lnfluence of polymorphisms in VEGF, ACE, TNF and GST genes on the susceptibility to retinopathy of prematurity among Chinese infants
Xiao-Jun Lei 1 , Yong-Xia Zhao 2 , Tong Qiao 3
1 Department of Ophthalmology, Friendship Hospital of Dalian,Dalian 116000, Liaoning Province, China
2 Department of Ophthalmology, Center Hospital in Cangzhou,Cangzhou 061000, Hebei Province, China
3 Department of Ophthalmology, Shanghai Children’s Hospital,Shanghai Jiao Tong University, Shanghai 200000, China
Abstract · AlM: To investigate common polymorphisms in VEGF,ACE, TNF and GST genes with retinopathy of prematurity(ROP) risk among Chinese infants.· METHODS: Nine polymorphisms in the above genes were genotyped on 724 advanced cases of ROP and 878 prematurely-born infants of low birth weight who were without any ophthalmologic disease. The frequencies of the polymorphisms were compared between cases and controls to identify the association present, if any.· RESULTS: Of the nine polymorphisms, only two showed significant associations: ACE insertion deletion (lD)polymorphism ( P =0.031) and TNF -308G/A polymorphism( P <0.001). The former was associated with a reduced ROP risk [lD genotype, adjusted OR (aOR): 0.603, 95%Cl: 0.427-0.893, P =0.034; DD genotype, aOR: 0.468, 95%Cl: 0.229-0.626, P =0.002], while the latter showed an increased risk (GA genotype, aOR: 1.956, 95%Cl: 1.396-2.465, P <0.001; AA genotype, aOR: 2.809, 95%Cl: 1.802-4.484, P <0.001). The association was also noted at the allele level (ACE D allele aOR: 0.698, 95%Cl: 0.294-0.883, P <0.001; TNF -308A allele aOR: 1.776, 95%Cl: 1.446-2.561, P <0.001).· CONCLUSlON: The ACE lD polymorphism can protect against ROP development while the TNF -308G/A can increase the risk of the disease among Chinese infants.
· KEYWORDS: inflammation; oxidation; polymorphisms;retinopathy of prematurity; vascularization
INTRODUCTION
Retinopathy of prematurity (ROP) is an ophthalmologic disease of abnormal retinal vascularization which affects preterm infants. The disease can potentially lead to detachment of the retina, and represents one of the most common causes of permanent blindness among prematurely-born babies [1-2] .Currently, the etiology of ROP is not well understood,although short gestational age and low birth weight have been consistently linked to the development of the disease [3-4] .Despite this, not all infants of short gestational age and low birth weight develop ROP. Additionally, among infants who develop ROP, spontaneous regression of the disease can be seen in up to 86.7% of the cases, especially during early stages of ROP [5] . The reason why only a small subset of infants develops ROP and eventually progresses into an advanced stage of the disease is not entirely known.
Recently, it has been suggested that genetic factors may provide an explanation for such a phenomenon [4] . Individualto-individual variations in the sequences of genes involved in retinal development may alter the normal level or structure of the protein products, which may either increase the likelihood of ROP development, or protect against the ophthalmologic disease. Hence, a number of studies have been conducted to examine the association of these genetic variations, or polymorphisms, with the risk of ROP [6-13] . However, little studies have been conducted in the Chinese population,although it was thought that Asian babies are more susceptible to the disease compared to Caucasian babies [4,14] . This study addressed this gap in the literature by conducting a genetic association study of popularly studied genes with ROP risk in a Chinese population. Among the popular genes studied include VEGF , ACE , TNF and GST genes.
VEGF , vascular endothelial growth factor , is a natural candidate of genetic association studies in ROP because it encodes a potent inducer of vascularization, and ROP is essentially a disease of abnormal retinal vascularization [15] . In fact, anti-VEGF therapy is now considered a common off-label therapy for ROP [15-16] . The level of VEGF protein is highly regulated throughout different phases of normal retinal development.Abnormal expression of VEGF has been observed in advanced ROP cases and is known to contribute to the disease [17] . Hence,it can be hypothesized that polymorphisms which can affect the expression of VEGF gene might influence the risk of ROP. Some of the commonly studied VEGF polymorphisms in ROP are the -460T/C, -634G/C, +405G/C and +936C/T polymorphisms [6-10] , and are examined in the present work.
ACE encodes angiotensin-converting enzyme, which also plays a significant role in vascularization of the retina. The enzyme is responsible for the formation of vascular wall and maintenance of a proper vascular tone by carefully regulating the conversion of angiotensin I to angiotensin II [12] . In addition, animal studies have demonstrated that a well-regulated level of ACE is essential for a precise functioning of neuropeptides during the development and maturation of the retina [18] . The insertiondeletion (ID) polymorphism of the ACE gene can influence the synthesis and cellular concentration of ACE enzyme [19-21] , and in turn, influence normal retinal development. For this reason,we also investigated the association of ACE ID polymorphism with ROP risk in the present study.
