Table 1 Primer sequence pairs used for quantitative real-time PCR
Cong Nie 1 , Xin-Chun Zhang 2 , Si-Ying Xu 1 , Ya-Dan Quan 1 , Zhi-Xin Tang 1 , Rong Lu 1
1 State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou 510060, Guangdong Province, China
2 Department of Prosthodontics, Hospital of Stomatology,Guangdong Provincial Key Laboratory of Stomatology,Guanghua School of Stomatology, Sun Yat-sen University,Guangzhou 510060, Guangdong Province, China
· KEYWORDS: pterygium; epithelium; proliferation;transcription factor 4
Pterygium is a common ocular surface disease. It exists as a kind of neoplasm located on the bulbar conjunctiva and cornea at the palpebral fissure. The disease can not only affect the ocular surface condition and appearance of the patients,but also cause discomfort and corneal astigmatism owing to the tension caused by the pterygial volumn. Meanwhile,it can influence the ocular movement to different degrees,sometimes even affect vision [1-2] . Up till now, the pathogenesis is not yet clear. There is no widely recognized theory. Some researchers thought the occurrence of pterygium were related with proliferation of epithelium. Kase et al [3] indicated that the proliferation of epithelial cells could lead to the occurrence and development of pterygium.
Concerning pterygium proliferation, several researchers thought that it mainly relies on pterygial head. As pathology evidence shows, there is an increase in epithelial cell layers of the pterygial head [4] . Some researchers proposed that pterygium proliferation is related to vimentin positive pterygial cells in the pterygial head epithelium [5] . There is a theory that with pterygial head dragging the body, pterygium affects conjunctiva and cornea. Nonetheless, there is no extensively accepted consensus. Others proposed alternative hypothesis that the high proliferative capacity of pterygium owes to the body. It corresponds with the phenomenon that the pterygial body are seen proliferates and swells in clinical observation.Some researches indicated that Ki-67 and cyclin D1 were expressed in the epithelium of pterygial body, which may promote the proliferation of epithelial cells [6-7] . However,there is no quantitative study or further discussion about the orientation of pterygium.
Wnt signaling pathway is known involved in regulation of several physiological processes, including embryonic development, cell proliferation, differentiation, and migration [8] . Transcription factor 4 (TCF4) is a key factor of Wnt signaling pathway.TCF4 plays an important role in the balance of normal tissues’development and differentiation, promoting cell proliferation and differentiation [9] . In one of our early researches, we also found that TCF4 localized in putative conjunctival epithelial stem/precursor cells. The expression of TCF4 may correlate with the highly proliferative potential of conjunctival epithelial cells and serve as a potential signature of highly proliferative conjunctival epithelial cells [10] . Moreover, expression of TCF4 in pterygial epithelium was also examined in a research in 2016 [11] . Therefore, it is meaningful to make it clear whether TCF4 plays an important role in the proliferation of pterygium.In the present study, we characterized the proliferative potential of different part of pterygial epithelial cells by cell culture,clonal analysis, and immunofluorescence, and then identified the role of TCF4 in pterygial epithelium proliferation. The research may enable us to know more about the occurrence and progress of pterygium and helps find new methods for the management of pterygium in the future.
Materials and Reagents Polyvinyl chloride (PVC) plastic film and filter paper were purchased from Shanghai Peninsula Industry Company (Shanghai, China). Rat anti-human monoclonal antibody ATP-binding cassette sub-family G member 2 (ABCG2), rat anti-human monoclonal antibody cytokine13 (CK13), and rat anti-human monoclonal antibody mucoprotein 5AC (MUC5AC) were from Abcam (Cambridge,MA, USA). Rat anti-human monoclonal antibody p63α, rat anti-human monoclonal antibody proliferating cell nuclear antigen (PCNA), FITC-conjugated anti-rabbit IgG secondary antibody, FITC-conjugated anti-sheep IgG secondary antibody and DAB were purchased from Dako Cytomation (Carpinteria,CA, USA). Rabbit anti-human monoclonal antibody TCF4 was from Santa Cruz Biotechnology (Santa Cruz, CA, USA).FITC-conjugated anti-rat IgG secondary antibody, acetone,100% ethyl alcohol, hydrogen peroxide, Triton X-100 and bovine serum albumin (BSA) were from Sigma-Aldrich Corp (USA).Paraformaldehyde was from Shanghai Biological Engineering Company (Shanghai, China). OCT was from SAKURA Company (USA). Mounting Medium with DAPI/PI and ABC Kit were from Vector Laboratories (Burlingame, CA, USA).
Donor Tissues in Our Research We collected pterygium tissues from 30 primary pterygium patients. They received surgical treatments in Zhongshan Ophthalmic Center during August 2014 to October 2015. We also selected 10 normal bulbar conjunctival tissues provided by Guangdong Eye Bank.The study was approved by the ethics committee of Zhongshan Ophthalmic Center, Sun Yat-sen University. The research followed the tenets of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study. All tissues obtained from patients in surgeries were with no corneal disease, conjunctival disease, trauma history, long-term ocular medication history or other diseases.
Conjunctival and Pterygial Epithelial Cell Culture Tissues got from surgeries and Guangdong Eye Bank were rinsed in phosphate buffer solution (PBS) containing 1000 U/L gentamicin 3 times for 2min each and then cut into pieces about 3 mm ×3 mm after removing the underlined connective tissues. Subsequently, the pieces were placed in the 0.025%neutral protease solution at the room temperature for 40min and then centrifuged at the speed of 15 000 rpm for 5min.Then the pieces were resuspended with nutrient solution and digested into single cells by 0.25% trypsin. Explants and cells were cultured in the keratinocyte serum free medium (KSFM)solution under the condition of 37℃ (5% CO 2 ). Explants and cells were grown for 7d and the nutrient solution was hereby replaced every 2d.
