Table 1 Cell proliferation values at different culture time points in two groups mean±SD, A 450
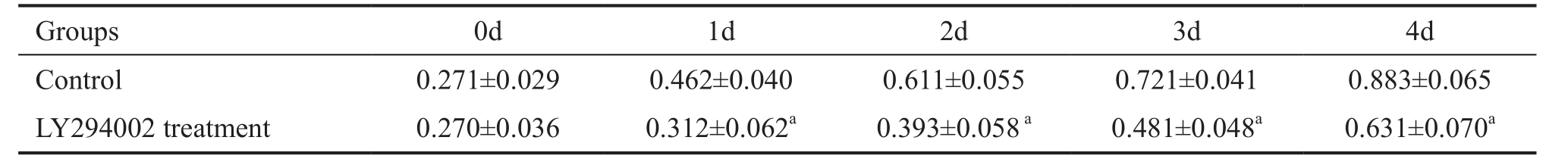
a P <0.05 vs control group.
Yu Di, Xiao-Long Chen
Department of Ophthalmology, Shengjing Hospital of China Medical University, Shenyang 110004, Liaoning Province,China
Abstract · AlM: To study the effects of LY294002 [phosphatidylinositol 3-kinase (Pl3K) inhibitor] on the function and mechanisms of retinal endothelial cells (RECs) in vitro .· METHODS: RECs were randomly divided into control group and LY294002 treatment group. RECs in the control group were placed the incubator for hypoxic exposure in vitro . RECs in the LY294002 treatment group were pretreated with LY294002 (40 μmol/L) under hypoxic condition. The expression of matrix metalloproteinase (MMP)-2, MMP-9,vascular endothelial growth factor (VEGF), and apoptosis and proliferation of RECs were evaluated with Western blot,real-time reverse transcription-polymerase chain reaction(RT-PCR), and flow cytometric analysis, correspondently.· RESULTS: Compared with the control group, treating the RECs with LY294002 was able to remarkably inhibit cell proliferation rates ( t 1d =2.13, t 2d =2.65, t 3d = 2.36, t 4d =2.06, all P <0.05). Flow cytometric analysis indicated that a moderate increase in apoptosis in the LY294002 treatment group compared to the control group ( t =2.51, P <0.05). The expression of MMP-2, MMP-9 and VEGF were downregulated in the LY294002 treatment group by Western blot and real-time RT-PCR (all P <0.05).· CONCLUSlON: LY294002 regulates the function of RECs by reducing the expression of MMP-2, MMP-9, and VEGF in vitro . LY294002 may provide an effective method for preventing pathological angiogenesis.
· KEYWORDS: LY294002; phosphatidylinositol 3-kinase;retinal endothelial cell; angiogenesis
Ocular angiogenesis is a pathological feature of numerous pathological angiogenic diseases, including retinop athy of prematurity (ROP), proliferative diabetic retinopathy (PDR),and age-related macular degeneration (AMD) [1-2] . Currently,the treatment of ocular angiogenesis includes laser surgery or inhibition of the pro-angiogenic factor vascular endothelial growth factor (VEGF) molecular therapy [3-6] . However,targeting the angiogenesis pathway or binding to VEGF may lead to more effective and safer ophthalmic angiogenesis inhibitors. Recent studies have demonstrated that LY294002[phosphatidylinositol 3-kinase (PI3K) inhibitor] is an effective and safe inhibitor for eye development and ectopic hyaloid angiogenesis [7-8] .
However, the specific mechanisms, which are involved in LY294002-mediated pathological angiogenesis in retinal endothelial cells (RECs) need to be fully clarified. This study hypothesized that LY294002 could regulate the function of RECs through reducing matrix metalloproteinase (MMP)-2,MMP-9, and VEGF expression in vitro . To confirm this hypothesis, this study investigated the effect of LY294002 on the function of RECs under hypoxic condition.
Drug Preparation LY294002 (Sigma-Aldrich, St. Louis,MI, USA) diluted in dimethyl sulfoxide (DMSO) to create an inventory solution (the final concentration was 40 μmol/L).Subsequent experiments were carried out using the concentration of LY294002 on RECs in vitro .
