Table 1 Summary of the numbers of anterior lens capsule and the methods used in this study
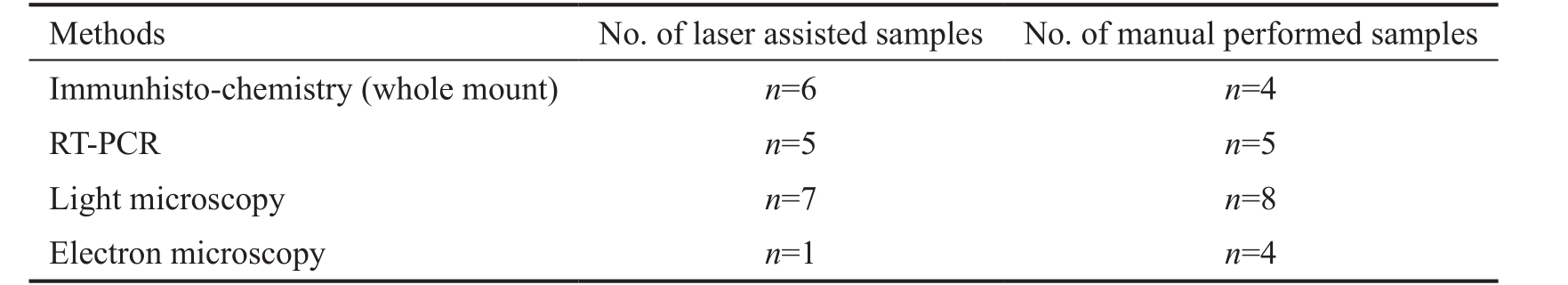
RT-PCR: Reverse transcription polymerase chain reaction.
Andrea Krisztina Sükösd 1 , Judit Rapp 2,3 , Diána Feller 2,3 , György Sétáló Jr 3,4 , Beáta Gáspár 5 , Judit E.Pongrácz 2,3 , Hajnalka Ábrahám 4 , Zsolt Biró 1,5
1 Department of Ophthalmology, the University of Pécs Medical School and Clinical Centre, Pécs 7623, Hungary
2 Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, the University of Pécs, Pécs 7624, Hungary
3 János Szentágothai Research Centre of the University of Pécs,Pécs 7624, Hungary
4 Department of Medical Biology and Central Electron Microscopic Laboratory, the University of Pécs Medical School, Pécs 7624, Hungary
5 Optimum Laser Centre, Budapest 1124, Hungary
Abstract · AlM: To study molecular and morphological changes in lens epithelial cells following femtosecond laserassisted and manually performed continuous curvilinear capsulotomy (CCC) in order to get information about these methods regarding their potential role in the induction of development of secondary cataract.· METHODS: Anterior lens capsules (ALC) were removed from 40 patients with age-related cataract by manual CCC and by femtosecond laser-assisted capsulotomy (FLAC).Samples removed by manual CCC were assorted in group 1, FLAC samples were classified in group 2.Morphology of lens epithelial cells was examined with light and electron microscopes. Following capsulotomy,expressions of p53, Bcl-2 and cyclin D1 genes were analyzed with reverse transcriptase polymerase chain reaction. lmmunohistochemistry was used to detect the pro-apoptotic p53 in the epithelial cells.· RESULTS: Light and electron microscopic examination showed that ALC of group 1 contained more degenerating cells following manual CCC than after FLAC. The expression level of p53 was higher after manual than laser-assisted surgery. lmmunocytochemistry indicated significantly higher number of cells containing p53 protein in the manual CCC group than following FLAC. Bcl-2 and cyclin D1 gene expression levels were slightly lower following manual CCC than after FLAC, but the difference was not significant.· CONCLUSlON: Manually removed ALC shows slightly,but not significantly larger damage due to the mechanical stretching and pulling of the capsule than those removed using FLAC.
· KEYWORDS: capsulotomy; capsulorhexis; femtosecond laser; immunhistology; ultrastructure; gene expression
Cataract is a common, multifactorial, age-related pathology with a high incidence among elderly people. Since the development of phacoemulsification, cataract surgery has undergone major improvements. From 2009, laser-assisted anterior capsulotomy with femtosecond laser system is also used. Initially from 2001, this technology dramatically changed the refractive surgery and it was widely used in the laser in situ keratomileusis [1-2] . Today, the goal of cataract surgery is to achieve near emmetropia. Femtosecond laser technology can deliver remarkable gains in reproducibility, centration,and safety in cataract surgery [3-6] . Regarding the incidence of posterior capsule opacification which is the most common postoperative complication following capsulotomy, results of different studies are contradictory. Investigations showed that laser-assisted surgery is not significantly better than manual capsulotomy, while others warn that femtosecond laser could increase the risk of this novel postoperative complication [7-11] .Secondary cataract develops by the migration and proliferation of residual lens epithelial cells after extracapsular cataract extraction. These residual lens epithelial cells become immature cells, and in addition to their proliferating ability,they undergo a large increase in cell volume. In the absence of normal lens architecture, these enlarged cells can take on a globular shape. The presence of crystallin-rich Elschnig pearls at the rear of the lens capsule interferes with vision due to light scattering [12] .
