Table 1 Comparison of B10 cells frequency with demographic characteristics and clinical features of TAO patients
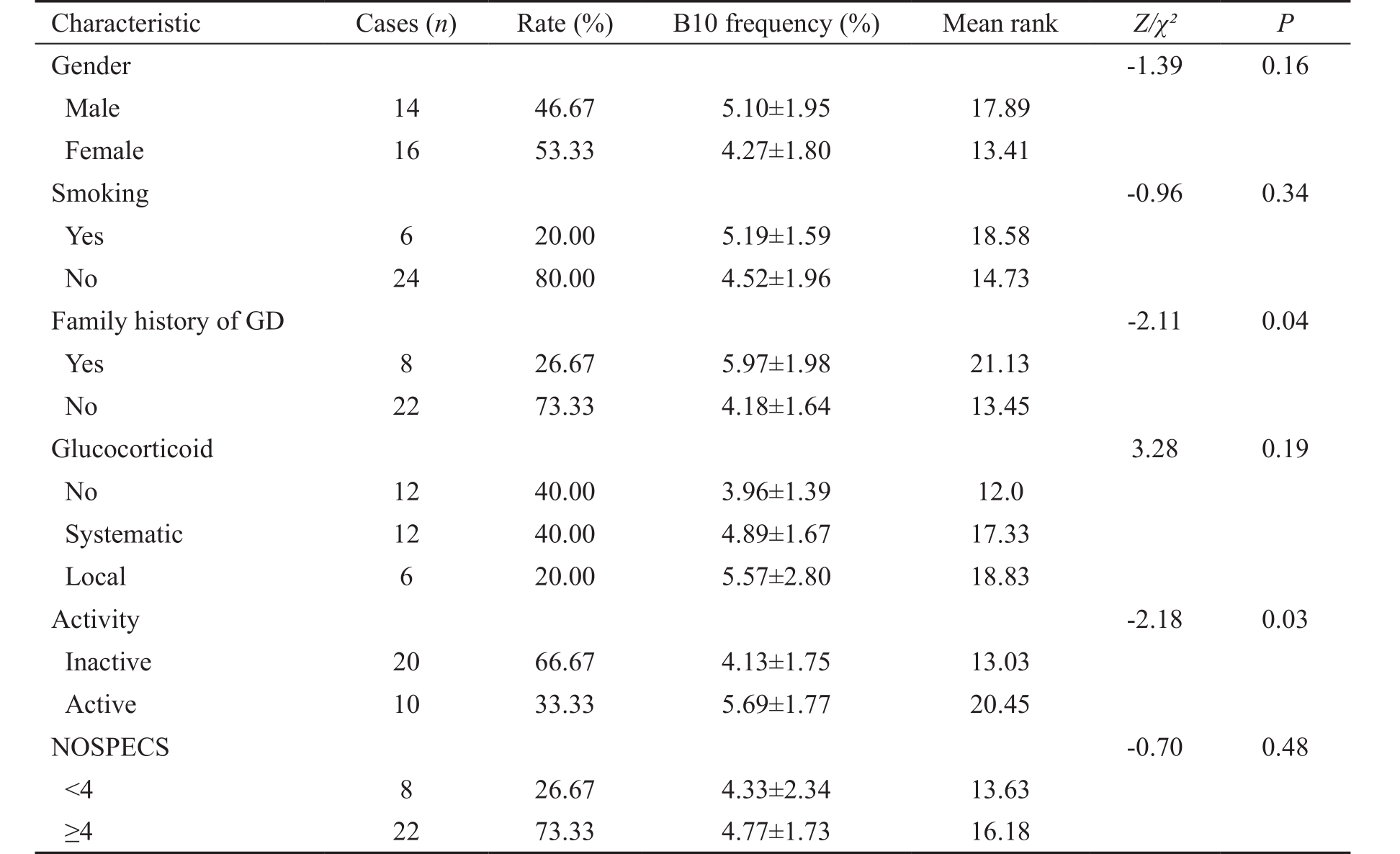
GD: Graves’ disease.
