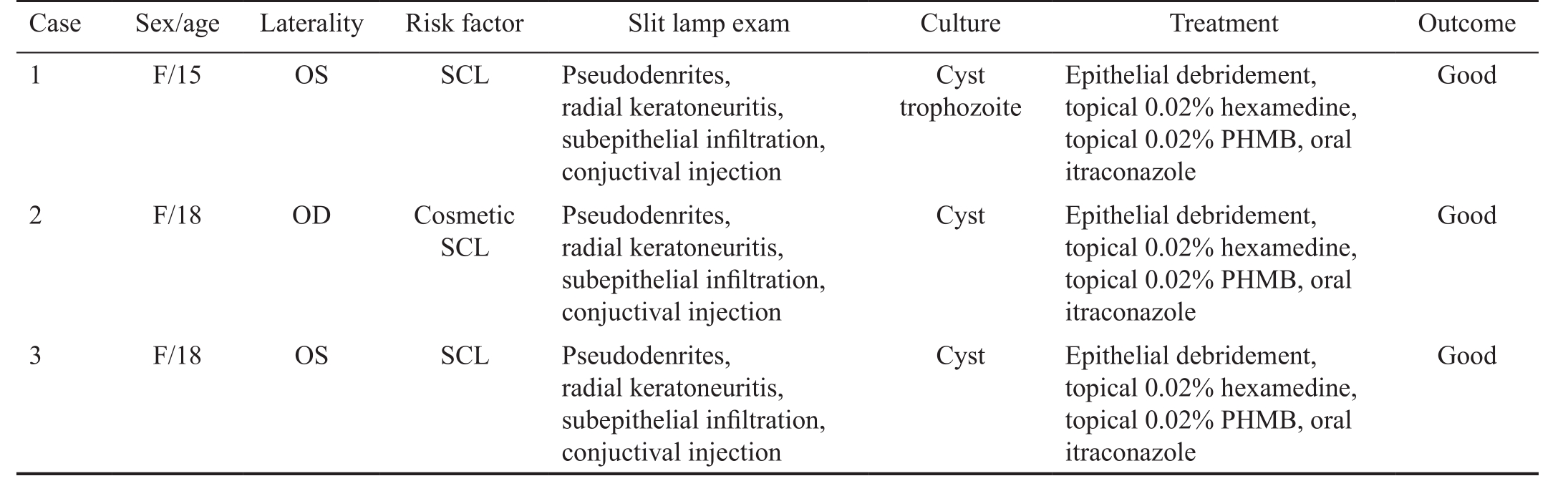
SCL: Soft contact lenses; PHMB: Polyhexamethylene biguanide.
Case Sex/age Laterality Risk factor Slit lamp exam Culture Treatment Outcome 1 F/15 OS SCL Pseudodenrites,Good radial keratoneuritis,subepithelial infiltration,conjuctival injection 2 F/18 OD Cosmetic SCL Cyst trophozoite Epithelial debridement,topical 0.02% hexamedine,topical 0.02% PHMB, oral itraconazole Pseudodenrites,radial keratoneuritis,subepithelial infiltration,conjuctival injection Cyst Epithelial debridement,topical 0.02% hexamedine,topical 0.02% PHMB, oral itraconazole Good 3 F/18 OS SCL Pseudodenrites,radial keratoneuritis,subepithelial infiltration,conjuctival injection Cyst Epithelial debridement,topical 0.02% hexamedine,topical 0.02% PHMB, oral itraconazole Good
RESULTS
Acanthamoeba Keratitis PatientsTable 1 summarizes the characteristics, clinical ocular histories, and treatments for all AK patients. The use of soft contact lenses was the cause of AK for all the patients. A definitive diagnosis of AK was confirmed by plate culturing tissue obtained by corneal scraping. The corneal tissue was placed onto the center of a 1.5% non-nutrient agar plate covered with a lawn of Escherichia coli. Plates were sealed with parafilm, incubated at 30℃, and screened by inverted phase contrast microscopy.