Measurement of Colony Forming Efficiency Fresh epithelial cells isolated from head, neck and body of pterygium were planted on 100% growth factor-reduced Matrigel (BDBioscience, San José, CA, USA) with a concentration of 5×10 4 cells/cm 2 in 24-well plates. On the 7 th day of culture,cells were fixed with 2% paraformaldehyde and stained with Rhodamine B to determine the clonal types. Colonies were then observed and examined under a microscope (BX53,Olympus). Digital pictures were analyzed for colony size and cell number with Graphpad prism6.
Cell Counting and Fold Growth Analysis Tissues were cut into pieces and cultured in the KSFM solution under the condition of 37℃ (5% CO 2 ) for 7d. The nutrient solution was replaced every 2d. In the outgrowth measurement, explant size and outgrowth size were quantified on the 7 th day of culture with ImageJ software (200 fields; National Institutes of Health,Bethesda, MD, USA). Explant size and outgrowth size were both measured with ImageJ software. Fold growth was defined as outgrowth size/explant size (magnification ×40).
Immunofluorescence Staining Immunofluorescence staining was performed as described in our previous research [10] . The tissue specimens were put in optimal cutting temperature(OCT) compound and stored at -80℃. Cryostat sections(10 μm) were fixed in 4% paraformaldehyde for 15min and washed three times in PBS. Then they were placed in 0.2%Triton X-100 for 15min and then rinsed 3 times with PBS for 5min each. After that, 3% BSA were added for 1h to avoid nonspecific binding. Subsequently, the sections were covered with primary antibodies at different dilutions (CK13 at 1:150,P63α and CK19 at 1:50, MUC5AC at 1:200, ABCG2, PCNA and TCF4 at 1:100) overnight at 4℃. On the second day the sections were rinsed with PBS three times for 5min each and then added with matching FITC-IgG secondary antibody and Cy3-IgG secondary antibody for 1h at 37℃ without light. After washed 3 times with PBS for 30min each, the sections were fitted with mounting medium which contains PI or DAPI. Then they were photographed with Nikon TE-2000 U Eclipse epifluorescence microscope (Nikon Instruments, Tokyo, Japan). In this experiment, we set up blank control groups in which the primary antibody is replaced by PBS. To evaluate the colony composition, cells were fixed for 20min in methanol on plates at -20℃ and then stained. The fixation and staining protocol for colony immunofluorescence was the same as frozen section staining. Colonies were observed under Nikon TE-2000 U Eclipse epifluorescence microscope (Nikon Instruments, Tokyo, Japan).
Table 1 Primer sequence pairs used for quantitative real-time PCR

Real-time Polymerase Chain Reaction In our experiment,RNA was acquired from epitheliums of different parts of the pterygium with TRIzol (Invitrogen, Carlsbad, CA, USA).Concentrations and parameters of RNA were inspected by NanoDrop 1000TM (Thermo Fisher Scientific, Waltham, MA,USA). Equivalent amount of RNA was reverse transcribed to cDNA with ExScript RT Reagent kit. Referring to the instructions, real-time polymerase chain reaction (PCR) was processed with a Step One Real-Time PCR detection system(Applied Biosystems, Darmstadt, Germany) with SYBR Premix Ex Taq Kit (Takara Bio, Otsu, Shiga, Japan). In each test, RT-minus controls ( i.e. RNA samples that are dealt with similarly except for the addition of reverse transcriptase) and template-minus controls were also taken in as negative controls for the following PCR reactions. The expanding program included a denaturation step firstly at 95℃ for 10s, followed by another denaturation process at 95℃ for 15s. Then an annealing and extending process at 60℃ for 35s and for 40 cycles was performed. After each extending step, the SYBR Green fluorescence was measured. The specificity of extension was assessed by the melting curve analysis. The results of the related quantitative real-time PCR were assessed with the comparative CT method. GAPDH was used as the internal control. The primer sequence used for PCR are listed in the Table 1 below.
Microscopy and Image Analysis For immunofluorescence analysis, pictures were taken at room temperature using the fluorochromes DAPI, FITC-IgG (green), and Cy3-IgG(red). Confocal pictures were taken with a Zeiss Meta LSM 510 microscope (Carl Zeiss, Jena, Germany; software: Zen 2009; objectives: 10×/0.3; 20×/0.5; 40×/1.3 oil; 63×/1.4 oil).Fluorescence pictures were taken with an Olympus BX-53 microscope (software: Applied Precision Software; the magnifications: 20 /0.75; 10×/0.40; 40×/1.35 oil; 60×/1.42 oil).In the studies of explant proliferation and clonal analysis,bright field microscopy was done with an Olympus BX-53 microscope (the software: Olympus Cell D; the objectives:10×/0.4; 20×/0.75; 40×/0.4). Cell and explant culture pictures were made in an Olympus E-620 camera. Photoshop(CS6, Adobe) and Image Pro Plus V 6.0 were used only for additional picture processing. No image medium was involved.For illustration purposes, pictures were adjusted with the level and brightness/contrast tools in the Photoshop software; the constant adjustments were implemented to every pixel in each of the RGB channels.