A number of clinical studies focus on the cut surface, on the intraocular lens decentration parameters, or on the continuouscurvilinear capsulorhexis (CCC) parameters that effect intraocular lens centration when manual or laser assisted methods are compared [13-15] . Little is known about the changes of the lens epithelial cells following capsulotomy using the two different methods. According to Mayer et al [16] , epithelial cells on the cutting surface of the removed anterior lens capsule(ALC) are affected differently when femtosecond laser-assisted capsulotomy (FLAC) and manual capsulotomy (manual CCC)were performed. Detection of apoptotic epithelial cells at the cut surface of the capsule with the TUNEL method indicated that more cells have undergone apoptosis using FLAC when compared to manual CCC [16] .
Table 1 Summary of the numbers of anterior lens capsule and the methods used in this study

RT-PCR: Reverse transcription polymerase chain reaction.
In addition to the cells on the cut edge of the removed ALC,cells locating medially of the cutting may also be affected by the surgery. The deleterious effect of the mechanical damage on epithelial cells due to surgery, and its consequence on the increased cell proliferation rate was also observed in other organs [17] .
It is plausible that the mechanical damage caused by the removal of the ALC may induce the formation of secondary cataract through the dedifferentiation and increased proliferation rate of the residual epithelial cells. We hypothesize that the damage caused by FLAC and manual CCC can be different. However, the effect of these surgical methods on residual epithelial cells cannot be directly examined in human.Alternatively, study of the epithelial cells of the removed ALC is a useful method to get information about the changes that occur in the residual epithelial cells. Therefore, in this work light microscopic and ultrastructural morphological alterations were detected in the epithelial cells of the removed ALC. In addition, expression of genes coding proteins related to cell proliferation (cyclin D1) and apoptosis, such as pro-apoptotic p53 and anti-apoptotic Bcl-2 were studied.
Patients Samples of ALC ( n =40) were taken during routine manual CCC and FLAC (VICTUS ® Femtosecond Laser,Bausch+Lomb’s, USA) with the following parameters: energy,7.2 µJ; spot spacing, 6 µm; path spacing, 4 µm; time, 400-550 femtoseconds. In all cases we obtained written consent of the patients for further examination of the lens capsule.Investigations were carried out according to procedures approved by the Institutional Ethics Committee (University of Pécs 5426, Hungary). Manually removed ALC were assorted in group 1 ( n =21), while FLAC samples were classified in group 2 ( n =19). Our inclusion criteria were the following: all patients with cataract, independent of gender and age. Table 1 summarizes the number of ALC and the methods used in this study.
Immunohistochemistry After removal, ALC were immersed in fixative containing 4% paraformaldehyde. Expression of p53 was detected on whole mount preparation with immunohistochemistry using primary antibody against p53 protein (rabbit polyclonal antibody, 1:50, Santa Cruz Biotechnology, USA), while the secondary antibody was fluorophore-conjugated (Alexa Fluor ® 488, 1:100, Life Technologies, Budapest, Hungary). Nuclear staining was performed with 4’,6-diamidino-2-phenylindole (DAPI;1:1000, Sigma, Budapest, Hungary), and tissues were coverslipped with Fluoromount-G (Southern Biotech, USA). For control experiments, primary antibody was omitted, and cross-reactivity of the non-corresponding secondary antibody with the primary was also checked. Photographs were taken digitally with a Fluoview FV-1000 Laser Confocal Scanning Microscope (Olympus, Japan).
Transmission electron microscopy The lens capsules were fixed in a buffered solution (phosphate buffer 0.1 mol/L,pH=7.4) of 2% formaldehyde and 2.5% glutaraldehyde for 24h at 4℃. Specimens were post-fixed with 1% osmium tetroxide diluted in phosphate buffer for 30min at room temperature.Following dehydration with ethyl-alcohol, samples were washed and placed in propylene oxide and then embedded in Durcupan resin (Sigma, Budapest, Hungary). Semithin sections were stained with toluidine-blue and examined with light microscope. Ultrathin sections were placed on mesh grids, and contrasted using solutions of lead-citrate and uranylacetate and examined in a JEOL JEM 1200EX transmission electron microscope (TEM). Photographs were taken digitally with the iTEM software (Olympus, Japan).
Gene Expression Analyses Using Real-time Reverse Transcriptase Polymerase Chain Reaction After removal,ALC were immersed into SAGM TM cell culture medium(Lonza, Basel, Switzerland) that contains all the ingredients that are necessary for the survival of human epithelial cells.From ALC total mRNA was isolated using NucleoSpin RNA Kit (Macherey-Nagel) within 24h following removal.cDNA was synthesized using High Capacity cDNA Kit (Life Technologies, Budapest, Hungary). Reverse transcription polymerase chain reaction (RT-PCR) was performed using a StepOnePlus TM and Real Time PCR System (Life Technologies, Budapest, Hungary) to quantify the PCR product once per cycle. Reaction consisted of 1 μL of each primer (in a final concentration of 500-500 nmol/L),10 μL 2× SensiFAST SYBR Hi-ROX master mix (Bioline Reagents Ltd., USA) in a 20 μL final volume. The following pairs of primers were used: p53, Bcl-2, cyclin D1 (Table 2).Relative product quantities were determined using StepOne Software and the Livak analysis method. β-actin was used as endogenous control.
Table 2 The primers used in reverse transcription polymerase chain reaction

p53: p53 tumor suppressor protein; ACTB: Human beta-actin protein coding gene; CCND1: Human cyclin D1 protein coding gene; IL: Interleukin; Bcl-2: Human Bcl-2 apoptosis regulator protein.