In recent years, more and more evidence has implicated a role for inflammation in ROP development [1] . It has been shown that inflammatory responses can result in an abnormal retinal vessel development and can promote pathological characteristics of ROP [22] . Since TNF encodes a potent proinflammatory cytokine, its involvement in the development of ROP has been extensively investigated previously. An animal study demonstrated that hyperoxia mice models of ROP showed an improved vascular recovery and a reduced pathological neovascularization when the TNF gene was knocked-out [23] . Besides, in humans, it has been shown that a high plasma level of TNF was associated with the later development of ROP [24] . These evidences suggest that the level of TNF must be strictly controlled for a normal development of the retina. Thus, polymorphisms which could affect TNF production may alter the risk of ROP. One polymorphism which has been commonly thought to affect TNF production is the TNF -308G/A polymorphism [25] , but its association with the risk of ROP among Chinese is not well understood.
In addition to improper vascularization and inflammation,oxidative stress has also been implicated in the development of ROP [26] . This is because retinal tissues are highly susceptible to oxidative damage, since they consume a high level of oxygen due to their high polyunsaturated fatty acid contents [27] .Glutathione-S-transferases (GSTs) are a family of enzymes which play important antioxidant roles [28] . Three primary classes of GSTs are GSTT1, GSTM1 and GSTP1. Several polymorphisms of these GST genes have been commonly thought to influence their functional efficiencies. These include GSTT1 null/present polymorphism, GSTM1 null/present polymorphism and GSTP1 Ile/Val polymorphism, which were examined in this study.
The overall objective of this study was to investigate the association of VEGF -460T/C, -634G/C, +405G/C and +936C/T polymorphisms, ACE ID polymorphism, TNF -308G/A polymorphism, GSTT1 null/present polymorphism, GSTM1 null/present polymorphism and GSTP1 Ile/Val polymorphism with the risk of ROP among Chinese infants.
SUBJECTS AND METHODS
Cases and Controls Cases and controls were recruited from Department of Ophthalmology, Shanghai Children’s Hospital, Shanghai Jiao Tong University between May 2012 and November 2016. All subjects were preterm infants who were born at or before the 32 nd gestational week and weighed not more than 1500 g at birth. Cases comprised 724 babies who showed features of advanced ROP (stages 4 and 5) as determined by experienced ophthalmologists based on the International Classification of Retinopathy of Prematurity (ICROP) [29] . Controls were healthy babies with no ophthalmological disease who were unrelated to the cases. A total of 2327 eligible controls were identified from the hospital database, in which each control was assigned a serial number(1 to 2327). A Random Number Generator (www.random.org)was then used to generate 878 random numbers and babies with serial number treatment matched to the random number generated were selected for inclusion into the study. All cases and controls were of Han Chinese ethnicity. Recruitment of subjects and protocol of the study was approved by the Ethics Review Committee of Shanghai Children’s Hospital (No.2016R032-F01). Parents or legal guardians of all subjects gave informed consent before the study was conducted.
Genetic Analysis Genetic analysis was performed on the blood specimens of the subjects. VEGF -460T/C polymorphism was genotyped by sequencing method and the primers used were shown in Table 1. The other three VEGF polymorphisms(-634G/C, +405G/C and +936C/T), along with TNF -308G/A and GSTP1 Ile/Val polymorphisms were genotyped by PCRRFLP method and the results were validated by performing DNA sequencing on 10% of the samples. The primers used were also shown in Table 1. The VEGF -634G/C and +405G/C polymorphisms were both digested with BsmFI restriction enzyme, while the remaining three polymorphisms were digested with NlaIII, NcoI and Alw26I respectively. The band sizes obtained were used for categorization of genotypes as shown in Table 1. Besides, ACE ID, GSTT1 null/present and GSTM1 null/present polymorphisms were genotyped by PCR method. Results of ACE ID polymorphism was confirmed using a second PCR, while results of GSTT1 and GSTM1 polymorphisms were confirmed by amplification of albumin gene as an internal control. All primers used and the band sizes obtained are shown in Table 1.