Statistical Analysis In all the experiments, at least 3 independent experiments were done in each group. Fold growth and clone forming efficiency (CFE) were compared with one-way analysis of variance (ANOVA) and the Student-Newman-Keuls (SNK) q test. All statistical analyses were finished with SPSS Version 18.0(SPSS, Chicago, IL) and Prism 6.0 (GraphPad Software, La Jolla,CA, USA). Data are reported as means±standard deviation (SD)unless specially stated, and significance was established at P <0.05. Error bars show the SD of the mean.
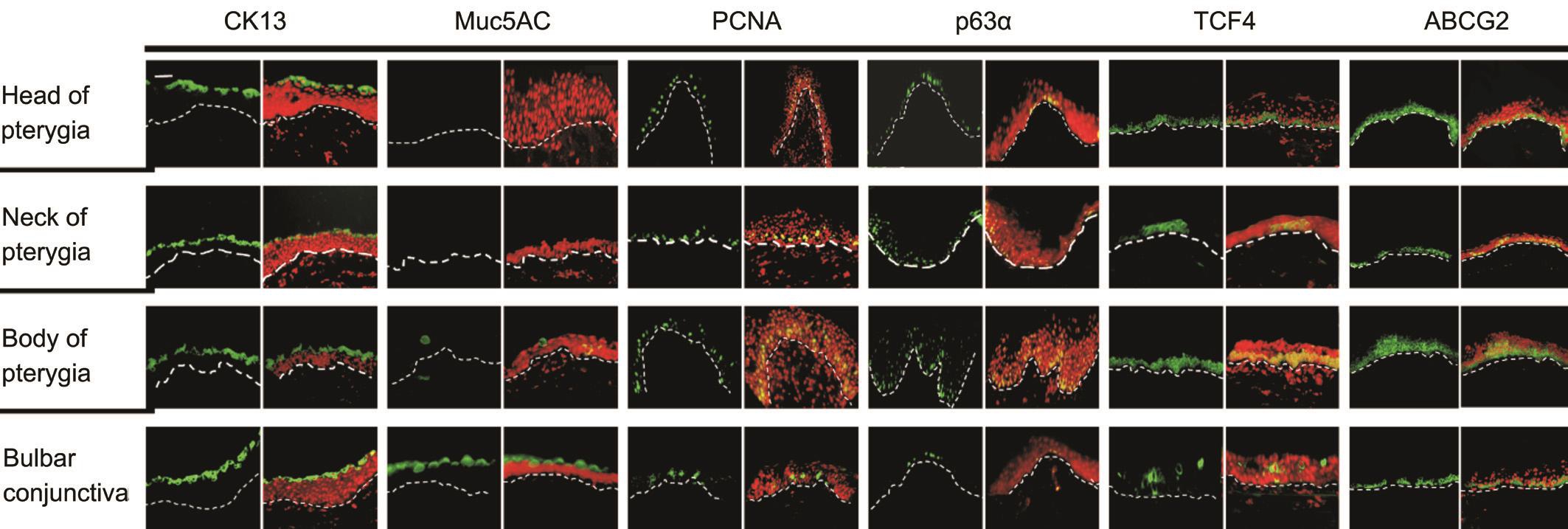
Figure 1 Expression profile of CK13, Muc5AC, PCNA, p63α, TCF4 and ABCG2 in head, neck and body pterygial epithelium The green color indicates the expression of markers. The red color indicates PI combined with cell nucleis. The dotted line marks the basal membrane.Scale bars: 20 μm.

Figure 2 The expression of TCF4 and other proliferation-related proteins (ABCG2, PCNA, and p63α) in pterygial epithelium A: RT-qPCR result of p63α, PCNA and TCF4 gene expression. The result reveals a significant difference of p63α, PCNA and TCF4 expression in pterygial head, neck and body epithelium; B: The double-staining result of TCF4 and another proliferation related marker (ABCG2 and PCNA) in pterygial body epithelium. The proliferation related markers (ABCG2 and PCNA) were visualized in green. The red color indicates the expression of TCF4. Nuclei in the images were combined with DAPI (blue). White dotted lines represent the basal membrane. Scale bars: 20 μm. a P <0.05, b P <0.01.
Proliferation Related Indicators Expressed in Head, Neck and Body Parts of Pterygium Epithelium We detected 2 conjunctival biomarkers (CK13, MUC5AC) and confirmed that epithelium of pterygium origins from conjunctiva (Figure 1).Cytokeratin 13 (CK13), which represents conjunctival epithelium [12] , presented positive expression in the superficial epithelial cells of pterygial head, neck and body (Figure 1).MUC5AC, considered as a molecular marker of goblet cells [13] ,showed positive expression in the superficial layer of conjunctival epithelium while presented nearly complete absence in all the pterygial epithelium. The result indicates that pterygium may possess very low mucin-producting function.Four proliferative markers (p63α, PCNA, ABCG2 and TCF4)were detected in different parts of pterygial epithelium (Figure 1).P63α is a type of nucleoprotein considered as a marker of proliferative potential [14-15] . A small amount of p63α positive cells were found in the basal and suprabasal layer of the normal conjunctival epithelium sporadically. The expression of p63α increased dramatically in the basal epithelium of body pterygium while decreased significantly in the neck of pterygial epithelium (Figure 1). PCNA protein is a highaccurate and sensitive indicator reflecting cell proliferative state intuitively [16] . Sporadic PCNA positive cells were found in suprabasal epithelial layer of conjunctiva. The expression increased in the body pterygial epithelium and decreased in head pterygial epithelium (Figure 1). ABCG2 is a marker of stemness and also considered as a proliferative marker.Expression of ABCG2 in basal layer of pterygial epithelium is highest in pterygial body while lowest in pterygial neck(Figure 1). Strong TCF4 expression was also found in the basal layer of pterygial epithelium. The expression was mostly in pterygial body epithelium while barely seen in pterygial neck(Figure 1). This expression pattern of proliferative markers in the pterygial epithelium revealed that highly proliferative cells were dispersed deep in the basal and suprabasal epithelium of body pterygium. Notably, the pattern of TCF4 positivity was similar to that of p63α/PCNA within different part of pterygial epithelium, which were all increased dramatically in pterygial body epithelium and decreased in neck of pterygium.Moreover, in body pterygial epithelium, the positive cells of proliferative biomarker were much more than those in normal conjunctival epithelium. This indicated that the proliferative potential of pterygial epithelium is higher than that of normal conjunctival epithelium.