Cell Culture RECs (Cell Systems Corporation, Kirkland,WA, USA) were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS) in incubator for hypoxic condition containing 5% CO 2 . The cells were divided into control and LY294002 treatment group. LY294002 treatment group were pretreated with LY294002 (40 μmol/L)for 30min prior to being cultured under hypoxic condition.
Proliferation Assay For cell counting kit-8 (CCK8) assay(Beyotime Institute of Biotechnology, Jiangsu, China), 1×10 4 cells were inoculated at 96-well plates after different treatment and cell proliferation was measured every day for 4d after transfection. A total of 10 μL CCK8 was added and incubated for 2h. Then, the samples were vortexed for 10min and the absorbance was measured in a Sunrise™ microplate reader(Tecan Group, Ltd., Männedorf, Switzerland).
Table 1 Cell proliferation values at different culture time points in two groups mean±SD, A 450

a P <0.05 vs control group.
Apoptosis Detection Assay RECs apoptosis was measured by the terminal deoxynucleotidyl transferase-mediated dutp nick end labeling (TUNEL) assay. DAPI (Beyotime Institute of Biotechnology, Jiangsu Province, China) stained celluar nuclei and laser confocal microscopy (FV1000; Olympus Crop., Tokyo,Japan) were used to detect apoptosis.
Western Blot Analysis The protein from each sample was extracted with lysis buffer. Proteins were isolated and transferred to polyvinylidene fluoride (PVDF) membranes (Merck Millipore, Billerica, MA, USA). Membranes were incubated for the night at 4℃ with MMP-2 (sc-13595), MMP-9 (sc-21733),VEGF (sc-57496) and β-actin (1:2000, Santa Cruz Biotech nology Inc.). Then the mem branes were incubated with horseradish peroxidase-conjugated secondary antibodies (1:2000,Zhongshan Jinqiao Biotechnology Co., Ltd., China) for 1h at 37℃ and visualized with an enhanced-chemiluminescence kit(Thermo Fisher Scientific).
Real-time Reverse Transcription-polymerase Chain Reaction Total RNA was extracted from each sample using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). cDNA synthesis was performed according to the RNA polymerase chain reaction protocol (PrimeScriptTM RT Reagent Kit-Perfect Real Time, Takara Bio, Otsu, Japan). The primer sequences were MMP-2: 5’-GGCTTGGACCGAATGCT-3’(forward) and 5’-TTGTTGAAGGCTGTGGC -3’ (reverse);MMP-9: 5’-AGCAAACAGGCTCACAGGT T-3’ (forward)and 5’-TAAGTCCTCCCCATCTCCCT-3’ (reverse);VEGF: 5’-CCCGACAGGGAA GACAAT-3’ (forward) and 5’-TCTGGAAGTGAGCCAACG -3’ (reverse); β-actin:5’-GAGAGGGAAATCGTGCGTGA-3’ (forward) and 5’-GCCTAGAAGCAT TTGCGGTG-3’ (reverse). Real-time reverse transcription-polymerase chain reaction (RT-PCR)reaction was performed using SYBR green RT-PCR Master Mix (Premix Ex TaqTM-Perfect Real Time, Takara Bio,Otsu, Japan) on the 7300 real-time RT-PCR system (Applied Biosystems, Foster City, CA, USA).
Statistical Analysis The data were represented by the mean±standard deviation (SD). Statistical analysis of cell proliferation, cell apoptosis, protein and mRNA were carried out using independent-sample t -test. P <0.05 was significant significance.