Table 1 Primers used for genetic analysis and band sizes obtained

Polymorphism Primers Method of genotyping Band sizes VEGF -460T/C P1F-5’-TGT GCA GAC GGC AGT CAC TA-3’P1R-5’-CCC GCT ACC AGC CGA CTT T-3’ VEGF -634G/C P2F-5’-TTG CTT GCC ATT CCC CAC TTG A-3’P2R-5’-CCG AAG CGA GAA CAG CCC AGA A-3’Direct sequencing Not required for genotyping PCR-RFLP with BsmFI G allele-196 and 274 bp C allele-470 bp VEGF +405G/C P3F-5’-ATT TAT TTT TGC TTG CCA TT-3’P3R-5’-GTC TGT CTG TCT GTC CGT CA-3’PCR-RFLP with BsmFI G allele-193 and 111 bp C allele-304 bp VEGF +936C/T P4F-5’-AAG GAA GAG GAG ACT CTG CGC-3’P4R-5’-TAT GTG GGT GGG TGT GTC TAC AGG-3’PCR-RFLP with NlaIII C allele-198 bp T allele-114 and 84 bp ACE ID P5F-5’-CTG GAG ACC ACT CCC ATC CTT TCT-3’P5R-5’-GAT GTG GCC ATC ACA TTC GTC AGA T-3’I allele-335 bp D allele-no band TNF -308G/A P7F-5’-AGG CAA TAG GTT TTG AGG GCC AT-3’P7R-5’-TCC TCC CTG CTC CGA TTC CG-3’I allele-490 bp D allele-190 bp P6F-5’-TGG GAC CAC AGC GCC CGC CCG CCA CTA C-3’P6R-5’-TCG CCA GCC CTC CCA TGC CCA TAA-3’ GSTT1 null/present P8F-5’-TTC CTT ACT GGT CCT CAC ATC TC-3’P8R-5’-TCA CCG GAT CAT GGC CAG CA-3’P9F-5’-GCC CTC TGC TAA CAA GTC CTA C-3’P9R-5’-GCC CTA AAA AGA AAA TCC CCA ATC-3’ GSTM1 null/present P10F-5’-GAA CTC CCT GAA AAG CTA AAG C-3’P10R-5’-GTT GGG CTC AAA TAT ACG GTG G-3’P9F-5’-GCC CTC TGC TAA CAA GTC CTA C-3’P9R-5’-GCC CTA AAA AGA AAA TCC CCA ATC-3’ GSTP1 Ile/Val P11F-5’-ACC CCA GGG CTC TAT GGG AA-3’P11R-5’-TGA GGG CAC AAG AAG CCC CT-3’PCR and confirmed with a second ARMS-PCR PCR-RFLP with NcoI G allele-87 and 20 bp A allele-107 bp PCR (multiplex with albumin gene)Null-no band Present-459 bp Internal control-350 bp PCR (multiplex with albumin gene)Null-no band Present-219 bp Internal control-350 bp PCR-RFLP with Alw26I Ile allele-176 bp Val allele-85 and 91 bp
Statistical Analysis Continuous data are presented as mean±SD. Differences of gestational age at birth and birth weight between cases and controls were measured with t -test.Gender and genotype differences between the subjects and also between ROP stages were measured with Chi-square test. In addition, deviation of the genotype from Hardy Weinberg equilibrium was determined also with Chi-square test. Polymorphisms which were found to differ significantly between cases and controls were analyzed with logistic regression model to find out their respective odds ratios (ORs).Besides crude ORs, logistic regression data were also adjusted for gestational age at birth, birth weight and gender of the subjects to eliminate the effect of these confounding factors.
RESULTS
Details of Study Subjects In this study, 724 babies with ROP were recruited as cases and 878 healthy preterm babies without any ophthalmological disease were included as controls.The average gestational age at birth, birth weight and sex distribution of the subjects are shown in Table 2. Cases and controls differed significantly in gestational age ( P <0.001) and birth weight ( P =0.005), although differences in sex were not statistically significant ( P =0.470).
We included only cases with advanced stages (stages 4 and 5)of ROP to eliminate false positive or negative results. Among the cases, 497 (68.6%) were in stage 4, while the remaining 227 (31.4%) were in stage 5.
Genotype Frequency All 724 cases and 878 controls were genotyped successfully. The genotype frequencies of the polymorphisms in cases and controls were shown in Table 2.Chi-square test revealed that there were significant differences in the frequencies of ACE ID polymorphism and TNF -308G/A polymorphism between cases and controls ( P =0.031 and P <0.001 respectively). The other polymorphisms did not differ significantly between cases and controls ( P >0.05, Table 2).
We tested the genotype distributions for their deviations from the Hardy Weinberg equilibrium. It was found that the P values for VEGF -460T/C, -634G/C, +405G/C and +936C/T polymorphisms, ACE ID polymorphism, TNF -308G/A polymorphism and GSTP1 Ile/Val polymorphism were larger than 0.05, suggesting that their deviations from the Hardy Weinberg equilibrium were not statistically significant.However, deviations of GSTT1 and GSTM1 polymorphisms from the Hardy Weinberg equilibrium could not be tested since the two polymorphisms only had two genotypes (either null or present).