Yun-Gang Ding 1,2 , Guo Chen 1 , Qian Li 1,3 , Xiao-Feng Wen 1 , Lai Wei 1 , Hua-Sheng Yang 1
1 State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou 510060, Guangdong Province, China
2 Qindao Ludong Eye Hospital, Qingdao 266600, Shandong Province, China
3 Ningxia Eye Hospital, People’s Hospital of Ningxia Hui Autonomous Region, the First Clinical College of Northwest University for Nationalities, the Cooperative Teaching Hospital of North Minzu University, Yinchuan 750001, Ningxia Hui Autonomous Region, China
Thyroid-associated orbitopathy (TAO) is an autoimmune disease which potentially threatens vision, disfigures appearance and results in a pronounced loss of quality of life [1-2] . It is characterized by orbital T cell infiltration, release of proinflammatory cytokines and interaction with various cells including orbital fibroblasts through autoimmune mechanisms [3-5] . However, multiple lines of evidence suggest that B cells contribute to the pathogenesis of TAO by producing autoantibodies to the thyroid-stimulating hormone receptor (TSHR) and insulin-like growth factor-I receptor(IGF-IR), which leads to hyperthyroidism and potentially fibroblast activation, and have emerged as a therapeutic target [6] . Depleting CD20 + B cells with anti-CD20 antibody rituximab has shown potential benefit in controlling the disease activity of TAO [7-8] .
Regulatory B (Breg) cells are critical regulators of immune responses in autoimmune diseases [9-11] . By producing suppressive cytokines interleukin-10 (IL-10) and transforming growth factor-β (TGF-β), Breg cells contribute to the maintenance of peripheral immune tolerance [12-14] . Analogous Breg cells in human could also suppress the differentiation of CD4 + T cells and secretion of interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α). These suppressive effects were mediated by IL-10 [10-11,15] . The functional IL-10-producing Breg cells are called B10 cells.
There are growing evidences that B10 cells play a key role in the pathogenesis of autoimmune diseases [16-18] . In this study,we evaluated the frequency of B10 cells in TAO patients according to the disease activity and severity.
Table 1 Comparison of B10 cells frequency with demographic characteristics and clinical features of TAO patients

GD: Graves’ disease.
Patients and Controls Patients diagnosed with TAO at Zhongshan Ophthalmic Center from May 2015 to December 2015 were prospectively enrolled in this study. The diagnosis depended on the Bartley criteria [19] . Systemic examinations and orbital computed tomography (CT) or magnetic resonance imaging (MRI) scans were performed to exclude other confounding diseases ( e.g. high myopia, orbital tumors,trauma, etc ). Clinical Activity Score (CAS) was used to assess the disease activity in these patients [20-21] . The CAS score above 3/7 at the first examination or above 4/10 in successive examinations was defined as active [22] . The disease severity was evaluated according to the NOSPECS classification(no physical signs or symptoms, only signs, soft tissue involvement, proptosis, extraocular muscle signs, corneal involvement, and sight loss) reported by Werner [23] . Healthy controls with normal thyroid function [thyroid stimulating hormone (TSH): 0.27-4.20 μIU/mL] were recruited at the same time. Subjects with goiter, neoplastic, inflammatory, infectious diseases or other autoimmune disease were excluded from the study. This study adhered to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Boards of Zhongshan Ophthalmic Center, Sun Yat-sen University.All participants provided written informed consent. Recorded demographic characteristics and clinical features of TAO patients are displayed in Table 1.
Sample Collection and Induction of B10 Cells After collecting whole blood from the TAO patients and healthy controls, peripheral blood mononuclear cells (PBMCs)were isolated by density gradient centrifugation using Histopaque-1077. Then PBMCs were washed with phosphatebuffered saline (PBS) solution twice and resuspended(2×10 6 cells/mL) in medium containing 10% fetal calf serum for culture. For B10 cell induction, PBMCs were stimulated with CpG (ODN 2006, 10 μg/mL; Invivogen), CD40 ligand(CD40L, 1 μg/mL; R&D Systems) or their combination for 48h at 37℃ as previously described [10] . For the last 5h,polymethyl acrylate (PMA; 50 ng/mL; Sigma-Aldrich-Aldrich), ionomycin (1 μg/mL; Sigma-Aldrich-Aldrich) and Brefeldin A (BFA; 1×solution/mL; BioLegend) (PIB) were added to increase intracellular protein expression and to stop intracellular cytokine secretion.