To verify this unique pattern of proliferative biomarkers expression in different part of pterygial epithelia, the levels of p63α, PCNA and TCF4 mRNA in head, neck or body pterygial epithelia were evaluated by reverse transcriptionquantitative real-time PCR (RT-qPCR) with GAPDH as an internal control. The results confirmed that levels of p63α,PCNA and TCF4 mRNA expression in pterygial body epithelia were significantly higher than those in head or neck pterygial epithelia ( P <0.05; Figure 2A).
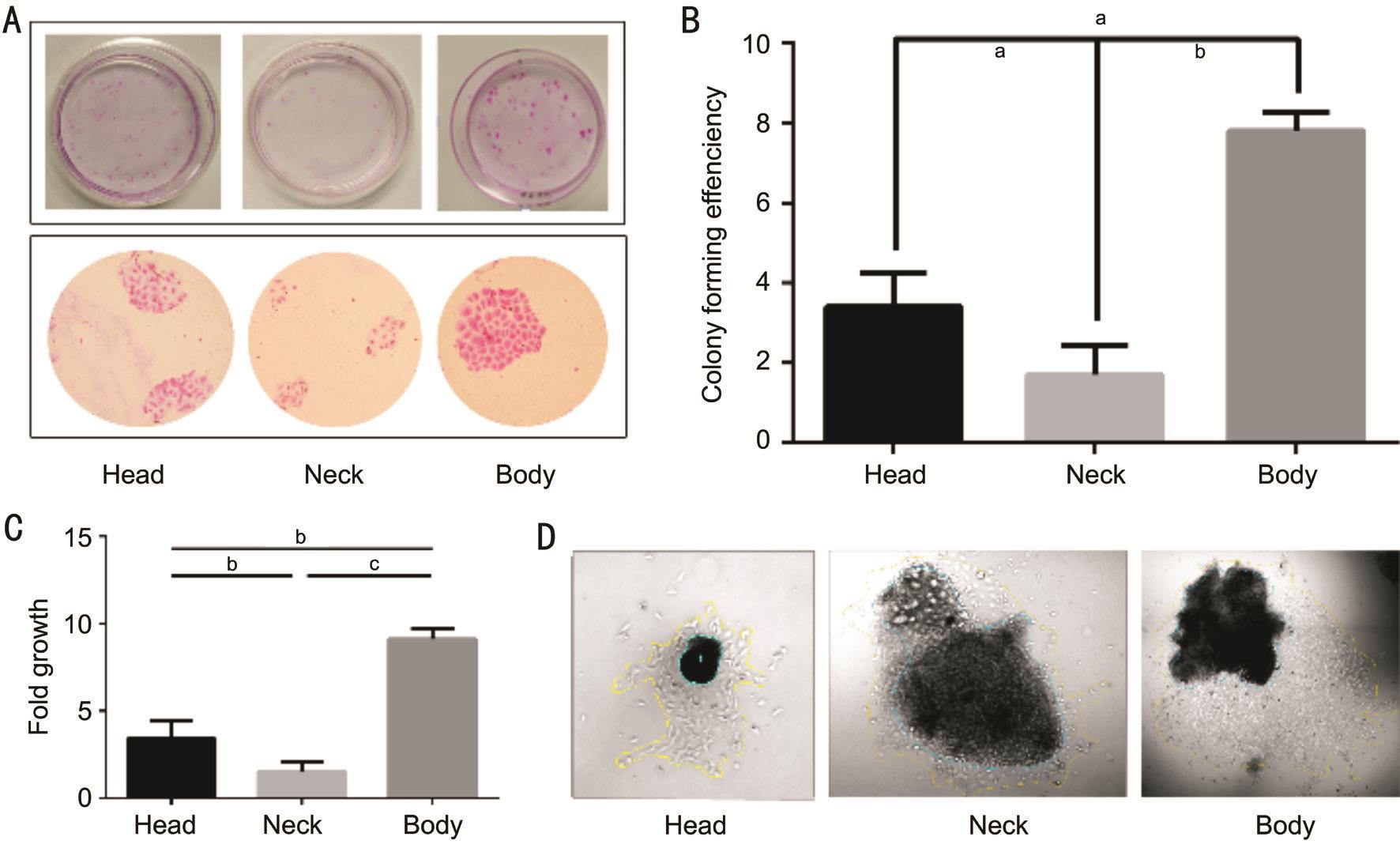
Figure 3 The proliferative capacity of pterygial epithelial cells in vitro A: Colonies formed by cells isolated from different parts of pterygial epithelium stained with Rhodamine B; B: Clone-forming capacity of cells isolated from different parts of pterygial epithelium. The bar chart reveals the colony forming efficiency (CFE) of the cells isolated from different parts of pterygial epithelium ( a P <0.05, b P <0.01). C: Comparision of fold growth of the tissue explants from different regions of the pterygial epithelium ( a P <0.05, b P <0.01, c P <0.001). D: Outgrowth of tissue explants from 3 different pterygial regions. Yellow line represents the boundary of the outgrowth of epithelial cells. Green line shows the boundary of tissue explant. The outgrowth sizes were measured with ImageJ software (magnification: ×40).