In all cases, patients were initially treated with 100 mg of oral itraconazole, topical 0.02% polyhexamethylene biguanide(PHMB) and 0.02% chlorhexidine. Clinical outcomes were fair with visual recovery in all AK cases.
In all three AK cases, we observed reflective bands in the corneal stroma that corresponded to the area of radial keratoneuritis (case 1: Figure 1A; case 2: Figure 2A; case 3:Figure 3A). The depth of the reflective bands varied in each case. After AK treatment, slit-lamp biomicroscopy confirmed that radial keratoneuritis had resolved and AS-OCT confirmed that reflective bands in the corneal stroma had also disappeared in all patients (case 1: Figure 1B; case 2: Figure 2B; case 3:Figure 3B).

Figure 3 AK case 3 A: Radial keratoneuritis observed by slit-lamp biomicroscopy. AS-OCT exhibits reflective bands in the corneal stroma that correspond to the area of radial keratoneuritis (white arrows); B: After AK treatment, radial keratoneuritis disappeared in slit-lamp biomicroscopy, and reflective bands in the corneal stroma disappeared in AS-OCT.
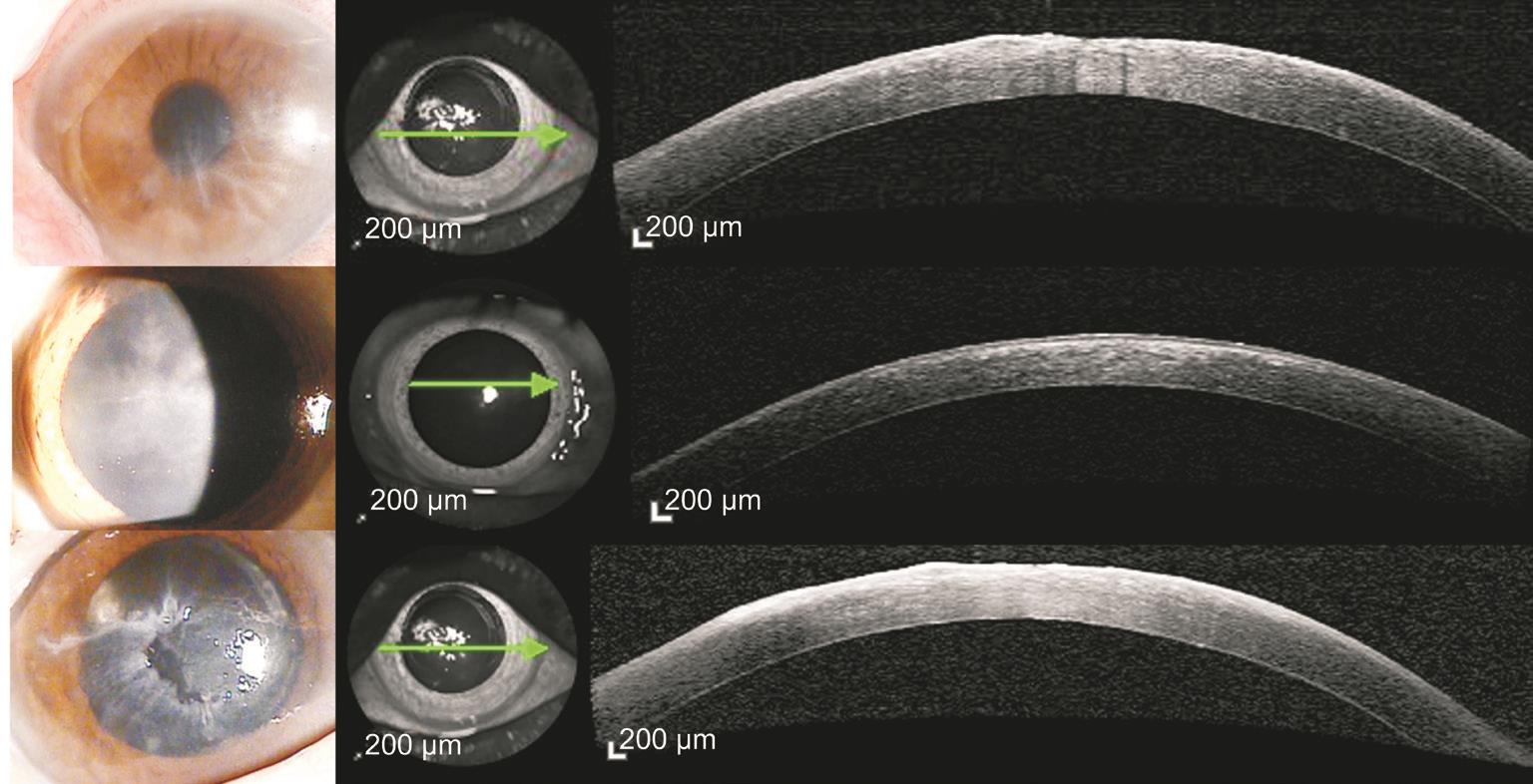
Figure 4 HEK cases Highly reflective lesion of HEK were observed only at subepithelial area but not at stroma. In some patients, corneal epithelial irregularity was also observed in both slit-lamp biomicroscopy and AS-OCT.
Table 2 Characteristics, clinical ocular histories and treatments of HEK patients
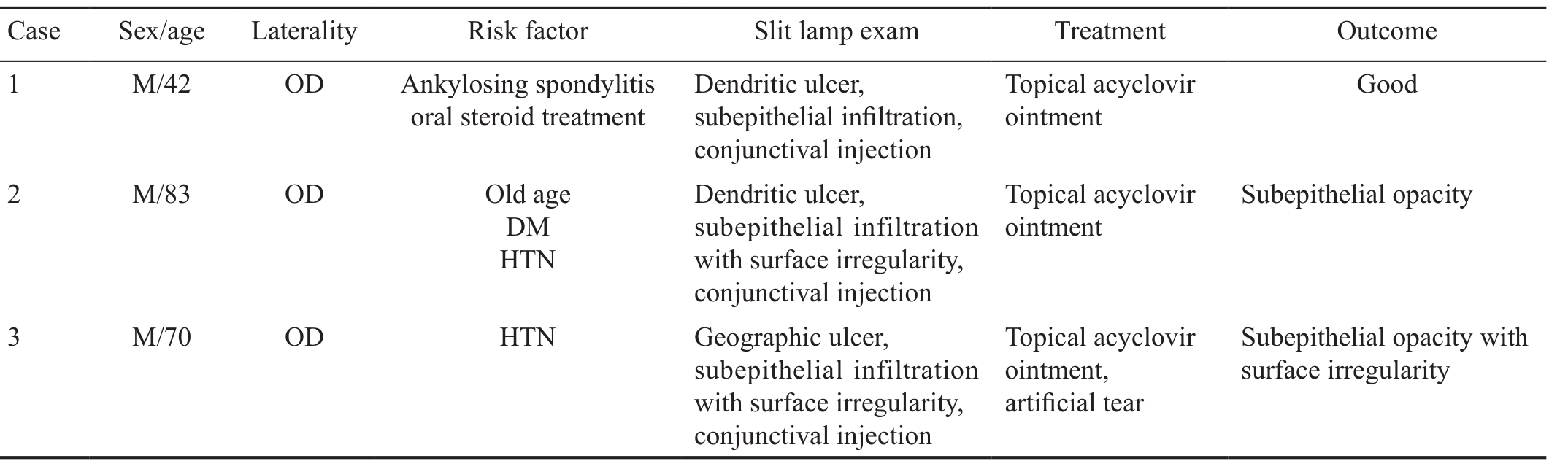
DM: Diabetes mellitus; HTN: Hypertension; HEK: Herpetic epithelial keratitis.