Flow Cytometry Analysis For IL-10 detection, Fc receptors were blocked using Fc Block (BD Biosciences, USA).Cells were stained with the appropriate anti-CD19 antibody for 30min on ice. The cells were washed twice, fixed and permeabilized using a Cytofix/Cytoperm kit (BD Biosciences,USA) according to the manufacturer’s instructions and stained with anti-IL-10-PE (BioLegend, USA) before analysis by flow cytometry (MACSQuant™, Miltenyi Biotec, Germany). All flow cytometry data were analyzed using FlowJo software.
Statistical Analysis All graphs were prepared with Graph-Pad Prism 5.0. The t -test and analysis of variance were used to compare the levels of IL-10 + B cells between groups. The correlation between clinical characteristics and the frequency of B10 cells was estimated by t -test and correlation analysis. P <0.05 was considered statistically significant. SPSS version 22.0 was used for all statistical tests (SPSS Inc.).
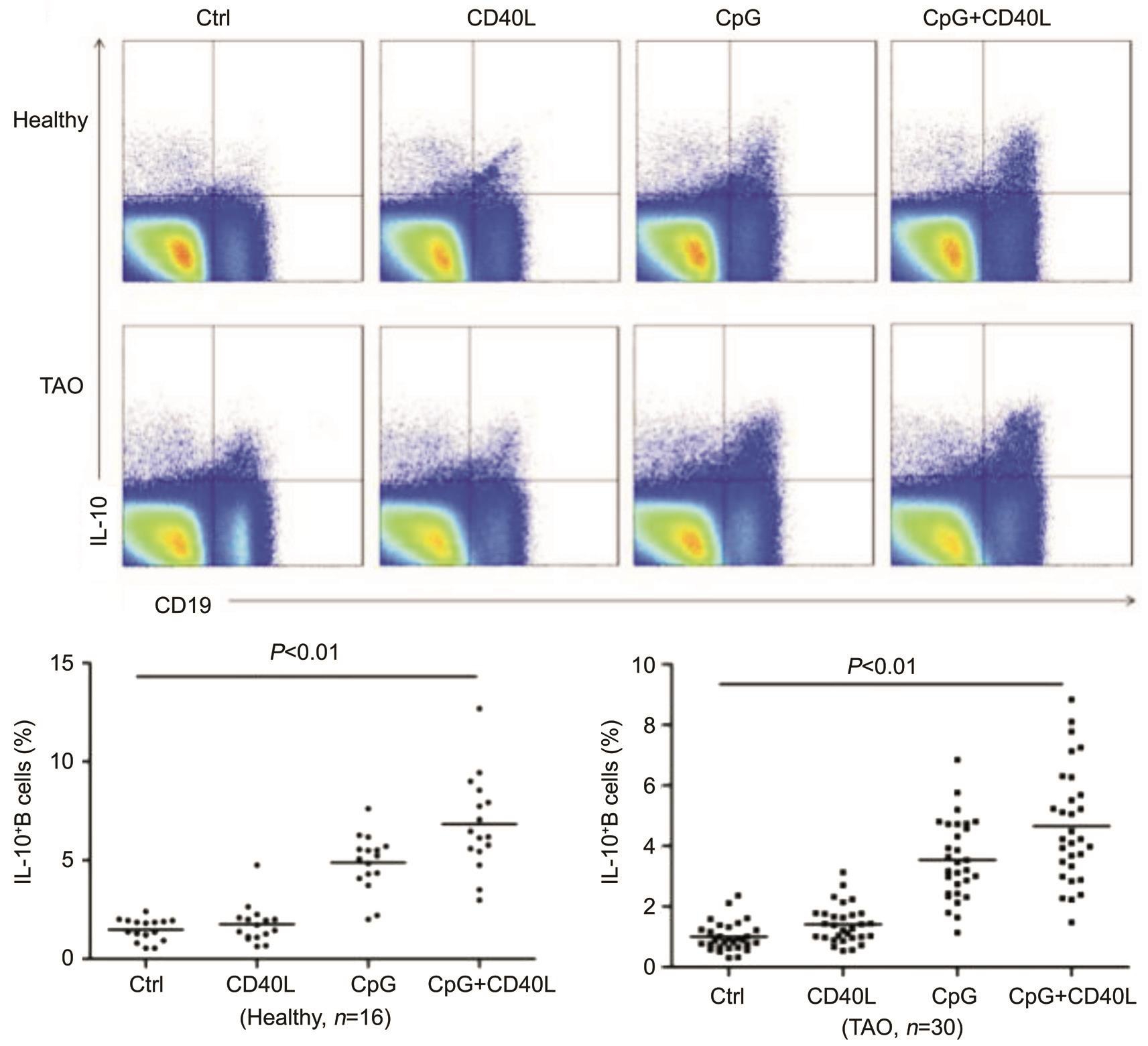
Figure 1 The percentage of B10 cells induced by different stimulations Representative dot plots showing the proportion of CD19 + IL-10 + cells from a healthy donor and a patient with TAO. B10 cells were identified after in vitro stimulation with PIB for 5h as control group.Alternatively, B10 cell frequencies in other group were determined after in vitro stimulation with CD40L, CpG, or CD40L+CpG, with PIB added during the final 5h of 48-hour cultures. Healthy: Healthy control; TAO: Patient with TAO.
Demographic Characteristics and Clinical Features of Thyriod-associated Orbitopathy Patients Totally 30 TAO patients and 16 healthy controls were enrolled in this study.The mean (±SD) ages of TAO patients and healthy controls were 39.77±12.69y (range, 19-64y) and 34.62±12.34y (range,20-58y), respectively. Female of TAO patients and healthy controls were 16 (53.33%) and 10 (62.50%), respectively.There was no significant difference in gender distribution( P =0.78) and age ( P =0.98) at sampling between TAO patients and healthy controls.