Measurement of Genetic Association The ACE ID and TNF -308G/A polymorphisms were selected for logistic regression analysis, since they showed statistically significant differences between cases and controls. The results are presented in Table 3.For the ACE polymorphism, the ID genotype was found to exhibit a lower risk of ROP, with a crude OR of 0.793 (95%CI:0.627-1.003), compared to the wild type II genotype. Thisassociation was at the borderline lack of statistical significance( P= 0.053). However, after adjusted for the gestational age at birth, birth weight and gender of the subjects, the association became statistically significant, with an adjusted OR (aOR) of 0.603 (95%CI: 0.427-0.893) at P =0.034. For the DD genotype,the association was statistically significant for both crude OR [0.700 (95%CI: 0.532-0.921; P =0.011)] and aOR [0.468(95%CI: 0.229-0.626; P =0.002)].
Table 2 Characteristics and differences between cases and controls n (%)
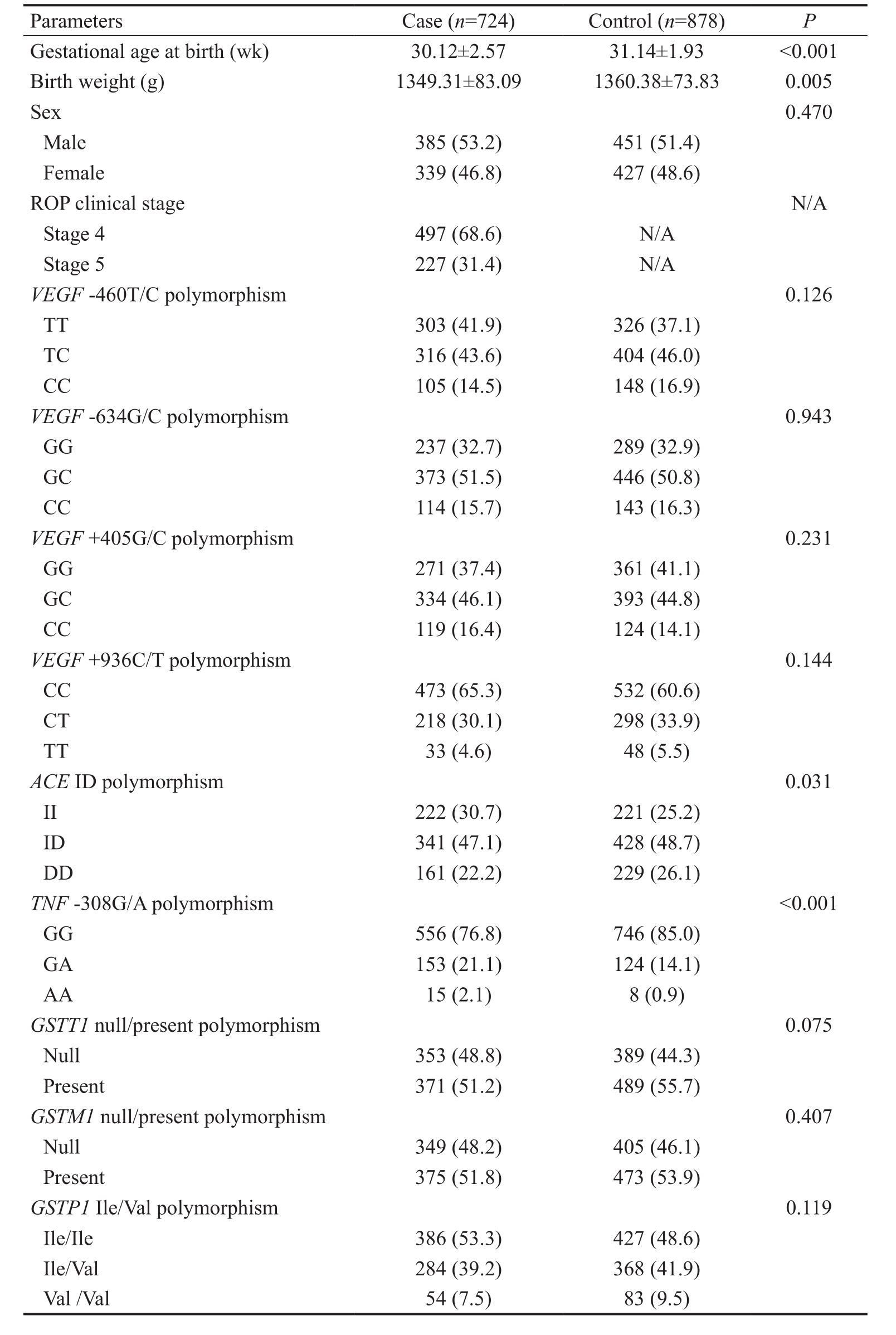
Parameters Case ( n =724) Control ( n =878) P Gestational age at birth (wk) 30.12±2.57 31.14±1.93 <0.001 Birth weight (g) 1349.31±83.09 1360.38±73.83 0.005 Sex 0.470 Male 385 (53.2) 451 (51.4)Female 339 (46.8) 427 (48.6)ROP clinical stage N/A Stage 4 497 (68.6) N/A Stage 5 227 (31.4) N/A VEGF -460T/C polymorphism 0.126 TT 303 (41.9) 326 (37.1)TC 316 (43.6) 404 (46.0)CC 105 (14.5) 148 (16.9) VEGF -634G/C polymorphism 0.943 GG 237 (32.7) 289 (32.9)GC 373 (51.5) 446 (50.8)CC 114 (15.7) 143 (16.3) VEGF +405G/C polymorphism 0.231 GG 271 (37.4) 361 (41.1)GC 334 (46.1) 393 (44.8)CC 119 (16.4) 124 (14.1) VEGF +936C/T polymorphism 0.144 CC 473 (65.3) 532 (60.6)CT 218 (30.1) 298 (33.9)TT 33 (4.6) 48 (5.5) ACE ID polymorphism 0.031 II 222 (30.7) 221 (25.2)ID 341 (47.1) 428 (48.7)DD 161 (22.2) 229 (26.1) TNF -308G/A polymorphism<0.001 GG 556 (76.8) 746 (85.0)GA 153 (21.1) 124 (14.1)AA 15 (2.1) 8 (0.9) GSTT1 null/present polymorphism 0.075 Null 353 (48.8) 389 (44.3)Present 371 (51.2) 489 (55.7) GSTM1 null/present polymorphism 0.407 Null 349 (48.2) 405 (46.1)Present 375 (51.8) 473 (53.9) GSTP1 Ile/Val polymorphism 0.119 Ile/Ile 386 (53.3) 427 (48.6)Ile/Val 284 (39.2) 368 (41.9)Val /Val 54 (7.5) 83 (9.5)
The association was also measured at the allele level. The D allele of the ACE ID polymorphism was found to be associated with a lower risk of ROP, in both the crude analysis and adjusted analyses. The crude OR of the D allele was 0.829(95%CI: 0.721-0.953; P =0.009), while the adjusted OR was 0.698 (95%CI: 0.294-0.883; P <0.001).