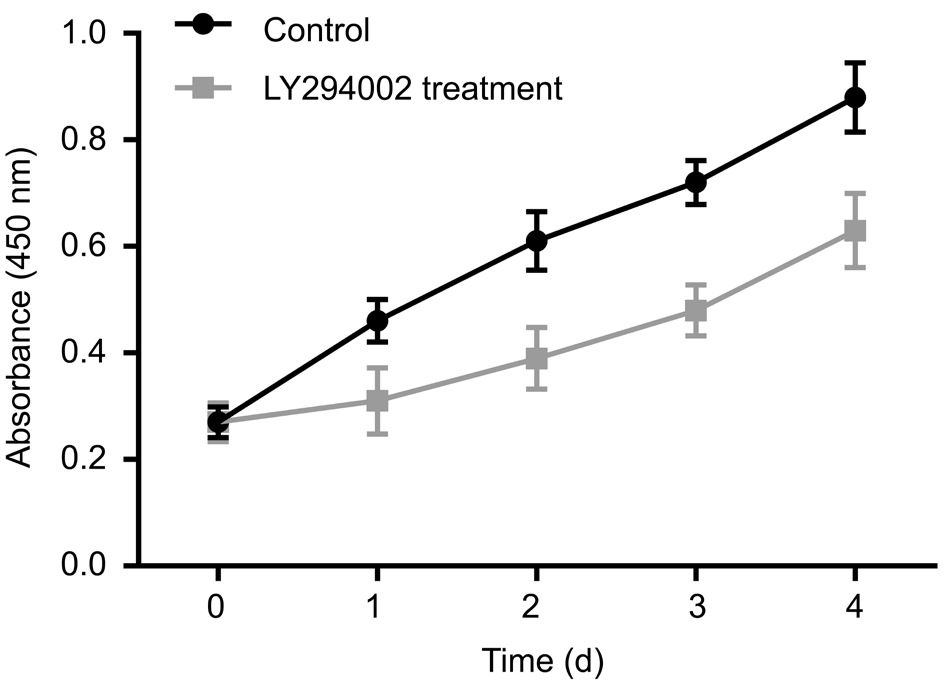
Figure 1 LY294002 inhibits RECs proliferation under hypoxia CCK8 assay was used to detect cell proliferation.
Inhibition of Cell Proliferation and Promotion of Cell Apoptosis by LY294002 RECs proliferation was measured using a CCK8 assay. The cell growth curves of 1, 2, 3 and 4d showed that the growth rate was slower in the LY294002 treatment group than in the control group at the same time point ( t 1d = 2.13, t 2d = 2.65, t 3d = 2.36, t 4d = 2.06, all P <0.05; Figure 1,Table 1). In order to detect the effect of LY294002 on RECs apoptosis, TUNEL staining was performed. The experimental result indicated that there were many apoptotic cells in the LY294002 treatment group. Statistical analysis showed that there were significant differences in apoptosis between the two groups ( t =2.51, P <0.05; Figure 2). These results demonstrated that LY294002 treatment cells had a more significant anti-proliferation and pro-apoptotic effects on RECs.
Inhibitory Effect of LY294002 on MMP-2, MMP-9 and Vascular Endothelial Growth Factor Protein Expression To verify the mechanism of LY294002 on RECs function,the expression of MMP-2, MMP-9 and VEGF were detected by Western blot. Our findings indicated that under hypoxic environment, MMP-2, MMP-9 and VEGF protein levels in the LY294002 treatment group were reduced by 45.01%, 36.91%,and 38.84%, respectively, contrasted with the control group(all P <0.05). After LY294002 treatment, MMP-2, MMP-9 and VEGF expressions were inhibited significantly (Figure 3). These results suggested that LY294002 could regulate the function of RECs by reducing the protein expression of MMP-2, MMP-9 and VEGF in vitro .
Inhibitory Effect of LY294002 on MMP-2, MMP-9 and Vascular Endothelial Growth Factor mRNA Expression In order to detect the interference effect of LY294002, we performed RT-PCR for quantitative detection. Real-time RTPCR revealed similar results in cell samples. MMP-2, MMP-9 and VEGF mRNA levels in the LY294002 treatment group(+56.23%, +59.45% and +46.45%) were reduced by 43.77%,40.55%, and 53.55%, respectively, contrasted with the control group (all P <0.05; Figure 4). These results suggested that LY294002 could regulate the function of RECs by reducing the mRNA expression of MMP-2, MMP-9 and VEGF in vitro .