For 30 TAO patients, 8 cases (26.67%) had a family history of Graves’ disease (GD). Duration of TAO was 15.90±13.08mo(range, 1-48mo). Totally 18 patients were treated with systemic(40.00%) or local (20.00%) glucocorticoid, while 12 of the patients (40.00%) received no glucocorticoid treatment. A total of 20.00% of the TAO patients smoke. The mean CAS and NOSPECS score was 2.57±1.96 (range, 0-6) and 3.80±0.55(range, 3-5), respectively. The mean free triiodothyronine(FT3), free thyroxine (FT4) and TSH was 4.94±1.71 pmol/L(3.15-10.50 pmol/L), 15.60±6.48 pmol/L (0.82-31.75 pmol/L)and 5.81±19.14 μIU/mL (0.00-102.00 μIU/mL), respectively.The demographic characteristics, clinical features and laboratory test of TAO patients were summarized in Table 1.
Percentage of B10 Cells Induced by Different Stimulations PBMC from the blood of 16 healthy donors and 30 TAO patients were stimulated with CD40L, CpG, or with their combinations for 48h. Cells were treated with PIB for the last 5h to stop cytokine secretion, and B10 cells were detected by flow cytometry. Control group only treated with PIB for 5h in media induced negligible percentage of B10 (healthy individuals 1.48%±0.56%; TAO patients 1.00%±0.48%)among CD19 + B cells. Compared to control group, CD40L,CpG group, the combination of CD40L and CpG group induced a significantly higher percentage of B10 cells ( P <0.01)in TAO and healthy individuals (Figure 1). So, we used the combination of CpG and CD40L to induce B10 cells in the following experiments.