Contrary to the ACE polymorphism, the GA and AA genotypes of TNF -308G/A polymorphism were found to be associated with an increased ROP risk compared to the wild type GG genotype. The GA and AA genotypes exhibited a crude OR of 1.656 (95%CI: 1.275-2.149; P <0.001) and 2.516 (95%CI:1.059-5.975; P =0.037) respectively. Consistent with thisobservation, when the analysis was done at the allele level,an increased risk of ROP was also observed for the A allele,with a crude OR of 1.670 (95%CI: 1.324-2.106; P <0.001).When adjusted for the gestational age at birth, birth weight and gender of the subjects, the associations of the GA and AA genotypes and the A allele all became highly significant, with a P value of <0.001 each. The GA genotype had an adjusted OR of 1.956 (95%CI: 1.396-2.465) while the AA genotype had an adjusted OR of 2.809 (95%CI: 1.802-4.484). Besides, the adjusted OR of the A allele was 1.776 (95%CI: 1.446-2.561).
Table 3 Crude and adjusted OR for the association of ACE ID and TNF -308G/A polymorphisms with ROP risk

a Adjusted for gestational age at birth, birth weight and gender of the subjects.
Polymorphism OR (95%CI) P aOR (95%CI) a P ACE ID II genotype 1.000 ID genotype 0.793 (0.627-1.003) 0.053 0.603 (0.427-0.893) 0.034 DD genotype 0.700 (0.532-0.921) 0.011 0.468 (0.229-0.626) 0.002 I allele 1.000 D allele 0.829 (0.721-0.953) 0.009 0.698 (0.294-0.883) <0.001 TNF -308G/A GG genotype 1.000 GA genotype 1.656 (1.275-2.149) <0.001 1.956 (1.396-2.465) <0.001 AA genotype 2.516 (1.059-5.975) 0.037 2.809 (1.802-4.484) <0.001 G allele 1.000 A allele 1.670 (1.324-2.106) <0.001 1.776 (1.446-2.561) <0.001
Correlation Between the Polymorphisms and Retinopathy of Prematurity Stages We also investigated whether there is any correlation between ACE ID and TNF -308G/A polymorphisms and ROP stages. The results are shown in Table 4. For both polymorphisms, there was no significant difference in polymorphic distribution between stage 4 and stage 5 patients ( P =0.747 for ACE polymorphism, P =0.432 for TNF polymorphism). Therefore, there was no correlation between the polymorphisms and ROP stages.
DISCUSSION
This study investigated the association of VEGF , ACE , TNF ,and GST genes polymorphisms with ROP risk among Chinese.These genes have been firmly linked to the development of ROP, but the associations of their polymorphisms with ROP risk have been insufficiently studied in the Chinese population.This is surprising, because Asian babies are at a higher risk of developing ROP. To fill this important gap in the literature, we performed the first study on the association of VEGF , ACE , TNF , and GST polymorphisms with the risk of ROP among Chinese. Polymorphisms in these genes have been investigated in several other populations, but their influence on ROP risk among Chinese was entirely unknown.