For further study, we selected pterygium body, which possesses the highest proliferative capacity, to process the immunofluorescence double staining test with TCF4 and the other proliferation related marker (ABCG2 or PCNA) (Figure 2B).We found that TCF4 were strongly positive in clusters or in interspersed single cells in the basal and suprabasal pterygial epithelia and followed the expression pattern of the putative stem cell-related marker ABCG2 and proliferative marker PCNA. We suggested that the most proliferative cells of pterygial epithelia resided within the basal and suparbasal layers in body of pterygial epithelium.
Proliferative Capacity of Pterygium Epithelium in vitro To assess whether cells isolated from the pterygial epithelium would efficiently proliferate in vitro , we isolated single cell from head, neck and body of pterygial epithelium and quantified their self-renewal capacity by clonal assays (Figure 3A, 3B). Clones can be classified into three types. Holoclone possesses the highest proliferative capacity while meroclone and paraclone own intermediate and the lowest separately [17] .In this experiment, the clones produced by head or body of pterygial epithelium were mainly holoclones. The clones produced by neck pterygial epithelium were mainly paraclones with irregular shape. CFE represented proliferative potential of cells. CFE of head pterygial epithelium was 3.4%±0.85%,CFE of neck pterygial epithelium was 1.7%±0.73% and CFE of body pterygial epithelium was 7.8±0.45% (Figure 3B). It can be noticed directly that clones formed by body pterygial epithelial cells possessed the largest average area (Figure 3A). In contrast, clones from pterygial neck epithelial cells possessed the smallest (Figure 3A). Number and average size of colonies can represent proliferation speed relatively. Therefore, these findings demonstrated that pterygial body epithelium cells may be endowed with the highest proliferative capacity in vitro .
To further confirm the proliferative potential, we compared the explant outgrowth fold from different part of pterygial epithelium (Figure 3C, 3D). Consistent with clonal analysis,the most effective expansion of pterygial epithelial monolayers was achieved by tissue explant from the body pterygial epithelia (Figure 3C).
Immunofluorescence staining of clone cells after 5d in culture demonstrated that the expression of TCF4 during propagation in vitro more clearly. TCF4 was highly co-expressed with the proliferation signature PCNA (Figure 4A). The expression of PCNA, a ring-shaped protein that encircles DNA, is indicative of the proliferative state of cells. Notably, cytoplasmic TCF4 showed stronger positivity with increasing eccentric distance as the peripheral area of the colony expanded (Figure 4B).
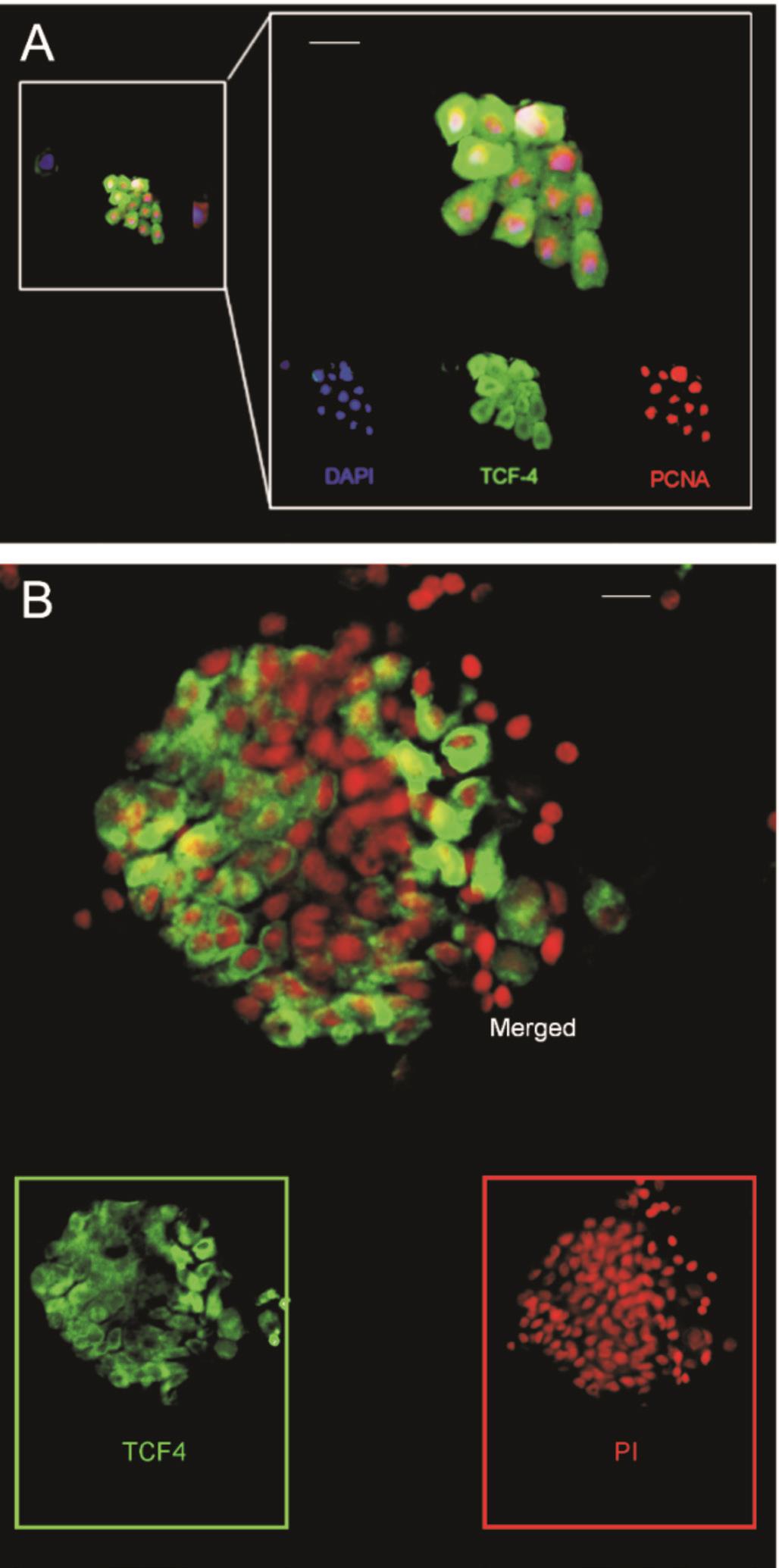
Figure 4 Immunofluorescence shows the expression of TCF4 on 5 th and 9 th day of culture A: On the 5 th day of cloning, the expression of TCF4 and PCNA in the pterygial body epithelial cells. Green:TCF4 positive. Red: PCNA positive. Blue represents the nucleus. The result indicates that on the 5 th day, TCF4 was positive in most cells in the clone and the expressing mode is similar to PCNA. Scale bars: 20 μm.B: On the 9 th day of cloning culture, the expression of TCF4 in the holoclone of pterygial body epithelium. The positive expression of TCF4 is showed in green. Nucleus were stained with PI (in red). Scale bars: 20 μm.