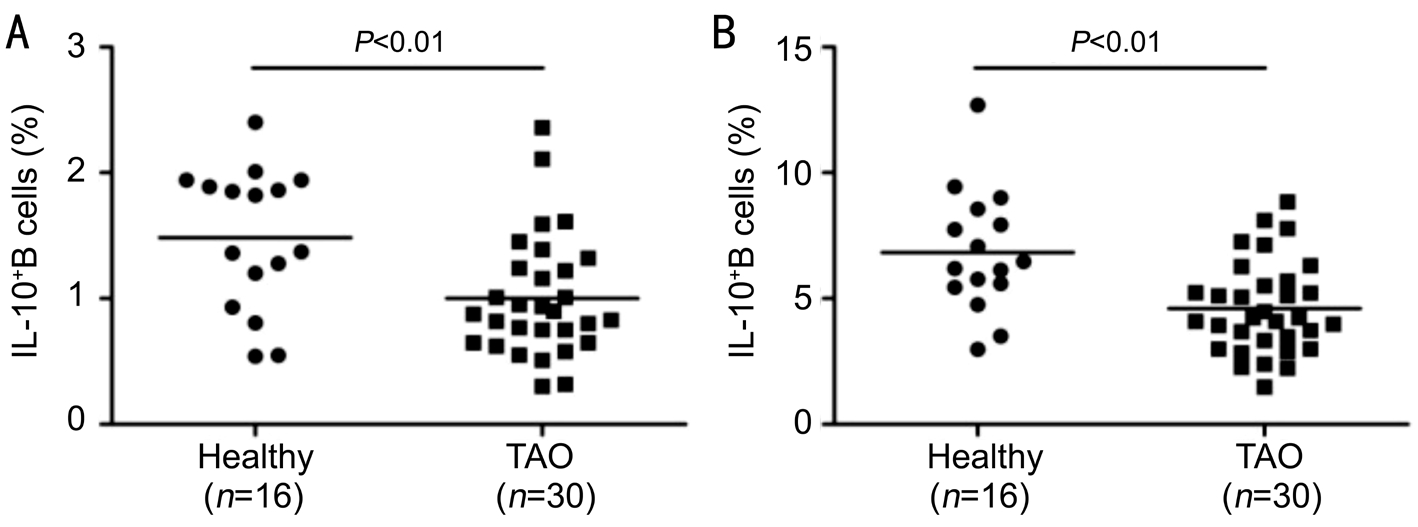
Figure 2 Frequency of B10 cells in TAO patients A: The frequency of B10 cells among CD19 + B cells in healthy controls and TAO patients in control group; B: The frequency of B10 cells among CD19 + B cells in healthy controls and TAO patients in the combination of CD40L and CpG group.
Our results showed that the frequency of B10 cells among CD19 + B cells in TAO patients was significantly lower than healthy individuals in the combination of CD40L and CpG group (TAO, 4.66%±1.88% versus healthy individuals,6.82%±2.40%, P <0.01; Figure 2).
Association of B10 Cells with the Clinical Activity of Thyriod-associated Orbitopathy Our results indicated that the frequency of B10 cells was significantly higher in TAO patients with family history of GD. When we compared active and inactive TAO patients, a significantly higher percentage of B10 cells was found in active TAO patients (Table 1). In addition, the TAO patients had a positive correlation between CAS and B10 cells frequency ( r =0.50, P =0.004, by correlation analysis; Table 2). There were no differences in the proportions of B10 cells between patients with different clinical features in gender, smoking, glucocorticoid treatment, or NOSPECS (Table 1).The correlation of B10 cells frequency with age, disease duration, FT3, FT4, or TSH was not significant (Table 2).