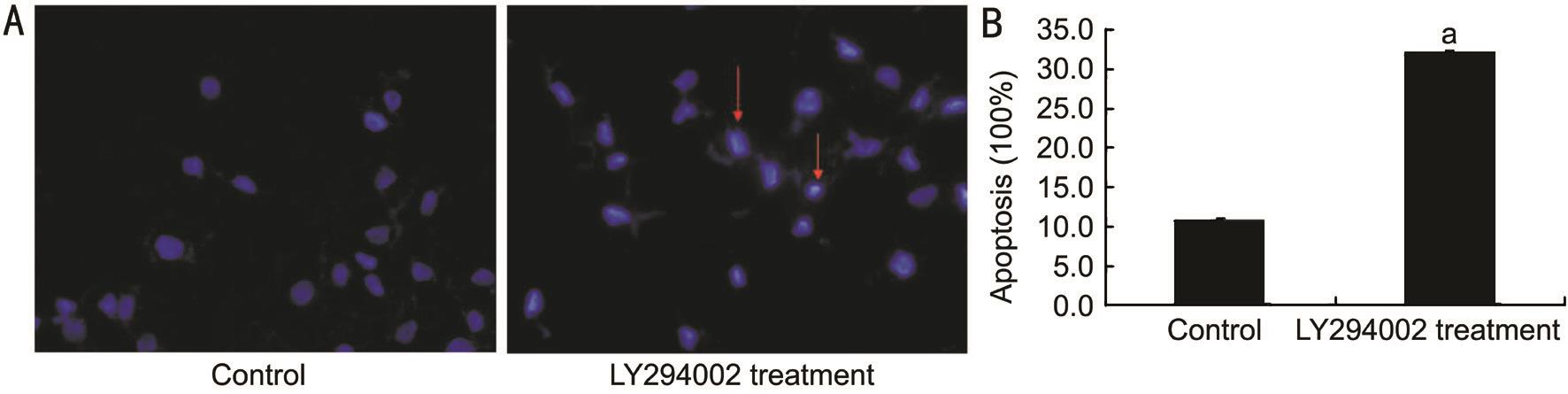
Figure 2 LY294002 induces RECs apoptosis under hypoxia A: Cell apoptosis was determined by TUNEL staining. The red arrows show the nucleus of the apoptotic cells. B: Cell viability in each groups. LY294002 remarkably induced cell apoptosis under hypoxic conditions. a P <0.05 vs control group.
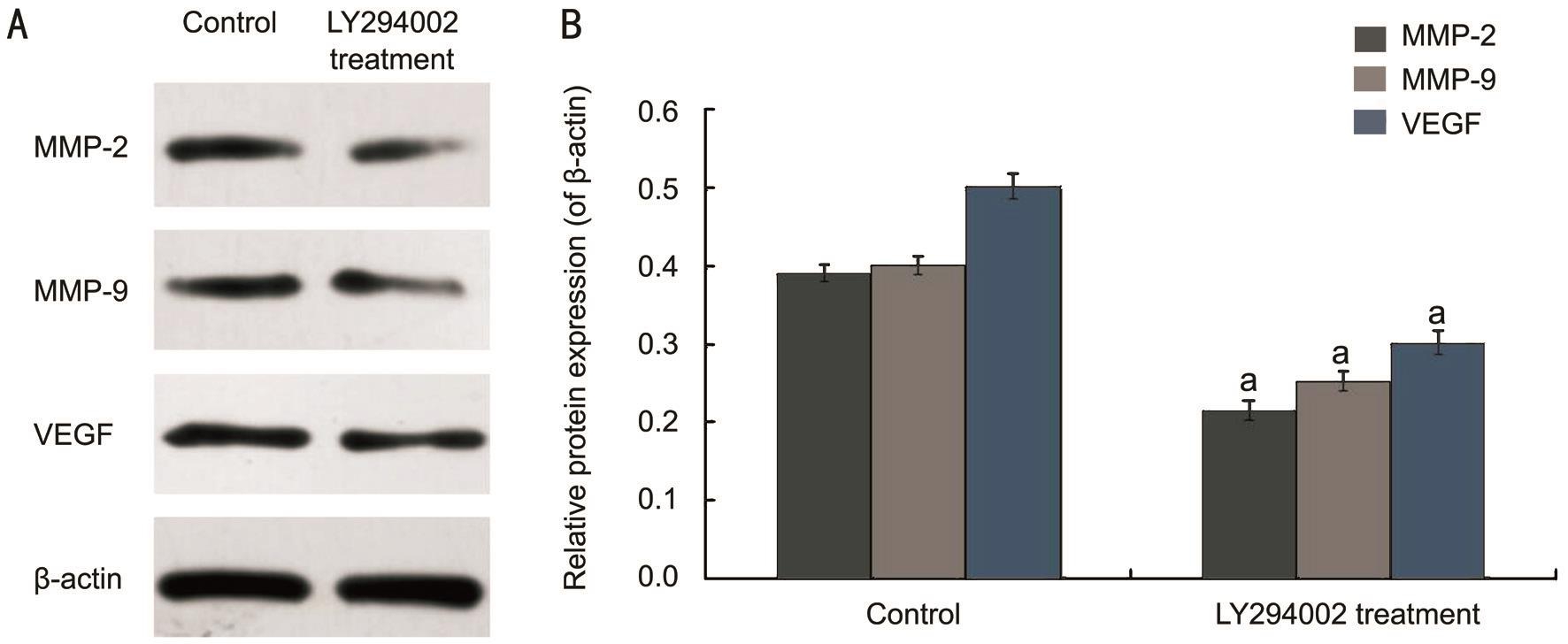
Figure 3 LY294002 regulated the function of RECs through inhibition of the expression of MMP-2, MMP-9 and VEGF in vitro A:Western blot assay for protein expression; B: Statistical analysis. a P <0.05 vs control group.
Pathological angiogenic diseases is characterized by progressive changes in retinal microvessels, including endothelial dysfunction,breakdown of the blood-retina barrier, retinal rupture, ischemia,and retinal neovascularization (RNV) [5-6,9] . The biological behavior of RECs plays a dominant role in the formation of RNV.