Pterygium is an ocular surface illness with high proliferative behavior. The proliferation of pterygium may be a critical factor for its occurrence and development. However, the underlying mechanisms involved in pterygium proliferation remain unknown. In the present study, we characterized the phenotype of head, neck and body part of pterygium epithelial cells and evaluated the proliferative potential with clonal assays. The present study revealed that the basal and suprabasal epithelium of body pterygium localized the most proliferative biomarkers positive cells than head and neck pterygial epithelium, and the body pterygial epithelial cells showed the highest proliferative capacity in vitro . Moreover,TCF4, the key transcript factor in Wnt signaling, revealed highly correlated with proliferation-related markers (ABCG2 and PCNA) in body pterygial epithelium. Meanwhile, TCF4 revealed strong positive expression in colonies from body single cells and coexpression with PCNA. All these findings identified that the body pterygial epithelium occupies the most proliferative capacity, and TCF4 may be associated with pterygial proliferation.
CK13 marks conjunctival epithelial cells while MUC5AC protein indicates goblet cells. According to the result, CK13 shows similar expressing pattern in epithelium of pterygium and conjunctiva. This indicates that the pterygial epithelial cells may origin from conjunctival epithelial cells. It is also found that the expression of MUC5AC reduces in all layers of the epithelium, which proves decrease of goblet cells in the pterygial epithelium. The results indicate that though pterygium origins from conjunctival epithelium, its characteristic changes to some extent. PCNA and p63α are associated with proliferative status. P63α, a nucleoprotein,plays an important role in the proliferation and advance of epithelial tissues [18] . King et al [19] found through experiment in vitro that rats’ p63α positive cells can maintain the proliferative state. In the study of zebra fish, the result indicated that p63α is necessary for the proliferation of epithelial cells [20] . Meanwhile,researchers found that p63α leads to over proliferation and the delay of terminal differentiation [21] . In terms of ocular surface,Pellegrini et al [22] found that high expression of p63α can be found in stem cells at corneal cells in limbal basal layer.Moreover, Dua et al [23] found that the expression of p63α presented not only in stem cells, but also in transit amplifying cells with high proliferative capacity. p63α may be an indicator of stem cell potential [24] . In our experiment, through the immunofluorescence staining, we found that there is p63α expression in pterygial epithelial cells. The expression is higher in pterygial body than in head and neck. These results indicated that pterygial body may be the region mainly responsible for the high proliferative capacity of pterygium. PCNA, an acidic nonhistone protein in the nucleus, is a crucial nuclear antigen of cell proliferation. It plays a major role in DNA replication,cell division and proliferation [25] . The expression of PCNA can be an indicator for cell proliferating state. It is clear that the result of immunofluorescence staining that PCNA possess similar expressing pattern of p63α. The PCNA expression is high in the pterygial body epithelium, on the contrary, in the neck the expression amount decreases considerably. ABCG2,as a marker of stemness and also proliferative capacity,expressed highest in basal layer of pterygial body epithelium while lowest in pterygial neck.
The result of RT-PCR is analogous to immunofluorescence staining. PCNA and p63α were expressed highest in the pterygial body while lowest in the pterygial neck. Meanwhile,the difference between pterygial neck and head, head and body,neck and body are all statistically significant. The observations revealed that pterygial body plays the key role in the whole proliferating process of pterygium. It seems that when pterygium grows onto the cornea, its proliferative capacity will be weakened.
For further study proliferative characteristic of pterygial epithelial cells, we isolated the pterygial epithelial cells from 3 parts (head, neck and body). Through different experiments in vitro , we analyzed the proliferative capacity and self-renewing capacity of epithelial cells. Colony forming efficiency is a common index reflecting the cell proliferation. Concerning the result of clone analysis, generally the pterygial body epithelial cells show the highest CFE (7.8%±0.45%) while the pterygial neck epithelial cells reveal the lowest (1.7%±0.73%). Similarly,in single epithelial clones, cells isolated from pterygial body formed the largest average area while cells from pterygial neck formed the smallest. Moreover, from the cultural experiment of tissue explants we got the similar conclusions. Tissue explants from pterygial body showed the highest fold growth.It can be concluded that the proliferative capacity of pterygial body epithelium is stronger than head and body. This indicates that high proliferation of pterygium is mainly attributed to the pterygial body epithelium. The pterygial body may push the pterygium to grow forward and expand onto the cornea.