In recent years, many studies have recognized the critical role of Breg cells in suppressing immune responses and maintaining peripheral tolerance by producing regulatory cytokines IL-10(B10 cells). Our study indicated that the frequency of B10 cells was significantly associated with TAO disease activity assessed by CAS score, whereas the correlation with disease severity evaluated by NOSPECS was not significant. It suggests that the frequency of B10 cells may be a reflection of the disease activity in TAO patients. The clinical course of TAO involves two stages. After a progressive active phase characterized by inflammation and orbital tissue remodeling, the condition gradually stabilizes and eventually trends towards quiescence(inactive phase) [24] . CAS is a validated scoring system designed to distinguish inflammatory from noninflammatory TAO [21] .Based on the classical signs of acute inflammation (pain,redness, swelling, and impaired function), this scoring system was proposed as a clinical classification to discriminate easily between the active and quiescent stages of the disease and was modified in 1997 [21] . The sum of all points defines clinical activity: the score above 3/7 at the first examination or above4/10 in successive examinations is defined as active [22] . The frequency of B10 cells increased in active TAO patients and was significantly correlated with disease activity assessed by CAS score. B10 cells may contribute to the induction of regulatory T cells (Tregs) and down-regulate the inflammation [25] . The underlying mechanisms between B10 cells and Tregs in TAO merit further investigation.
Table 2 The correlation between the B10 cells frequency and characteristics of the TAO patients
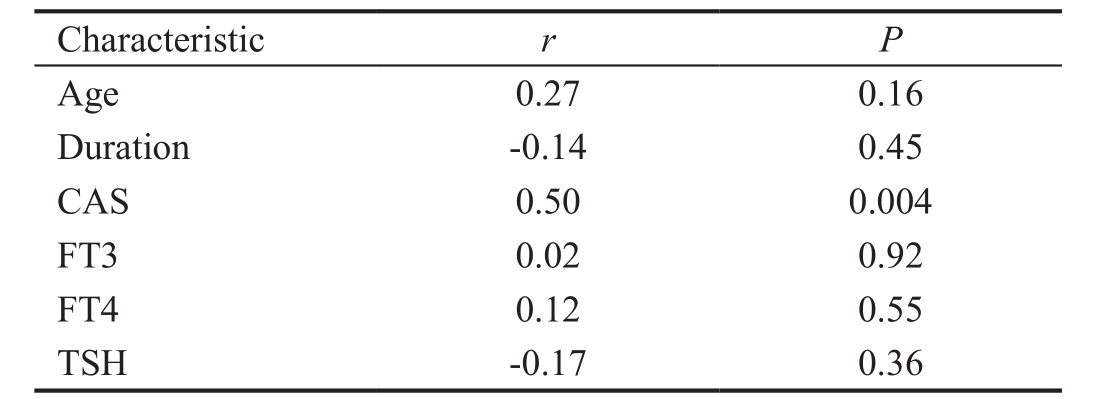
CAS: Clinical Activity Score; FT3: Free triiodothyronine; FT4: Free thyroxine; TSH: Thyroid stimulating hormone.
An increased frequency of Breg cells was also found in patients with systemic or organ-specific autoimmune diseases [10,25-27] .However, other reports suggested a reduction in Breg numbers in rheumatoid arthritis [17] , neuromyelitis optica [28] ,Graves’ disease and Hashimoto’s thyroiditis [29] patients. The discrepancies between these studies may due to the disease activity of patients at test or the methods pulsing B cells for Breg detection.
This study has several limitations. The number of sample was small and there is no longitudinal follow-up. The frequency of B10 cells decreases in TAO patients and is associated with disease activity, and further studies should investigate the mechanism by which B10 cells contribute to the pathogenesis and disease activity of TAO.
ACKNOWLEDGEMENTS
Foundations: Supported by the National Natural Science Foundation of China (No.81470664; No.81670887;No.81700875)
Conflicts of Interest: Ding YG, None; Chen G, None; Li Q, None; Wen XF, None; Wei L, None; Yang HS, None.
REFERENCES
1 Bahn RS. Graves’ ophthalmopathy. N Engl J Med 2010;362(8):726-738.
2 Du Y, Ye H, Li K, Xiao X, Chen R, He JF, Yang H. Vision-related quality of life tends to be more severely impaired in patients with dysthyroid optic neuropathy. Curr Eye Res 2014;39(5):532-536.