Quantification of Data and Statistical Analysis Expression of the genes were measured using RT-PCR analysis in both group and relative expression were compared to β-actin.Quantification and melting curve analysis were performed using StepOnePlus TM and Real Time PCR quantification software 4.1.
Degenerating cell number was determined on toluidineblue-stained semithin sections in both groups, using light microscope (40×magnification). All the cells and those with degenerating morphology along the removed ALC were counted and the percentage of cells with degenerating morphology was determined.
DAPI stained and the p53-immunoreactive cells were counted separately on the entire thickness of the whole mount preparation in both groups (ten pictures were taken from every sample). The percentage of anterior lens epithelial cells expressing p53 protein was determined. Data were presented as mean±standard error of measurement (SEM), and statistical significance was set using Student t -test analysis.
Examination of toluidine blue-stained sections of the ALC using light microscopy clearly showed two types of cells in both groups. One of these was a cell with normal, light staining of the nucleus and cytoplasm (Figure 1A, 1C). These cells were normal appearing cuboidal epithelial cells with round nuclei, and we could see apparently well-preserved junctions between the neighboring cells and between the cells and the capsule (Figure 1C). The other cell type was characterized by a darker staining in both the nucleus and the cytoplasm (Figure 1D, 1E). In many cases, loss of the cuboidal shape of lens epithelial cells and the shrinkage of the cytoplasm were seen(Figure 1B). In addition, detachment of the lens epithelial cells from the capsule was observed (arrow on Figure 1E). Shrunken dark cells frequently contained vacuoles in their cytoplasm(Figure 1D), indicating the presence of swollen intracellular organelles. These morphological characteristics indicate severe cell damage. ALC removed by manual CCC and with FLAC contained epithelial cells with both normal and damaged morphology indicating that both methods induce certain level of cell damage. Apparently, more damaged (darker, shrunken)cells were seen in the ALC removed by manual CCC than after FLAC.
In order to get more accurate information about the morphological differences caused by manual CCC and FLAC, we performed TEM. In the manually removed ALC, severe morphological alterations of the nuclei were observed. In contrast to the round nuclei of the control ALC that mostly could be observed in group 2 (Figure 2A), the shape of the nuclei in group 1 were irregular due to shrinkage, while the nuclear membrane remained intact (Figure 2B). Regarding the cytoplasm,difference could be seen between the cells of groups 1 and 2. In group 2, most of the cells contained well-preserved organelles in the relatively light cytoplasm (Figure 2A, 2C, 2E), while in group 1, electron dense cytoplasm contained shrunken organelles (Figure 2B, 2D). Due to the shrinkage of the cells,that is frequently observed in group 1, disruption of cellcell and cell-extracellular matrix junctions was observed. In contrast to the well-preserved, control-like junctions between epithelial cells of group 2 (Figure 2E), separation of cells by large gaps between them could be seen in group 1 (Figure 2F).Even the apparently preserved junctions on the apical part of the cells by overlapping processes revealed abnormality.Many junctions were formed by processes of cells with dark cytoplasm, indicating that the junction is formed by damaged cells.
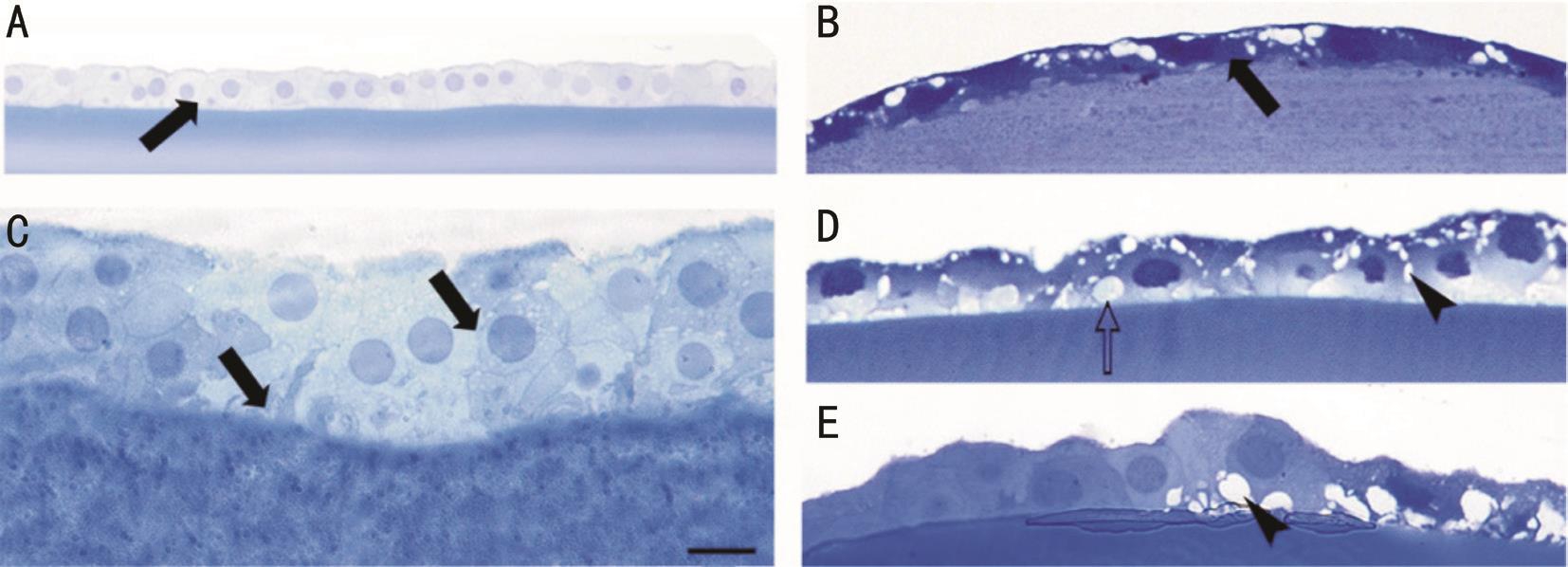
Figure 1 Light microscopic photos (toluidine blue staining) showing morphological alterations on semithin sections due to manual CCC(B, D, E) and FLAC (A, C) Cuboidal epithelial cells with round nuclei and light cytoplasm (arrows in A and C) were present in large numbers after FLAC; Following manual CCC, many cells showed degenerative signs (B-E), they became flat and darker (arrow in B) with empty spaces(arrowhead) and cytoplasmic vacuoles (open arrows) could be seen. Scale bar: 30 μm (A), 10 μm (C) and 15 μm (B, D, E).