The best inhibitor for PI3K is LY294002. Previous studies have directly [10-12] and indi rectly [13-14] demonstrated that LY294002 inhibited the growth of various tumor cells by inhibiting the functions of PI3K and downstream components [15-16] .LY294002 may inhibit RECs proliferation and induce RECs apoptosis by inhibiting PI3K. But, the downstream molecules that LY294002 inhibit the apoptosis of RECs by inhibition of PI3K/Akt still to be identified.
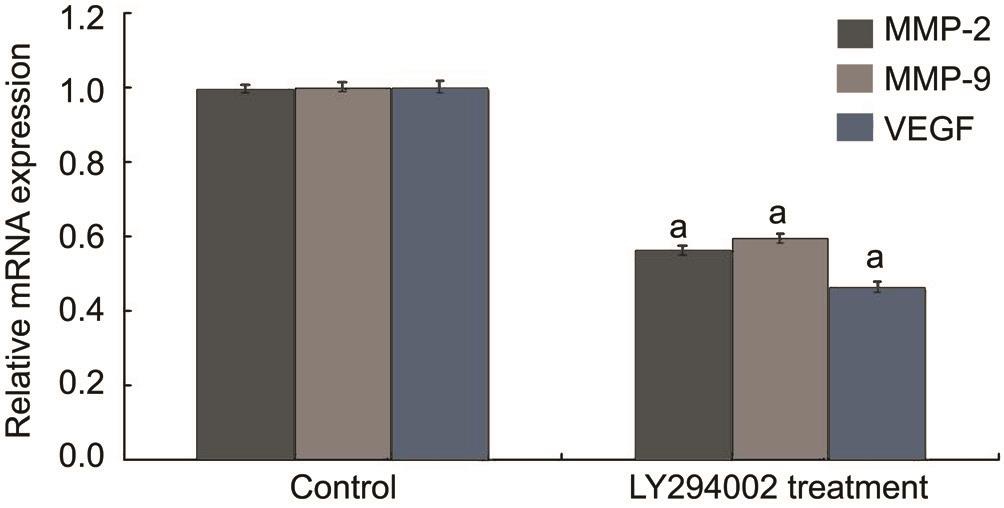
Figure 4 LY294002 regulated the function of RECs through inhibition of MMP-2, MMP-9 and VEGF mRNA expression in vitro mRNA expressions of MMP-2, MMP-9 and VEGF were determined by real-time RT-PCR. a P <0.05 vs control group.