3 Bahn RS, Heufelder AE. Pathogenesis of Graves’ ophthalmopathy. N Engl J Med 1993;329(20):1468-1475.
4 Kahaly GJ, Shimony O, Gellman YN, Lytton SD, Eshkar-Sebban L,Rosenblum N, Refaeli E, Kassem S, Ilany J, Naor D. Regulatory T-cells in Graves’ orbitopathy: baseline findings and immunomodulation by anti-T lymphocyte globulin. J Clin Endocrinol Metab 2011;96(2):422-429.
5 Kim SE, Yoon JS, Kim KH, Lee SY. Increased serum interleukin-17 in Graves’ ophthalmopathy. Graefes Arch Clin Exp Ophthalmol 2012;250(10):1521-1526.
6 Hegedüs L, Smith TJ, Douglas RS, Nielsen CH. Targeted biological therapies for Graves’ disease and thyroid-associated ophthalmopathy.Focus on B-cell depletion with Rituximab. Clin Endocrinol (Oxf) 2011;74(1):1-8.
7 Salvi M, Vannucchi G, Campi I, Rossi S, Bonara P, Sbrozzi F,Guastella C, Avignone S, Pirola G, Ratiglia R, Beck-Peccoz P. Efficacy of rituximab treatment for thyroid-associated ophthalmopathy as a result of intraorbital B-cell depletion in one patient unresponsive to steroid immunosuppression. Eur J Endocrinol 2006;154(4):511-517.
8 Minakaran N, Ezra DG. Rituximab for thyroid-associated ophthalmopathy. Cochrane Database Syst Rev 2013;(5):CD009226.
9 Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med 2003;197(4):489-501.
10 Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, Hall RP,St Clair EW, Tedder TF. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 2011;117(2):530-541.
11 Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA,Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 2010;32(1):129-140.
12 Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 2008;28(5):639-650.
13 Lee JH, Noh J, Noh G, Choi WS, Cho S, Lee SS. Allergen-specific transforming growth factor-β-producing CD19(+)CD5(+) regulatory B-Cell (Br3) responses in human late eczematous allergic reactions to cow’s milk. Journal of Interferon and Cytokine Research 2011;31(5):441-449.
14 Noh J, Choi WS, Noh G, Lee JH. Presence of Foxp3-expressing CD19(+)CD5(+) B cells in human peripheral blood mononuclear cells:human CD19(+)CD5(+)Foxp3(+) regulatory B cell (Breg). Immune Netw 2010;10(6):247-249.
15 Bouaziz JD, Calbo S, Maho-Vaillant M, Saussine A, Bagot M, Bensussan A, Musette P. IL-10 produced by activated human B cells regulates CD4(+) T-cell activation in vitro. Eur J Immunol 2010;40(10):2686-2691.
16 Kristensen B, Hegedüs L, Lundy SK, Brimnes MK, Smith TJ, Nielsen CH. Characterization of regulatory B cells in Graves’ disease and Hashimoto’s thyroiditis. PLoS One 2015;10(5):e0127949.
17 Yu S, Qi Y, Wang H, Jiang J, Sun L, Zhou Q. Dysfunction of CD24+CD38+ B cells in patients with Hashimoto’s thyroiditis is associated with a lack of interleukin 10. Int J Biochem Cell Biol 2017;90:114-120.
18 Sun Y, Huang Y, Chen WW, Jia J, Wei T. The IL-10-producing regulatory B cells (B10 cells) and regulatory T cell subsets in neuromyelitis optica spectrum disorder. Neurol Sci 2018;39(7):1307-1308.
19 Bartley GB, Gorman CA. Diagnostic criteria for Graves’ ophthalmopathy. Am J Ophthalmol 1995;119(6):792-795.
20 Mourits MP, Koornneef L, Wiersinga WM, Prummel MF, Berghout A, van der Gaag R. Clinical criteria for the assessment of disease activity in Graves’ ophthalmopathy: a novel approach. Br J Ophthalmol 1989;73(8):639-644.