In this study, we included only cases with advanced stages(stages 4 and 5) of ROP to eliminate false positive or negativeresults, because spontaneous remission of the disease is commonly observed at earlier stages [5] . For controls subjects,we included only prematurely-born babies and those whose birth weight were lower than 1500 g, in order to match them with the cases for more accurate comparisons to be done. In addition, the present work was also the largest-scale study conducted on these polymorphisms so far. Studies in other populations may not be able to have such stringent criteria for selection of subjects. However, China has the advantage of having a large population, which made recruitment of subjects convenient.
Table 4 Correlation between the polymorphisms and ROP stages n (%)
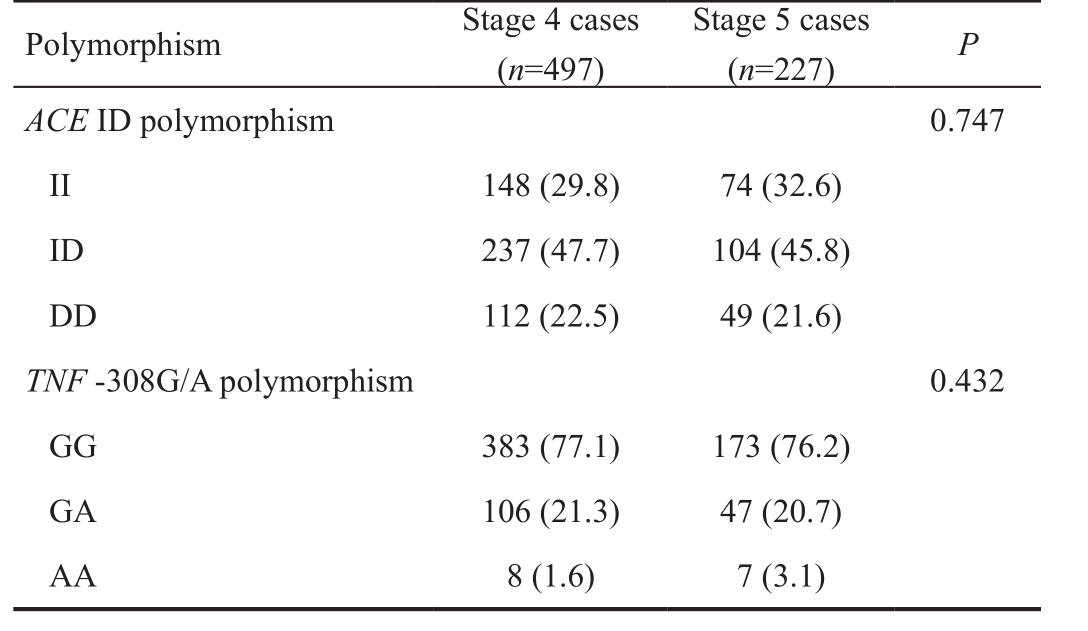
Polymorphism Stage 4 cases( n =497)Stage 5 cases( n =227) P ACE ID polymorphism 0.747 II 148 (29.8) 74 (32.6)ID 237 (47.7) 104 (45.8)DD 112 (22.5) 49 (21.6) TNF -308G/A polymorphism 0.432 GG 383 (77.1) 173 (76.2)GA 106 (21.3) 47 (20.7)AA 8 (1.6) 7 (3.1)
We found a significant association of ACE ID polymorphism with a decreased risk of ROP, and of TNF -308G/A polymorphism with an increased risk of ROP. These associations remained significant after adjustment for potential confounding factors,which included gestational age at birth, birth weight and gender of the subjects, all of which have been known to play a role in the development of ROP.
There were two previous studies which investigated the association of the ACE ID polymorphism with ROP risk: one in Kuwaiti population [11] and another one in Turkey population [12] .
Our results were consistent with neither of the reports. Both of these reports found no significant association between the ACE ID polymorphism and ROP risk. It is unknown why such a discrepancy in study findings occurred. However, it is known that ethnic background can have a substantial impact on ROP susceptibility [4] , and our report was the first one in the Chinese population. As mentioned previously in the Introduction, a carefully-regulated level of ACE is essential for optimal retinal maturation. The ACE ID polymorphism can influence the level of the ACE enzyme [19-21] . It is perhaps for this reason that the risk of ROP could be affected by the ACE ID polymorphism.Besides, apart from our present study, there were also two reports which examined the association of TNF -308G/A polymorphism with ROP risk [9,13] . Both reports also demonstrated no significant association between the polymorphism and the disease, which was again contradictory with ours. However,as inflammation has been shown to promote the development of ROP, it is reasonable that the A allele of the TNF -308G/A polymorphism could lead to an increased ROP risk, since the allele has been known to cause an increase in the level of the proinflammatory cytokine [26] .