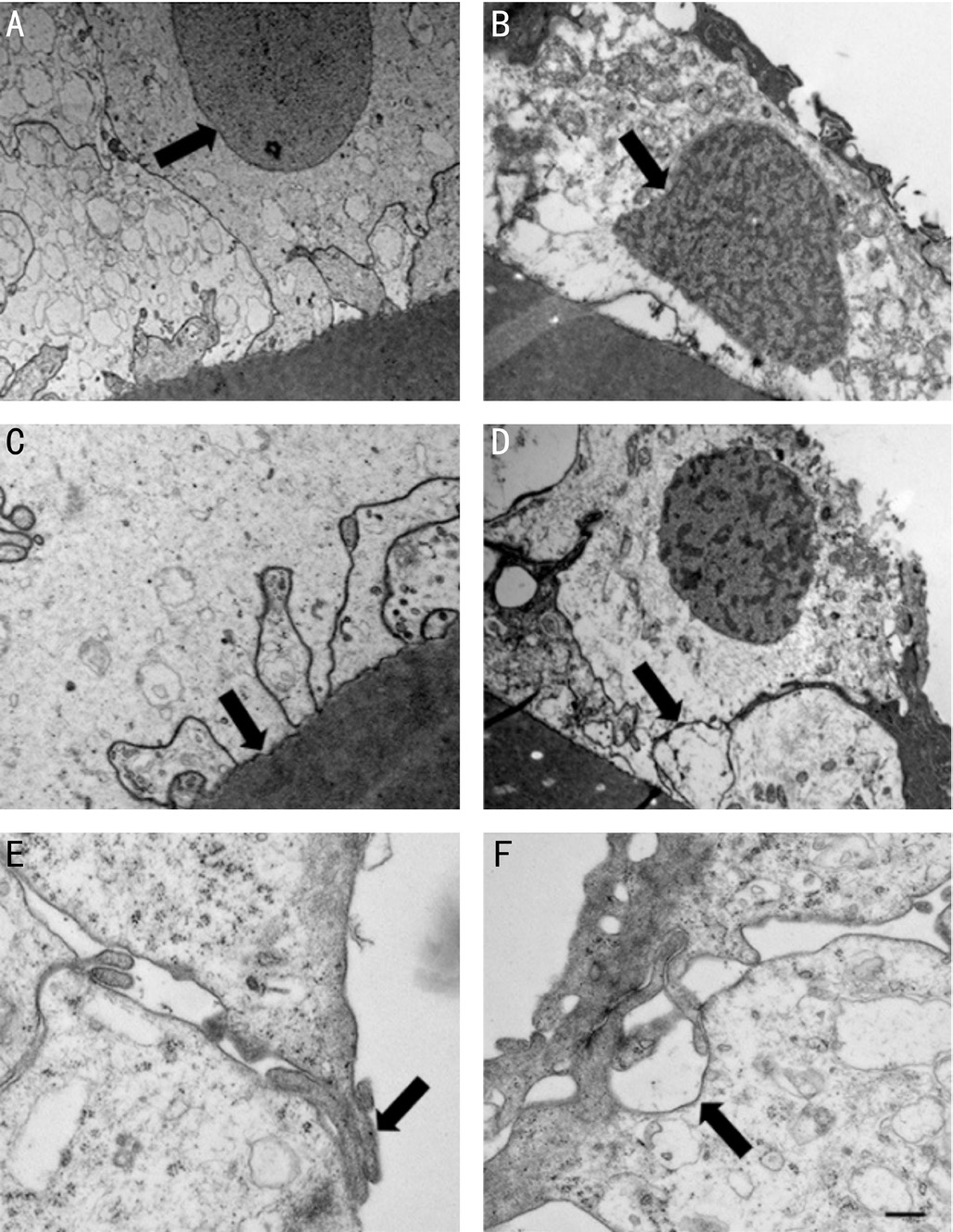
Figure 2 Electron micrographs of epithelial cells after FLAC(A, C, E) and manual CCC (B, D, F) A: Light cytoplasm of a cell with a round nucleus (arrow); B: Shrunken nucleus (arrow) with an irregular but apparently intact nuclear envelop; C, D: Basal surface of the epithelial cells are attached to the capsule (arrow); E, F:Overlapping cytoplasmic processes of the apical surfaces of the cells form junctions (arrow in E). Arrow (D) points to a gap between the two cells formed after manual CCC. Scale bar: 1000 nm (A, B, D)and 250 nm (C, E, F).