In our experiment, TCF4 was found to be a signature indicating highly proliferative capacity of pterygial epithelial cells. TCF4 is an influential member of TCF/LEF family. In our early researches, we found that TCF4 may correlate with the highly proliferative potential of conjunctival epithelial cells [10] . There is no relevant research about whether TCF4 is involved in the proliferation of pterygial epithelial cells. In this experiment, positive expression of TCF4 can be found in basal pterygial epithelia, mostly in the epithelium of pterygial body.In addition, the results of immunofluorescence double staining showed high coexpression of ABCG2, PCNA and TCF4.ABCG2 positive cells are considered as of high proliferative potential. The results indicated that TCF4 may be associated with proliferation of pterygial epithelium. Moreover, to identify the relationship between TCF4 and the proliferative characteristic of pterygial epithelium, we isolated epithelial cells from pterygial body (where epithelial cells with highest proliferative capacity reside) for culture in vitro and processed the immunofluorescence double staining of TCF4 and PCNA.The result revealed that on the 5 th day, TCF4 was expressed by most cells in the clones. PCNA shows similar expressing pattern. With the prolonging of the culture time, there are more TCF4 positive cells at the boundary of the holoclones. It is commonly recognized that the small and round cells at the boundary of the clones are at minimal differentiated state and possess highest proliferative capacity [26] . The results showed that TCF4 may be related to the proliferation of pterygial epithelial cells.
In conclusion, in our research, it was found that high proliferation of pterygium is mainly owing to the pterygial body. Clinically,the operator should remove conjunctival part of the pterygium completely to avoid recurrence. Moreover, we proposed TCF4 to be a protein responsible for proliferative capacity of pterygium, which is supported by the expressing pattern of TCF4 in the pterygial epithelium and coexpression of TCF4 with proliferation-related markers (ABCG2 and PCNA). In our further research we will explore the mechanism of TCF4 on human pterygial proliferation. The research may enable us to know more about the occurrence and progress of pterygium and helps find new methods for the management of pterygium in the future.
ACKNOWLEDGEMENTS
We thank the Zhongshan Ophthalmic Center for great support in providing tissues for experiments. We thank the volunteers for their cooperation. We also thank all the staff and facilties of the Zhongshan Ophthalmic Center that supported our work.
Foundation: Supported by National Natural Science Foundation of China (No.81670823).
Conflicts of Interest: Nie C, None; Zhang XC, None; Xu SY, None; Quan YD, None; Tang ZX, None; Lu R, None.
REFERENCES
1 Cornand G. Pterygium. Clinical course and treatment. Rev Int Trach Pathol Ocul Trop Subtrop Sante Publique 1989;66(3-4):31-108.
2 Lin A, Stern G. Correlation between pterygium size and induced corneal astigmatism. Cornea 1998;17(1):28-30.
3 Kase S, Takahashi S, Sato I, Nakanishi K, Yoshida K, Ohno S. Expression of p27(KIP1) and cyclin D1, and cell proliferation in human pterygium. Br J Ophthalmol 2007;91(7):958-961.
4 Lluch S, Julio G, Pujol P, Merindano D. What biomarkers explain about pterygium OCT pattern. Graefes Arch Clin Exp Ophthalmol 2016;254(1):143-148.
5 Chen YT, Tseng SH, Tsai YY, Huang FC, Tseng SY. Distribution of vimentin-expressing cells in pterygium: an immunocytochemical study of impression cytology specimens. Cornea 2009;28(5):547-552.
6 Ljubojevic V, Gajanin R, Amidzic L, Vujkovic Z. The expression and significance of p53 protein and Ki-67 protein in pterygium. Vojnosanit Pregl 2016;73(1):16-20.
7 Kase S, Takahashi S, Sato I, Nakanishi K, Yoshida K, Ohno S.Expression of p27(KIP1) and cyclin D1, and cell proliferation in human pterygium. Br J Ophthalmol 2007;91(7):958-961.
8 Helfer G, Tups A. Hypothalamic Wnt signalling and its role in energy balance regulation. J Neuroendocrinol 2016;28(3):12368.
9 Nguyen H, Merrill BJ, Polak L, Nikolova M, Rendl M, Shaver TM, Pasolli HA, Fuchs E. Tcf3 and Tcf4 are essential for long-term homeostasis of skin epithelia. Nat Genet 2009;41(10):1068-1075.
10 Quan Y, Zhang X, Xu S, Li K, Zhu F, Li Q, Cai X, Lu R. Tcf7l2 localization of putative stem/progenitor cells in mouse conjunctiva. Am J Physiol Cell Physiol 2016;311(2):C246-C254.
11 Li J, Li C, Wang G, Liu Z, Chen P, Yang Q, Dong N, Wu H, Liu Z, Li W. APR-246/PRIMA-1Met inhibits and reverses squamous metaplasia in human conjunctival epithelium. Invest Ophthalmol Vis Sci 2016;57(2):444-452.
12 Wakae H, Higashide T, Tsuneyama K, Nakamura T, Takahashi K,Sugiyama K. Immunohistochemical characterization of the ectopic epithelium devoid of goblet cells from a posttraumatic iris cyst causing mucogenic glaucoma. J Glaucoma 2016;25(3):e291-e294.