21 Mourits MP, Prummel MF, Wiersinga WM, Koornneef L. Clinical activity score as a guide in the management of patients with Graves’ophthalmopathy. Clin Endocrinol (Oxf) 1997;47(1):9-14.
22 Barrio-Barrio J, Sabater AL, Bonet-Farriol E, Velázquez-Villoria Á, Galofré JC. Graves’ ophthalmopathy: VISA versus EUGOGO classification, assessment, and management. J Ophthalmol 2015;2015:249125.
23 Werner SC. Modification of the classification of the eye changes of Graves’ disease. Am J Ophthalmol 1977;83(5):725-727.
24 Douglas RS, Gupta S. The pathophysiology of thyroid eye disease:implications for immunotherapy. Curr Opin Ophthalmol 2011;22(5):385-390.
25 Cho EB, Cho HJ, Seok JM, Min JH, Kang ES, Kim BJ. The IL-10-producing regulatory B cells (B10 cells) and regulatory T cell subsets in neuromyelitis optica spectrum disorder. Neurol Sci 2018;39(3):543-549.
26 Figueiró F, Muller L, Funk S, Jackson EK, Battastini AM, Whiteside TL. Phenotypic and functional characteristics of CD39(high) human regulatory B cells (Breg). Oncoimmunology 2016;5(2):e1082703.
27 Rosser EC, Mauri C. Regulatory B cells: origin, phenotype, and function. Immunity 2015;42(4):607-612.
28 Quan C, Yu H, Qiao J, Xiao B, Zhao G, Wu Z, Li Z, Lu C. Impaired regulatory function and enhanced intrathecal activation of B cells in neuromyelitis optica: distinct from multiple sclerosis. Mult Scler 2013;19(3):289-298.
29 Zha B, Wang L, Liu X, Liu J, Chen Z, Xu J, Sheng L, Li Y, Chu Y. Decrease in proportion of CD19+ CD24(hi) CD27(+) B cells and impairment of their suppressive function in Graves’ disease. PLoS One 2012;7(11):e49835.
Citation: Ding YG, Chen G, Li Q, Wen XF, Wei L, Yang HS.Frequency of IL-10-producing regulatory B cells associated with disease activity in thyroid-associated orbitopathy. Int J Ophthalmol 2018;11(9):1458-1462
Received: 2018-04-23 Accepted: 2018-07-19
DOl: 10.18240/ijo.2018.09.05
Abstract · AlM: To investigate the association between lL-10-producing regulatory B (B10) cells and the clinical features of thyroid-associated orbitopathy (TAO).· METHODS: A total of 30 patients with TAO were recruited at Zhongshan Ophthalmic Center from May 2015 to December 2015. Peripheral blood mononuclear cells (PBMCs) were separated from blood samples of 30 TAO patients and 16 healthy controls and stimulated with CD40 ligand and CpG for 48h. The frequency of lL-10+ B cells was examined by flow cytometry and the correlation between the frequency of lL-10+ B cells and clinical features of TAO was analyzed by SPSS.· RESULTS: The frequency of lL-10+ B cells among CD19+B cells in TAO patients was significantly lower than in healthy controls (TAO: 4.66%±1.88% vs healthy control:6.82%±2.40%, P<0.01). The frequency of lL-10+ B cells showed a positive correlation with disease activity of TAO measured by Clinical Activity Score (CAS) (r=0.50, P<0.01),and became higher in TAO patients with family history of Graves’ disease (GD) (P=0.04).· CONCLUSlON: The decrease of the frequency of lL-10+ B cells in TAO patients indicates the deficiency of B10 cells in TAO, and the positive association with disease activity suggests its important role in TAO inflammation regulation.· KEYWORDS: thyroid-associated orbitopathy; regulatory B cells; interleukin-10; flow cytometry
Correspondence to: Hua-Sheng Yang and Lai Wei. State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center,Sun Yat-sen University, 54 South Xianlie Road, Guangzhou 510060, Guangdong Province, China. yanghs64@126.com;weil9@mail.sysu.edu.cn
Co-first authors: Yun-Gang Ding, Guo Chen and Qian Li