Quantification of the rate of cells with degenerative profiles was performed on the whole ALC on semithin sections. We have found that in the manual CCC specimens 85.39% of the cells presented degenerative signs, in the FLAC only 77.82%,however, the difference between the two groups was not statistically significant. Based on the detected morphological changes, we assumed that apoptosis is responsible for observed cell degeneration of ALC. Therefore, we performed the RTPCR to determine the level of expression of genes playing a role in apoptotic cell death, cell proliferation and survival.Slightly lower expression of cyclin D1 was detected following manual CCC compared to FLAC but the difference was not significant. Anti-apoptotic Bcl-2 expression levels were reduced in the manual CCC group compared to FLAC. In harmony with this, we have found an increased level of proapoptotic p53 mRNA in the manual CCC specimens compared to those removed using FLAC (Figure 3), although the difference was not statistically significant.
Therefore, we performed immunohistochemistry on whole mount preparation to detect p53 protein. In both groups we detected intense staining of the epithelial cells in the peripheral area of the ALC (Figure 4A, 4B), which gradually became weaker towards the central area. Following manual CCC, 48.5% of the cells showed immunoreactivity to p53. In contrast, in case of FLAC, p53 reactivity was present only in 31.42% of the cells, and the difference between the two groups was significant ( P =0.019).
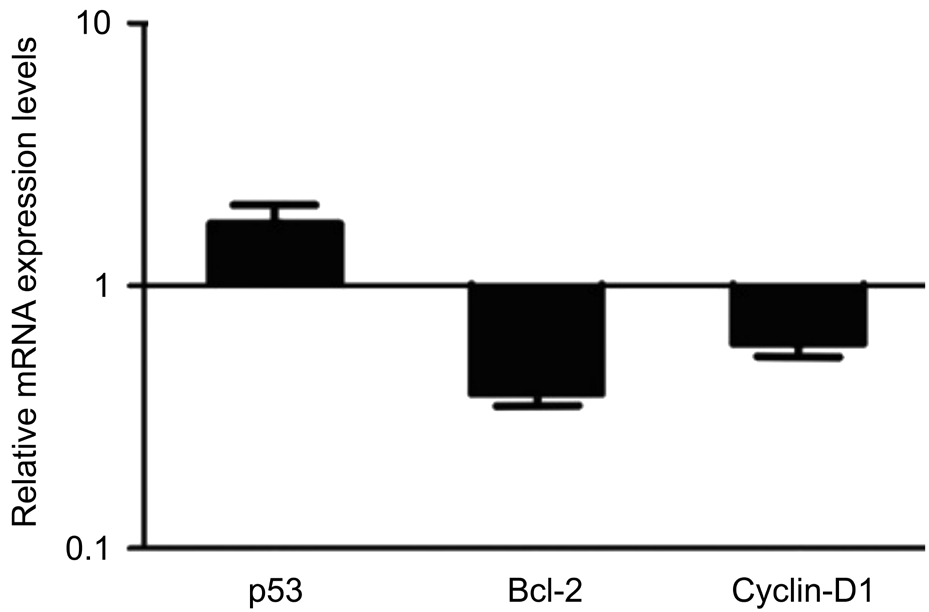
Figure 3 mRNA expression levels of various genes related to apoptosis and cell survival detected with qRT-PCR mRNA of p53,Bcl-2 and cyclin D1 genes in epithelial cells of ALC in manual CCC compared to that of cells after FLAC. The horizontal lines indicate the expression levels of genes following FLAC.
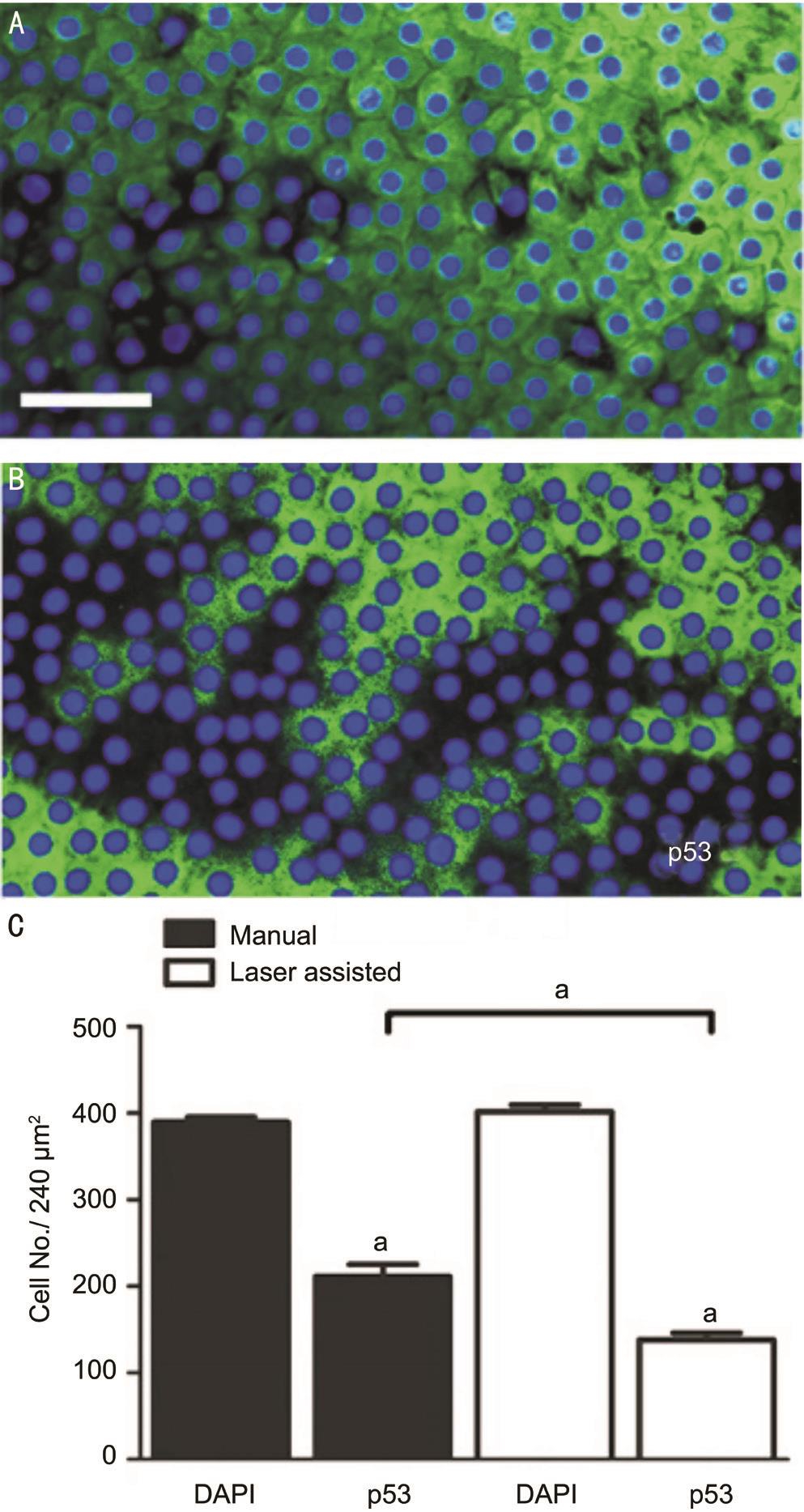
Figure 4 Photomicrographs showing p53 detected in epithelial cells using immunohistochemistry following manual CCC (A) and FLAC (B). Quantification of data on preparations shows that p53 positive cell number was higher following manual CCC than after FLAC (C) Scale bar: 50 μm; a Statistically significant.
The goal of our study is to compare morphological and molecular changes induced during manual CCC and FLAC in order to obtain information about the potential effect of these methods in the formation of posterior capsule opacification.In addition to the light and electron microscopic signs of ALC cells’ damage, we measured the expression level of the several genes playing a role in cell death and survival.
Morphological examination and quantification of cells with degenerating morphology revealed that manual CCC induces stronger cell damage than FLAC. The majority of cells with the signs of degeneration clearly showed the morphological features of apoptosis, such as the shrinkage and darker staining of the cytoplasm and the nucleus. These light and electron microscopic notions were in harmony with our molecular biological results and the p53 immunohistochemistry.