In the present study, we used RECs under hypoxic condition to simulate pathological angiogenic diseases in vitro model.Examination of the proliferation of RECs following LY294002 treatment under hypoxic conditions demonstrated that LY294002 significantly inhibited cell proliferation. Furthermore, it was demonstrated that LY294002 promoted apoptosis of the cells,thus interfering with angiogenesis. RNV is a complex series of processes in which many of the gene products are involved in the regulation of MMPs, which play a major role in destroying the extracellular matrix, are overexpressed in pathological angiogenic diseases and are considered to contribute to angiogenesis [17-18] . MMP-2 and MMP-9 are related very closely to RNV, and have been considered as important factors in promoting RNV [19-20] .
VEGF is one of the agents to promote angiogenesis and plays a central role in RNV [21-22] . In this study, MMP-2, MMP-9 and VEGF were significantly different between the RECs under hypoxic condition treated by LY294002 and that of the control group ( P <0.05). It is suggested that LY294002 can inhibit the gene expression of MMP-2, MMP-9, and VEGF. Furthermore,we found that LY294002 induced apoptosis in the RECs and had the potential to inhibit pathological angiogenesis.
Taken together, this study showed that LY294002 inhibited the proliferation of RECs, induced the apoptosis of RECs, and downregulated cytokines expression, including MMP-2, MMP-9 and VEGF, which acted through inhibiting PI3K/Akt activation.Therefore, inhibition of the PI3K pathway is an effective strategy for the treatment of pathological angiogenesis.
ACKNOWLEDGEMENTS
Foundations: Supported by National Natural Science Foundation of China (No.81600747); Startup Foundation for Doctors of Liaoning Province (No.201501020).
Conflicts of Interest: Di Y, None; Chen XL, None.
REFERENCES
1 Hartnett ME. Advances in understanding and management of retinopathy of prematurity. Surv Ophthalmol 2017;62(3):257-276.
2 Li Y, Liu X, Zhou T, Kelley MR, Edwards P, Gao H, Qiao X. Inhibition of APE1/Ref-1 redox activity rescues human retinal pigment epithelial cells from oxidative stress and reduces choroidal neovascularization. Redox Biol 2014;2:485-494.
3 Fagerholm R, Vesti E. Retinopathy of prematurity-from recognition of risk factors to treatment recommendations. Duodecim 2017;133(4):337-344.
4 Yonekawa Y, Thomas BJ, Thanos A, Todorich B, Drenser KA, Trese MT, Capone A Jr. The cutting edge of retinopathy of prematurity care:expanding the boundaries of diagnosis and treatment. Retina 2017;37(12):2208-2225.
5 Eldweik L, Mantagos IS. Role of VEGF inhibition in the treatment of retinopathy of prematurity. Semin Ophthalmol 2016;31(1-2):163-168.
6 Tran KD, Cernichiaro-Espinosa LA, Berrocal AM. Management of retinopathy of prematurity-use of anti-VEGF therapy. Asia Pac J Ophthalmol (Phila) 2018;7(1):56-62.
7 Ornelas IM, Silva TM, Fragel-Madeira L, Ventura AL. Inhibition of PI3K/Akt pathway impairs G2/M transition of cell cycle in late developing progenitors of the avian embryo retina. PLoS One 2013;8(1):e53517.
8 Alvarez Y, Astudillo O, Jensen L, Reynolds AL, Waghorne N, Brazil DP, Cao Y, O’Connor JJ, Kennedy BN. Selective inhibition of retinal angiogenesis by targeting PI3 kinase. PLoS One 2009;4(11):e7867.