Case Sex/age Laterality Risk factor Slit lamp exam Treatment Outcome 1 M/42 OD Ankylosing spondylitis oral steroid treatment Dendritic ulcer,subepithelial infiltration,conjunctival injection 2 M/83 OD Old age DM HTN Topical acyclovir ointment Good Dendritic ulcer,subepithelial infiltration with surface irregularity,conjunctival injection Topical acyclovir ointment Subepithelial opacity 3 M/70 OD HTN Geographic ulcer,subepithelial infiltration with surface irregularity,conjunctival injection Topical acyclovir ointment,artificial tear Subepithelial opacity with surface irregularity
Herpetic Epithelial Keratitis PatientsUnlike the AS-OCT results found in AK, highly reflective HEK lesions were observed only in the subepithelial area, not in the stroma(Figure 4). In some patients, we observed corneal epithelial irregularity using slit-lamp biomicroscopy and AS-OCT. Table 2 summarizes the characteristics, treatment process and results of the HEK patients.
DISCUSSION
Solely using slit-lamp biomicroscopy examination to clinically diagnose AK is limited, especially at the early phase of the disease. Among other anterior segment features, radial keratoneuritis is considered a characteristic sign of early stage AK and it can easily be confused with the dendritic lesions or subepithelial infiltration seen in HEK. AK is known to resemble HEK and is often misdiagnosed and treated as HEK[4]. More than 50% of AK cases are misdiagnosed as HEK at initial diagnosis[5], rendering it impossible to properly treat AK early. Although an AK diagnosis can be confirmed by staining, corneal biopsy, or tissue culture[4], these procedure require a lot of time and resources. Additionally, if AK is misdiagnosed as and treated for herpetic keratitis, the toxicity of HEK viral treatments can cause the disease to progress faster than normal while awaiting the test results. For this reason, it is important to distinguish AK from HEK early in the disease onset.
The recent development of AS-OCT enables the visualization of microscopic corneal structures. Yamazaki et al[3]reported that AS-OCT provides novel and detailed visual information of radial keratoneuritis in patients with early-stage AK[3].However, to date, there have been no reports comparing ASOCT findings in AK and HEK patients.
Consistent with the findings from the study by Yamazaki et al[3], we observed highly reflective bands in the corneal stroma that corresponded to the area of radial keratoneuritis and these bands disappeared as the radial keratoneuritis resolved after proper AK treatment in all of our patients.Corneal nerve bundles are known to enter the cornea at the periphery and move towards the center, below the anterior third of the stroma[12]. In our study, reflective bands in the corneal stroma also appeared at the anterior third of the stroma, which is consistent with the corneal nerve passage[12].However, in the current study, it was impossible to identify AK cysts or trophozoites using AS-OCT because of the limited power resolution of the device, meaning that currently, ASOCT cannot replace tissue culture. In addition, in-vivo confocal microscopy enables to identify AK cysts or trophozoites by its higher resolution compared to the AS-OCT, but AS-OCT has its strength by allowing an overall analysis of the ocular surface due to larger visualization windows[13-14]. We believe that if the AS-OCT resolution increases enough to distinguish AK cysts and trophozoites, then tissue culturing could be replaced by AS-OCT. Until then, additional in-vivo confocal microscopy examination along with AS-OCT may be a way to improve the diagnosis rate besides tissue culture.
Unlike AK cases, highly reflective lesions were only observed in the subepithelial area in HEK patients and these lesions remained after the treatment. Although some cases report that HEK initially presents as keratoneuritis, most herpetic keratitis involves the corneal epithelium in the early stage of the disease(e.g. corneal vesicles and dendritic ulcers)[15-17]. To differentiate AK from HEK early in the disease, it is important to confirm if the corneal lesion involves the corneal epithelium or stroma.