Although the results’ differences were not significant, the expression of the pro-apoptotic p53 gene expression was higher in manual CCC samples than in FLAC. The higher expression of p53 gene was accompanied by a higher level of p53 protein in the ALC epithelial cells after manual CCC than compared to that after FLAC. The p53 expression was detected in the cytoplasm and not in the nuclei of the epithelial cells, which indicates the synthesis (translation) of the p53 transcription factor and not yet it’s binding to the DNA.However, we cannot exclude that a low amount of p53 protein was already present in the nucleus bound to the relevant enhancers. Previous studies have shown that epithelial cells became TUNEL positive after cataract surgery, indicating apoptosis [16,18-20] . Two of these studies have shown lower number of apoptotic cells after manual CCC than after FLAC,and their goal was to present that cell damage depends on the energy used for the FLAC [16,20] . Their results are apparently in contradiction with to our present observations. The explanation for this can be that being a transcription factor, p53 can induce several processes, not only apoptosis. Depending on the damage, it can stop the cell cycle or can stimulate DNA repair,and, finally, when it is not avoidable, it induces apoptosis.We can suppose that the epithelial cells of our samples were mildly affected in both groups, and this mild damage does not induce immediate apoptosis, only arrest of the cells in G1 phase of the cell cycle, or DNA repair. Since we could observe only the elevated p53 mRNA and the presence of p53 protein product in the cytoplasm most probably during its synthesis, the exact role of p53 in our samples, e.g. whether it induces apoptosis or repair, is not clear. Expression of another apoptosis-related gene, the anti-apoptotic Bcl-2 did not differ significantly between the two groups, although it was slightly higher following FLAC than after the manual method. Since Bcl-2 protein is downstream to p53 in the apoptotic pathway,it is plausible that the major function of p53 in ALC is not the induction of apoptosis but the arrest of the cell-cycle. In harmony with this, the cell proliferation marker cyclin D1 expression was slightly lower in manual CCC than in FLAC.
In addition, the time-window needed for the removal of the ALC plus the time that lasts until mRNA is isolated from the cells may be too short for the whole apoptotic process.However, the transcription and translation of p53 can occur. According to a study performed in rat lenses, thirty minutes after the injury the mRNAs and proteins of another transcription factor-prototypical early response gene productswere already expressed [21] . In harmony with our results, it indicates a very fast and robust change in the gene expression of ALC following capsulotomy.