We did not find any significant association of the remaining seven polymorphisms with ROP risk. The VEGF -460T/C,-634G/C, +405G/C and +936C/T polymorphisms had been investigated in two [6,8] , three [8-10] , two [6-7] and three [7,9-10] previous studies respectively. All previous studies indicated an absence of significant association between VEGF -460T/C and +936C/T polymorphisms and ROP risk, which concurred with our findings [6-10] . However, discrepancies in study findings were observed for the VEGF -634G/C and +405G/C polymorphisms.For the VEGF -634G/C polymorphism, a study by Shastry and Qu [8] demonstrated a lack of significant association, but another study by Cooke et al [9] showed that the G allele was associated with an increased risk of ROP, and yet another study by Ali et al [10] found that while the GC genotype decreased ROP risk, the CC genotype increased the risk. On the other hand,for VEGF +405G/C polymorphism, a report from Vanney et al [6] showed that the C allele was overrepresented in the cases, while the study from Kalmeh et al [7] was in line with our results that no statistically significant association was present. These discordances necessitate a need for a study with a much larger sample size to confirm the presence or absence of significant associations. The present work included a large sample size and could serve this purpose.
Finally, there were also previous reports on the polymorphisms of the three GST genes. All the previous studies did not find a significant association with ROP risk, which was in agreement with our findings. Collectively, we showed that there was no association of GSTT1 null/present polymorphism, GSTM1 null/present polymorphism and GSTP1 Ile/Val polymorphism with ROP risk.
In conclusion, we reported for the first time the association of ACE ID polymorphism with a decreased risk of ROP and of TNF -308G/A polymorphism with an increased risk of ROP.We also showed that VEGF -460T/C, -634G/C, +405G/C and+936C/T polymorphisms, GSTT1 null/present polymorphism, GSTM1 null/present polymorphism and GSTP1 Ile/Val polymorphism was not associated with the risk of ROP among Chinese infants.
ACKNOWLEDGEMENTS
Conflicts of Interest: Lei XJ, None; Zhao YX, None; Qiao T, None.
REFERENCES
1 Hellström A, Smith LE, Dammann O. Retinopathy of prematurity. Lancet 2013;382(9902):1445-1457.
2 Zepeda-Romero LC, Barrera-de-Leon JC, Camacho-Choza C, Gonzalez Bernal C, Camarena-Garcia E, Diaz-Alatorre C, Gutierrez-Padilla JA, Gilbert C. Retinopathy of prematurity as a major cause of severe visual impairment and blindness in children in schools for the blind in Guadalajara city, Mexico. Br J Ophthalmol 2011;95(11):1502-1505.
3 Husain SM, Sinha AK, Bunce C, Arora P, Lopez W, Mun KS, Reddy MA, Adams GG. Relationships between maternal ethnicity, gestational age, birth weight, weight gain, and severe retinopathy of prematurity. J Pediatr 2013;163(1):67-72.
4 Holmström G, van Wijngaarden P, Coster DJ, Williams KA. Genetic susceptibility to retinopathy of prematurity: the evidence from clinical and experimental animal studies. Br J Ophthalmol 2007;91(12):1704-1708.
5 Ju RH, Zhang JQ, Ke XY, Lu XH, Liang LF, Wang WJ. Spontaneous regression of retinopathy of prematurity: incidence and predictive factors. Int J Ophthalmol 2013;6(4):475-480.
6 Vannay A, Dunai G, Bányász I, Szabó M, Vámos R, Treszl A, Hajdú J, Tulassay T, Vásárhelyi B. Association of genetic polymorphisms of vascular endothelial growth factor and risk for proliferative retinopathy of prematurity. Pediatr Res 2005;57(3):396-398.
7 Kalmeh ZA, Azarpira N, Mosallaei M, Hosseini H, Malekpour Z.Genetic polymorphisms of vascular endothelial growth factor and risk for retinopathy of prematurity in South of Iran. Mol Biol Rep 2013;40(7):4613-4618.
8 Shastry BS, Qu X. Lack of association of the VEGF gene promoter (-634 G-->C and -460 C-->T) polymorphism and the risk of advanced retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 2007;245(5):741-743.
9 Cooke RW, Drury JA, Mountford R, Clark D. Genetic polymorphisms and retinopathy of prematurity. Invest Ophthalmol Vis Sci 2004;45(6):1712-1715.
10 Ali AA, Hussien NF, Samy RM, Husseiny KA. Polymorphisms of vascular endothelial growth factor and retinopathy of prematurity. J Pediatr Ophthalmol Strabismus 2015;52(4):245-253.