9 Hellstrom A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet 2013;382(9902):1445-1457.
10 Xu Z, Liu D, Fan C, Luan L, Zhang X, Wang E. DIXDC1 increases the invasion and migration ability of non-small-cell lung cancer cells via the PI3K-AKT/AP-1 pathway. Mol Carcinog 2014;53(11):917-925.
11 Shin JY, Kim JO, Lee SK, Chae HS, Kang JH. LY294002 may overcome 5-FU resistance via down-regulation of activated p-AKT in Epstein-Barr virus-positive gastric cancer cells. BMC Cance r 2010;10:425.
12 Xing CG, Zhu BS, Fan XQ, Liu HH, Hou X, Zhao K, Qin ZH. Effects of LY294002 on the invasiveness of human gastric cancer in vivo in nude mice. World J Gastroenterol 2009;15(40):5044-5052.
13 Luo X, Dong Z, Chen Y, Yang L, Lai D. Enrichment of ovarian cancer stem-like cells is associated with epithelial to mesenchymal transition through an miRNA-activated AKT pathway. Cell Prolif 2013;46(4):436-446.
14 Lim W, Song G. Inhibitory effects of delphinidin on the proliferation of ovarian cancer cells via PI3K/AKT and ERK 1/2 MAPK signal transduction. Oncol Lett 2017;14(1):810-818.
15 Chen J, Shao C, Lu W, Yan C, Yao Q, Zhu M, Chen P, Gu P, Fu Y,Fan X. Adenosine triphosphate-induced rabbit corneal endothelial cell proliferation in vitro via the P2Y2-PI3K/Akt signaling axis. Cells Tissues Organs 2014;199(2-3):131-139.
16 Nagappan A, Lee WS, Yun JW, Lu JN, Chang SH, Jeong JH, Kim GS,Jung JM, Hong SC. Tetraarsenic hexoxide induces G2/M arrest, apoptosis,and autophagy via PI3K/Akt suppression and p38 MAPK activation in SW620 human colon cancer cells. PLoS One 2017;12(3):e0174591.
17 Aresu L, Aricò A, Comazzi S, Gelain ME, Riondato F, Mortarino M,Morello E, Stefanello D, Castagnaro M. VEGF and MMP-9: biomarkers for canine lymphoma. Vet Comp Oncol 2014;12(1):29-36.
18 Gao XH, Yang XQ, Wang BC, Liu SP, Wang FB. Overexpression of twist and matrix metalloproteinase-9 with metastasis and prognosis in gastric cancer. Asian Pac J Cancer Prev 2013;14(9):5055-5060.
19 Lin HB, Sharma K, Bialy D, Wawrzynska M, Purves R, Cayabyab FS,Wozniak M, Sawicki G. Inhibition of MMP-2 expression affects metabolic enzyme expression levels: proteomic analysis of rat cardiomyocytes. J Proteomics 2014;106:74-85.
20 Di Y, Nie QZ, Chen XL. Matrix metalloproteinase-9 and vascular endothelial growth factor expression change in experimental retinal neovascularization. Int J Ophthalmol 2016;9(6):804-808.
21 Hartnett ME. Studies on the pathogenesis of avascular retina and neovascularization into the vitreous in peripheral severe retinopathy of prematurity (an American ophthalmological society thesis). Trans Am Ophthalmol Soc 2010;108:96-119.
22 Kandasamy Y, Hartley L, Rudd D, Smith R. The association between systemic vascular endothelial growth factor and retinopathy of prematurity in premature infants: a systematic review. Br J Ophthalmol 2017;101(1):21-24.
Citation: Di Y, Chen XL. Effects of LY294002 on the function of retinal endothelial cell in vitro . Int J Ophthalmol 2018;11(9):1447-1450
Received: 2018-03-18 Accepted: 2018-05-28
DOl: 10.18240/ijo.2018.09.03
Correspondence to: Yu Di. Department of Ophthalmology,Shengjing Hospital of China Medical University, Shenyang 110004, Liaoning Province, China. diyu81@126.com