In this study we detected morphological and gene expression profile changes of epithelial cells of the removed anterior capsule using two different methods for the removal, manual CCC and FLAC. We propose that similar alterations may occur during and after the surgery in the residual lens epithelial cells and the damage caused by capsule removal may induce those pathways that can trigger the epithelialmesenchymal transformation (EMT) of residual capsular epithelial cells that eventually results in fibrosis and posterior capsule opacification [22] . Several studies indicated the central role of transforming growth factor-beta (TGF-β) signaling in EMT of lens epithelial cells and in the formation of posterior capsular opacification [23-27] . In addition, TGF-β is a key molecule in mechanotransduction, by which mechanical effects induce adaptive internal cellular changes in epithelial cells [28] . This means that mechanical effects that was induced either by manual CCC or FLAC activate TGF-β, and thereby induce intracellular transduction pathways that participate in pathological reactions such as EMT. We have to emphasize that morphological and molecular changes of the posterior capsule cannot be examined in human following cataract surgery. However, it is possible to examine the changes of the anterior capsule epithelial cells following the removal of the capsule, and information of the possible changes in the posterior capsule can be deduced from the changes that were found in the removed anterior capsular cells. While previous studies examined only the cutting rim of the removed anterior capsule [15-16,29] , we used whole mount preparation that allowed us to examine, in addition to the cutting rim, the changes of those cells that are several millimeters far from the cut.With both light and electron microscope, as well as with p53 immunocytochemistry, we could detect morphological changes and alteration of protein expression in the epithelial cells that locate overall including the central area of the removed anterior capsule. We can propose that if we can see changes in cells that locate several millimeters far from the cutting rim in the removed capsule, similar remote changes can be found in those cells that remain in the lens close to its equator. We observed stronger changes in the anterior lens epithelial cells following manual CCC, most probably because of the pulling and stretching of the anterior capsule. Among these changes disruption of cell-extracellular matrix ( e.g. cell-capsule)junctions was frequently seen following manual CCC that can trigger TGF-β signaling and result in EMT of the residual epithelial cells [30] . This indicates that the stronger deleterious effect of manual CCC may result in a slightly higher level of posterior capsule opacification in harmony with results of clinical observations [10] .
Since we detected the immediate effect of FLAC or manual CCC, the exact role and the extent of contribution of the detected molecular and morphological changes in acquiring the proliferative capacity of residual epithelial cells and the formation of posterior capsular opacification need further studies. We have to emphasize that the difference in the expression of genes related to apoptosis, cell survival and proliferation were for the most part insignificant following manual CCC and FLAC, therefore both methods appear to be fully suitable for the removal of the ALC in age-related cataract.
ACKNOWLEDGEMENTS
The authors appreciate the assist of surgeons and theatre nurses in sample collections, and the support of volunteers, and EFOP-3.6.1-16-2016-00004 project.
Foundation: Supported by grant EFOP-3.6.3-VEKOP-16-2017-00009 to University of Pécs Medical School.
Conflicts of Interest: Sükösd AK, None; Rapp J, None; Feller D, None; Sétáló GyJr, None; Gáspár B, None; Pongrácz JE, None; Ábrahám H, None; Biró Zs, None.
REFERENCES
1 Nagy ZZ. Intraocular femtosecond laser applications in cataract surgery. Cataract Refract Surg Today 2009;79-82.
2 Schaumberg DA, Dana MR, Christen WG, Glynn RJ. A systematic overview of the incidence of posterior capsule opacification. Ophthalmology 1998;105(7):1213-1221.
3 Kohnen T, Klaproth OK, Ostovic M, Hengerer FH, Mayer WJ.Morphological changes in the edge structures following femtosecond laser capsulotomy with varied patient interfaces and different energy settings. Graefes Arch Clin Exp Ophthalmol 2014;252(2):293-298.
4 Reddy KP, Kandulla J, Auffarth GU. Effectiveness and safety of femtosecond laser-assisted lens fragmentation and anterior capsulotomy versus the manual technique in cataract surgery. J Cataract Refract Surg 2013;39(9):1297-1306.
5 Abell RG, Darian-Smith E, Kan JB, Allen PL, Ewe SY, Vote BJ. Femtosecond laser-assisted cataract surgery versus standard phacoemulsification cataract surgery: outcomes and safety in more than 4000 cases at a single center. J Cataract Refract Surg 2015;41(1):47-52.
6 Grewal DS, Schultz T, Basti S, Dick HB. Femtosecond laser-assisted cataract surgery-current status and future directions. Surv Ophthalmol 2016;61(2):103-131.
7 Rostami B, Tian J, Jackson N, Karanjia R, Lu K. High rate of early posterior capsule opacification following femtosecond laser-assisted cataract surgery. Case Rep Ophthalmol 2016;7(3):213-217.
8 Ewe SY, Abell RG, Oakley CL, Lim CH, Allen PL, McPherson ZE, Rao A, Davies PE, Vote BJ. A comparative cohort study of visual outcomes in femtosecond laser-assisted versus phacoemulsification cataract surgery. Ophthalmology 2016;123(1):178-182.
9 Day AC, Gore DM, Bunce C, Evans JR. Laser-assisted cataract surgery versus standard ultrasound phacoemulsification cataract surgery. Cochrane Database of Systematic Reviews 2016;7:CD010735.
10 Kovács I, Kránitz K, Sándor GL, Knorz MC, Donnenfeld ED,Nuijts RM, Nagy ZZ. The effect of femtosecond laser capsulotomy on the development of posterior capsule opacification. J Refract Surg 2014;30(3):154-158.