AS-OCT is known to be able to identify disease boundaries in more detail than slit-lamp biomicroscopy even in the presence of corneal opacity, as in this study[18]. Similarly in our study,AS-OCT appears to be more helpful in analyzing detailed lesion depth than slit lamp biomicroscopy in diseases such as AK or HEK that accompanies corneal opacity.
Reflective bands in the corneal stroma that present with radial keratoneuritis, which can be seen using AS-OCT, can be useful to distinguish AK from herpetic keratitis. However, because keratoneuritis is also a characteristic of other diseases than AK,further information, such as a detailed medical history about contact lens use, severe ocular pain, and other symptoms of AK, are necessary for an AK diagnosis. Because this study only included a small number of patients, future studies with a larger number of AK patients are necessary.
ACKNOWLEDGEMENTS
Authors’ contributions:Kim SJ and Park YM were responsible for obtaining consent, acquiring the data and drafting the manuscript. Yoo JM, Park JM, Seo SW and Chung IY participated in patient care and helped establish the final clinical diagnosis. Lee JS and Kim SJ critically revised the manuscript. All authors read and approved the final version of the manuscript.
Foundation:Supported by Basic Science Research Program through the National Research Foundation of Korea (NRF)funded by the Ministry of Science, ICT & Future Planning(No.NRF-2015R1C1A1A02037702).
Conflicts of Interest:Park YM, None; Lee JS, None; Yoo JM, None; Park JM, None; Seo SW, None; Chung IY, None;Kim SJ, None.
REFERENCES
1 Brown AC, Ross J, Jones DB, Collier SA, Ayers TL, Hoekstra RM,Backensen B, Roy SL, Beach MJ, Yoder JS; Acanthamoeba Keratitis Investigation Team. Risk factors for acanthamoeba keratitis-a multistate case-control study, 2008-2011. Eye Contact Lens 2017.
2 Lee MH, Abell RG, Mitra B, Ferdinands M, Vajpayee RB. Risk factors,demographics and clinical profile of acanthamoeba keratitis in Melbourne:an 18-year retrospective study. Br J Ophthalmol 2017;102(5):687-691.
3 Yamazaki N, Kobayashi A, Yokogawa H, Ishibashi Y, Oikawa Y, Tokoro M, Sugiyama K. In vivo imaging of radial keratoneuritis in patients with Acanthamoeba keratitis by anterior-segment optical coherence tomography. Ophthalmology 2014;121(11):2153-2158.
4 Johns KJ, O’Day DM, Head WS, Neff RJ, Elliott JH. Herpes simplex masquerade syndrome: acanthamoeba keratitis. Curr Eye Res 1987;6(1):207-212.
5 Werkmeister RM, Sapeta S, Schmidl D, Garhöfer G, Schmidinger G, Aranha Dos Santos V, Aschinger GC, Baumgartner I, Pircher N,Schwarzhans F, Pantalon A, Dua H, Schmetterer L. Ultrahigh-resolution OCT imaging of the human cornea. Biomed Opt Express 2017;8(2):1221-1239.
6 Izatt JA, Hee MR, Swanson EA, Lin CP, Huang D, Schuman JS,Puliafito CA, Fujimoto JG. Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch Ophthalmol 1994;112(12): 1584-1589.
7 Li P, An L, Reif R, Shen TT, Johnstone M, Wang RK. In vivo microstructural and microvascular imaging of the human corneo-scleral limbus using optical coherence tomography. Biomed Opt Express 2011;2(11):3109-3118.
8 Yasuno Y, Madjarova VD, Makita S, Akiba M, Morosawa A, Chong C,Sakai T, Chan KP, Itoh M, Yatagai T. Three-dimensional and high-speed swept-source optical coherence tomography for in vivo investigation of human anterior eye segments. Opt Express 2005;13(26):10652-10664.