11 Haider MZ, Devarajan LV, Al-Essa M, Kumar H. Angiotensinconverting enzyme gene insertion/deletion polymorphism in Kuwaiti children with retinopathy of prematurity. Biol Neonate 2002;82(2):84-88.
12 Yildiz M, Karkucak M, Yakut T, Gorukmez O, Ozmen A. Lack of association of genetic polymorphisms of angiotensin-converting enzyme gene I/D and glutathione-S-transferase enzyme T1 and M1 with retinopathy of prematures. Genet Mol Res 2010;9(4):2131-2139.
13 Türe M, Yildiz M, Karkucak M, Gülten ET, Siğirli D, Özmen AT,Yakut T. Investigation of TNF-alpha gene (G308A) and GSTP1 gene(Ilel05Val) polymorphisms in Turkish patients with retinopathy of prematurity. Turk J Med Sci 2015;45(1):164-169.
14 Ng YK, Fielder AR, Shaw DE, Levene MI. Epidemiology of retinopathy of prematurity. Lancet 1988;2(8622):1235-1238.
15 Hartnett ME. Vascular endothelial growth factor antagonist therapy for retinopathy of prematurity. Clin Perinatol 2014;41(4):925-943.
16 Klufas MA, Chan RV. Intravitreal anti-VEGF therapy as a treatment for retinopathy of prematurity: what we know after 7 years. J Pediatr Ophthalmol Strabismus 2015;52(2):77-84.
17 Sato T, Kusaka S, Shimojo H, Fujikado T. Vitreous levels of erythropoietin and vascular endothelial growth factor in eyes with retinopathy of prematurity. Ophthalmology 2009;116(9):1599-1603.
18 Wheeler-Schilling TH, Sautter M, Guenther E, Kohler K. Expression of angiotensin-converting enzyme (ACE) in the developing chicken retina. Exp Eye Res 2001;72(2):173-182.
19 Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest 1990;86(4):1343-1346.
20 Tiret L, Rigat B, Visvikis S, Breda C, Corvol P, Cambien F, Soubrier F.Evidence, from combined segregation and linkage analysis, that a variant of the angiotensin I-converting enzyme (ACE) gene controls plasma ACE levels. Am J Hum Genet 1992;51(1):197-205.
21 Costerousse O, Allegrini J, Lopez M, Alhenc-Gelas F. Angiotensin I-converting enzyme in human circulating mononuclear cells: genetic polymorphism of expression in T-lymphocytes. Biochem J 1993;290(Pt 1):33-40.
22 Hong HK, Lee HJ, Ko JH, Park JH, Park JY, Choi CW, Yoon CH,Ahn SJ, Park KH, Woo SJ, Oh JY. Neonatal systemic inflammation in rats alters retinal vessel development and simulates pathologic features of retinopathy of prematurity. J Neuroinflammation 2014;11:87.
23 Gardiner TA, Gibson DS, de Gooyer TE, de la Cruz VF, McDonald DM, Stitt AW. Inhibition of tumor necrosis factor-alpha improves physiological angiogenesis and reduces pathological neovascularization in ischemic retinopathy. Am J Pathol 2005;166(2):637-644.
24 Silveira RC, Fortes Filho JB, Procianoy RS. Assessment of the contribution of cytokine plasma levels to detect retinopathy of prematurity in very low birth weight infants. Invest Ophthalmol Vis Sci 2011;52(3):1297-1301.
25 Abraham LJ, Kroeger KM. Impact of the -308 TNF promoter polymorphism on the transcriptional regulation of the TNF gene:relevance to disease. J Leukoc Biol 1999;66(4):562-566.
26 Poggi C, Giusti B, Vestri A, Pasquini E, Abbate R, Dani C. Genetic polymorphisms of antioxidant enzymes in preterm infants. J Matern Fetal Neonatal Med 2012;25(Suppl 4):131-134.
27 Pennathur S, Ido Y, Heller JI, Byun J, Danda R, Pergola P, Williamson JR, Heinecke JW. Reactive carbonyls and polyunsaturated fatty acids produce a hydroxyl radical-like species: a potential pathway for oxidative damage of retinal proteins in diabetes. J Biol Chem 2005;280(24):22706-22714.
28 Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi YC. Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid Redox Signal 2004;6(2):289-300.
29 International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005;123(7):991-999.
Citation: Lei XJ, Zhao YX, Qiao T. Influence of polymorphisms in VEGF , ACE , TNF and GST genes on the susceptibility to retinopathy of prematurity among Chinese infants. Int J Ophthalmol 2018;11(9):1451-1457
Received: 2017-11-19 Accepted: 2018-03-28
DOl: 10.18240/ijo.2018.09.04
Correspondence to: Yong-Xia Zhao. Department of Ophthalmology, Center Hospital in Cangzhou, Cangzhou 061000,Hebei Province, China. yongxia.zhao@hotmail.com