13 Zhang X, De Paiva CS, Su Z, Volpe EA, Li DQ, Pflugfelder SC.Topical interferon-gamma neutralization prevents conjunctival goblet cell loss in experimental murine dry eye. Exp Eye Res 2014;118:117-124.
14 Keyes WM, Pecoraro M, Aranda V, Vernersson-Lindahl E, Li W,Vogel H, Guo X, Garcia EL, Michurina TV, Enikolopov G, Muthuswamy SK, Mills AA. DeltaNp63alpha is an oncogene that targets chromatin remodeler Lsh to drive skin stem cell proliferation and tumorigenesis. Cell Stem Cell 2011;8(2):164-176.
15 Yoon JJ, Ismail S, Sherwin T. Limbal stem cells: Central concepts of corneal epithelial homeostasis. World J Stem Cells 2014;6(4):391-403.
16 Liu T, Wang Y, Duan HY, Qu ML, Yang LL, Xu YY, Zang XJ, Zhou QJ. Effects of preservation time on proliferative potential of human limbal stem/progenitor cells. Int J Ophthalmol 2012;5(5):549-554.
17 Wei ZG, Lin T, Sun TT, Lavker RM. Clonal analysis of the in vivo differentiation potential of keratinocytes. Invest Ophthalmol Vis Sci 1997;38(3):753-761.
18 Nair RP, Krishnan LK. Identification of p63+ keratinocyte progenitor cells in circulation and their matrix-directed differentiation to epithelial cells. Stem Cell Res Ther 2013;4(2):38.
19 King KE, Ponnamperuma RM, Yamashita T, Tokino T, Lee LA, Young MF, Weinberg WC. deltaNp63alpha functions as both a positive and a negative transcriptional regulator and blocks in vitro differentiation of murine keratinocytes. Oncogene 2003;22(23):3635-3644.
20 Westfall MD, Mays DJ, Sniezek JC, Pietenpol JA. The Delta Np63 alpha phosphoprotein binds the p21 and 14-3-3 sigma promoters in vivo and has transcriptional repressor activity that is reduced by Hay-Wells syndrome-derived mutations. Mol Cell Biol 2003;23(7):2264-2276.
21 Romano RA, Ortt K, Birkaya B, Smalley K, Sinha S. An active role of the DeltaN isoform of p63 in regulating basal keratin genes K5 and K14 and directing epidermal cell fate. PLoS One 2009;4(5):e5623.
22 Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A 2001;98(6):3156-3161.
23 Dua HS, Joseph A, Shanmuganathan VA, Jones RE. Stem cell differentiation and the effects of deficiency. Eye (Lond) 2003;17(8):877-885.
24 Stewart RM, Sheridan CM, Hiscott PS, Czanner G, Kaye SB. Human conjunctival stem cells are predominantly located in the medial canthal and inferior forniceal areas. Invest Ophthalmol Vis Sci 2015;56(3):2021-2030.
25 Melo RM, Martins YS, Luz RK, Rizzo E, Bazzoli N. PCNA and apoptosis during post-spawning ovarian remodeling in the teleost Oreochromis niloticus. Tissue Cell 2015;47(6):541-549.
26 Thangappan R, Kurzrock EA. Three clonal types of urothelium with different capacities for replication. Cell Prolif 2009;42(6):770-779.
Citation: Nie C, Zhang XC, Xu SY, Quan YD, Tang ZX, Lu R. Pterygial body epithelium domination of pterygial proliferation with TCF4 as a potential key factor. Int J Ophthalmol 2018;11(9):1467-1474
Received: 2018-01-04 Accepted: 2018-04-08
DOl: 10.18240/ijo.2018.09.07
Abstract · AlM: To characterize the proliferative capacity of pterygial epithelium in different regions (head, neck and body) of pterygium and explore the function of transcription factor 4 (TCF4) in pterygium proliferation.· METHODS: Thirty pterygium tissues and 10 normal conjunctival tissues were obtained from Zhongshan Ophthalmic Center (ZOC) and Guangdong Eye Bank,respectively. Proliferative capacity of head, neck and body in pterygial epithelium was measured using clonal analysis, fold growth analysis and expression profile of proliferative markers revealed by immunofluorescent staining and real-time PCR. The expression of TCF4 was highlighted by double immunofluorescent staining with other proliferation related markers such as proliferating cell nuclear antigen (PCNA) and ATP-binding cassette subfamily G member 2 (ABCG2).· RESULTS: The proliferative potential of pterygial epithelium was higher than that of normal conjunctival epithelium.High expression levels of proliferative markers (P63α,PCNA and ABCG2) in pterygial body epithelium were observed in immunofluorescent staining and real-time PCR ( P <0.05). Also, epithelial cells isolated from pterygial body demonstrated higher proliferative capacity in clonal analysis and fold growth analysis, than those isolated from the head and neck regions. The TCF4 expression in pterygial epithelium was similar to other proliferative markers (P63α,PCNA and ABCG2), as higher in pterygial body than head and neck. Moreover, TCF4 showed coexpression with other proliferation-related markers (PCNA and ABCG2) in the double immunofluorescent staining experiment.· CONCLUSlON: The proliferative capacity in pterygial body epithelium is prominent than the head and neck regions, and upregulated TCF4 may be associated with enhanced proliferation in the pterygium.
Correspondence to: Rong Lu. State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yatsen University, South Xianlie Road, Guangzhou 510060,Guangdong Province, China. lurong@gzzoc.com; rongluzz@yahoo.com
Co-first authors: Cong Nie and Xin-Chun Zhang