9 Gora M, Karnowski K, Szkulmowski M, Kaluzny BJ, Huber R,Kowalczyk A, Wojtkowski M. Ultra high-speed swept source OCT imaging of the anterior segment of human eye at 200 kHz with adjustable imaging range. Opt Express 2009;17(17):14880-14894.
10 Grulkowski I, Liu JJ, Baumann B, Potsaid B, Lu C, Fujimoto JG.Imaging limbal and scleral vasculature using Swept Source Optical Coherence Tomography. Photonics Lett Pol 2011;3(4):132-134.
11 Ruggeri M, Uhlhorn SR, De Freitas C, Ho A, Manns F, Parel JM.Imaging and full-length biometry of the eye during accommodation using spectral domain OCT with an optical switch. Biomed Opt Express 2012;3(7):1506-1520.
12 Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure,contents and function. Exp Eye Res 2003;76(5):521-522.
13 Tahiri Joutei Hassani R, Liang H, El Sanharawi M, Brasnu E, Kallel S, Labbé A, Baudouin C. En-face optical coherence tomography as a novel tool for exploring the ocular surface: a pilot comparative study to conventional B-scans and in vivo confocal microscopy. Ocul Surf 2014;12(4):285-306.
14 Petrovic A, Hashemi K, Blaser F, Wild W, Kymionis G. Characteristics of linear interstitial keratitis by in vivo confocal microscopy and anterior segment optical coherence tomography. Cornea 2018;37(6):785-788.
15 Darougar S, Wishart MS, Viswalingam ND. Epidemiological and clinical features of primary herpes simplex virus ocular infection. Br J Ophthalmol 1985;69(1):2-6.
16 Austin A, Lietman T, Rose-Nussbaumer J. Update on the management of infectious keratitis. Ophthalmology 2017;124(11):1678-1689.
17 Azher TN, Yin XT, Tajfirouz D, Huang AJ, Stuart PM. Herpes simplex keratitis: challenges in diagnosis and clinical management. Clin Ophthalmol 2017;11:185-191.
18 Hirano K, Ito Y, Suzuki T, Kojima T, Kachi S, Miyake Y. Optical coherence tomography for the noninvasive evaluation of the cornea.Cornea 2001;20(3):281-289.
Correspondenceto:Seong Jae Kim. Department of Ophthalmology, School of Medicine, Gyeongsang National University,Jinju 52727, South Korea. maya12kim@naver.com
Received:2018-03-28 Accepted: 2018-06-05
Abstract● This study is to investigate the characteristic features of Acanthamoeba keratitis (AK) that differentiating it from herpetic epithelial keratitis (HEK) using anterior segment optical coherence tomography (AS-OCT). Medical records of three eyes of each AK and herpetic keratitis who had AS-OCT examination were reviewed in this study. Slitlamp biomicroscopy and AS-OCT was performed on the initial visit and on every follow-up visits in all patients. ln all three AK cases, reflective bands in the corneal stroma that correspond to the area of radial keratoneuritis were observed. The depth of the reflective bands varied in each case. After AK treatment, slit-lamp biomicroscopy confirmed that radial keratoneuritis had resolved and AS-OCT confirmed that reflective bands in the corneal stroma had also disappeared in all patients. Unlike the AS-OCT results found in AK, highly reflective HEK lesions were observed only in the subepithelial area, not in the stroma. AS-OCT seems to be helpful analyzing the specific depth of the lesion which enables to distinguish AK from HEK.
● KEYWORDS:acanthamoeba; optical coherence tomography;herpes; herpetic keratitis
DOl:10.18240/ijo.2018.08.26
Citation:Park YM, Lee JS, Yoo JM, Park JM, Seo SW, Chung IY, Kim SJ. Comparison of anterior segment optical coherence tomography findings in acanthamoeba keratitis and herpetic epithelial keratitis. Int J Ophthalmol 2018;11(8):1416-1420