11 Yu Y, Chen X, Hua H, Wu M, Lai K, Yao K. Comparative outcomes of femtosecond laser-assisted cataract surgery and manual phacoemusification: a six-month follow-up. Clin Exp Ophthalmol 2016;44(6):472-480.
12 Boswell BA, Korol A, West-Mays JA, Musil LS. Dual function of TGFβ in lens epithelial cell fate: implications for secondary cataract. Mol Biol Cell 2017;28(7):907-921.
13 Nagy ZZ, Kránitz K, Takacs AI, Miháltz K, Kovács I, Knorz MC.Comparison of intraocular lens decentration parameters after femtosecond and manual capsulotomies. J Refract Surg 2011;27(8):564-569.
14 Kránitz K, Takacs A, Miháltz K, Kovács I, Knorz MC, Nagy ZZ.Femtosecond laser capsulotomy and manual continuous curvilinear capsulorrhexis parameters and their effects on intraocular lens centration. J Refract Surg 2011;27(8):558-563.
15 Ostovic M, Klaproth OK, Hengerer FH, Mayer WJ, Kohnen T. Light microscopy and scanning electron microscopy analysis of rigid curved interface femtosecond laser-assisted and manual anterior capsulotomy. J Cataract Refract Surg 2013;39(10):1587-1592.
16 Mayer WJ, Klaproth OK, Ostovic M, Terfort A, Vavaleskou T,Hengerer FH, Kohnen T. Cell death and ultrastructural morphology of femtosecond laser-assisted anterior capsulotomy. Invest Ophthalmol Vis Sci 2014;55(2):893-898.
17 Simmy T, Anup R, Prabhu R, Balasubramanian KA. Effect of surgical manipulation of the rat intestine on enterocyte populations. Surgery 2001;130(3):479-488.
18 Saika S, Miyamoto T, Ishida I, Ohnishi Y, Ooshima A. Lens epithelial cell death after cataract surgery. J Cataract Refract Surg 2002;28(8):1452-1456.
19 Harocopos GJ, Alvares KM, Kolker AE, Beebe DC. Human agerelated cataract and lens epithelial cell death. Invest Ophthalmol Vis Sci 1998;39(13):2696-2706.
20 Toto L, Calienno R, Curcio C, Mattei PA, Mastropasqua A,Lanzini M, Mastropasqua L. Induced inflammation and apoptosis in femtosecond laser-assisted capsulotomies and manual capsulorhexes: an immunohistochemical study. J Refract Surg 2015;31(5):290-294.
21 Shirai K, Okada Y, Saika S, Senba E, Ohnishi Y. Expression of transcription factor AP-1 in rat lens epithelial cells during wound repair. Exp Eye Res 2001;73(4):461-468.
22 Marcantonio JM, Vrensen GF. Cell biology of posterior capsular opacification. Eye (Lond) 1999;13 (Pt 3b):484-488.
23 Wormstone IM, Tamiya S, Anderson I, Duncan G. TGF-β2-induced matrix modification and cell transdifferentiation in the human lens capsular bag. Invest Ophthalmol Vis Sci 2002;43(7):2301-2308.
24 Suzuki S, Sagara H, Senoo T. Developmental factors of fibrous opacification in the atopic cataract lens capsule. Ophthalmic Res 2011;45(4):216-220.
25 Yao J, Yang W, Liu Y, Sun YX, Jiang Q. Dexamethasone inhibits TGF-β2-induced migration of human lens epithelial cells: implications for posterior capsule opacification prevention. Mol Med Rep 2012;5(6):1509-1513.
26 Wertheimer C, Liegl R, Kernt M, Docheva D, Kampik A, Eibl-Lindner KH. EGFR-blockade with erlotinib reduces EGF and TGF-β2 expression and the actin-cytoskeleton which influences different aspects of cellular migration in lens epithelial cells. Curr Eye Res 2014;39(10):1000-1012.
27 Ma B, Kang Q, Qin L, Cui L, Pei C. TGF-β2 induces transdifferentiation and fibrosis in human lens epithelial cells via regulating gremlin and CTGF. Biochem Biophys Res Commun 2014;447(4):689-695.
28 Huang C, Akaishi S, Ogawa R. Mechanosignaling pathways in cutaneous scarring. Arch Dermatol Res 2012;304(8):589-597.
29 Reyes Lua M, Oertle P, Camenzind L, Goz A, Meyer CH, Konieczka K, Loparic M, Halfter W, Henrich PB. Superior rim stability of the lens capsule following manual over femtosecond laser capsulotomy. Invest Ophthalmol Vis Sci 2016;57(6):2839-2849.
30 Mamuya FA, Wang Y, Roop VH, Scheiblin DA, Zajac JC, Duncan MK. The roles of αV integrins in lens EMT and posterior capsular opacification. J Cell Mol Med 2014;18(4):656-670.
Citation: Sükösd AK, Rapp J, Feller D, Sétáló GyJr, Gáspár B,Pongrácz JE, Ábrahám H, Biró Zs. Cell death and survival following manual and femtosecond laser-assisted capsulotomy in age-related cataract. Int J Ophthalmol 2018;11(9):1440-1446
Received: 2018-01-31 Accepted: 2018-07-12
DOl: 10.18240/ijo.2018.09.02
Correspondence to: Zsolt Biró. Departments of Ophthalmology,Clinical Centre, University of Pécs Medical School, Rákóczi út 2, Pécs 7623, Hungary. biro.zsolt@pte.hu