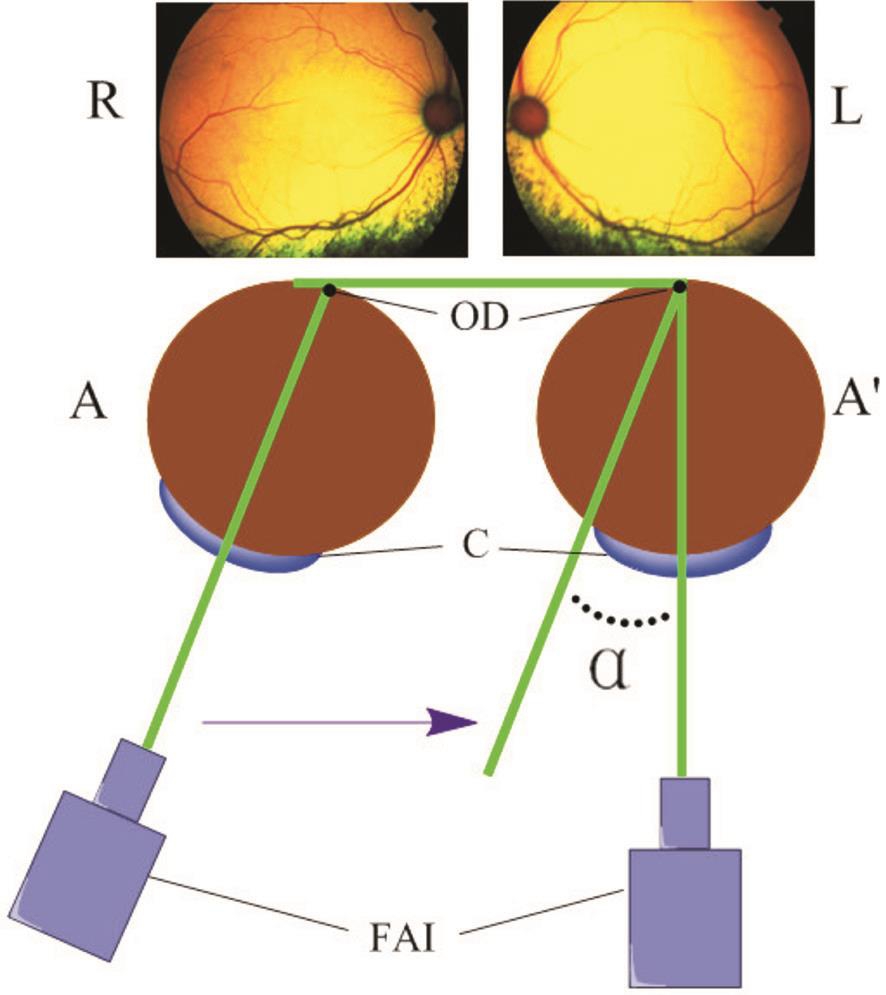
Figure 1 Measurement of ocular alignment R: Right eye; L: Left eye; A: Position of eye before injury; A’: Position of eye after injury.OD: Optic disc; C: Cornea; FAI: Fundus angiography instrument.
Jun-Jie Zhi 1,2 , Hong Yan 1,3 , Li-Hua Sun 3
1 Department of Ophthalmology, Xi’an No. 4 Hospital, Shaanxi Eye Hospital, Affiliated Guangren Hospital School of Medicine,Xi’an Jiaotong University, Xi’an 710004, Shaanxi Province, China
2 Department of Ophthalmology, No. 422 Hospital of PLA,Zhanjiang 524009, Guangdong Province, China
3 Department of Ophthalmology, Tangdu Hospital, Fourth Military Medical University, Xi’an 710038, Shaanxi Province, China
Abstract · AlM: To describe an acute extraocular muscle injury model in cats.· METHODS: Seventy-two cats were randomly divided into 6 groups (12 cats per group). Cats’ left lateral recti were clamped using a surgical needle holder with a clamping strength of 2 (Groups A and D), 4 (Groups B and E) and 6 kg(Groups C and F). The right lateral recti were treated as controls. On the 4th and 7th days, hematoxylin eosin (HE)staining, immunohistochemical staining for proliferating cell nuclear antigen (PCNA), muscle force measurements and ocular alignment changes were performed to evaluate the extent of injuries.· RESULTS: The morphological changes were graded as mild, moderate or severe by HE staining in all experiment groups. PCNA immunohistochemical staining indicated repairment of muscle fibers in the damaged area. On the 4th and 7th days after clamping, the injured lateral muscle exhibited an elevated threshold for electric stimulation.The muscle forces among groups 2, 4 and 6 kg injury at 4d(Groups A, B and C) were statistically significant (P<0.05),but no significant differences were noted among groups 2, 4 and 6 kg injury at 7d (Groups D, E and F) (P>0.05),respectively. ln addition, medial deviation in ocular alignment was also present to various degrees in all groups.· CONCLUSlON: A cat model of acute extraocular muscle injury can be established by rectus clamping. Different clamping strengths can make different degrees of muscle injury. This model may help the future study in the acute extraocular muscle injury.
· KEYWORDS: clamping; acute extraocular muscle injury;cat; deviation
Acute extraocular muscle injury is one of the most frequently observed clinical ocular problems. This condition is caused by orbital and craniocerebral trauma,and 15% of extraocular muscle paralysis cases result from this type of injury [1-3] . Ocular trauma may cause muscle tear or incarceration, which leads to restrictive strabismus and paralytic strabismus. Without appropriate treatment,extraocular muscle injury may lead to permanent extraocular muscle paralysis and atrophy and eventually diplopia and compensatory head posture [4] . These symptoms may disturb normal work and daily life. The current treatments for acute extraocular muscle injury include medication facilitating microcirculation, nerve nourishment and surgical repair.Despite the promise of treatment, serious damage to the extraocular muscle eventually leads to perpetual paralysis [5] .Therefore, studies to identify better therapies are warranted.Drug development and basic research studies require an appropriate and effective animal model. Several methods have been applied to establish models for skeletal muscle injury,such as muscle stretching, electric stimulation, downhillrunning, blunting and ischemia/reperfusion [6-10] . However,their intrinsic shortcomings restrict their implementation in extraocular muscles injury, for instance, extraocular muscles are hard to stretch in eye orbits, electric stimulation needs to isolate nerves and then subject to electric stimuli, etc . Thus an easy and convenient extraocular muscles injury model is desperately needed. In our previous study, we established a rabbit model of acute extraocular injury via clamping, and the extent of injury was evaluated [11] . However, the existing model has shortcomings, as prolonged clamping in the rabbit model may lead to damage that is similar to an ischemia/reperfusion injury rather than a traumatic injury. Rabbits have no binocular vision, which is essential for observing eye position. Therefore,cats were selected for this acute extraocular injury model given that their combined visual field is similar to that of human beings. The extent of injury was evaluated by measuring morphological changes, precursor cell proliferation, muscular strength and ocular alignment.
Animal Model Seventy-two cats ranged from 3 to 4mo in age and from 0.8 to 1.2 kg in weight were randomly divided into 6 groups ( n =12 each, Groups A to F) regardless of sex. All cats in the 6 groups were subjected to different degrees of injury using the same method, and 6 cats from each group were exclusively used to measure muscular strength. Procedures involving animals and their care were conducted in conformity with institutional guidelines. All animals involved in the study were handled in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
After 1% phenobarbital sodium (30 mg/kg) was injected into the abdominal cavity, the cat was strapped to the operation table. Next, the fur was cut open from the outer canthal angle of left eye for approximately 7 mm. After the conjunctiva sac was sheared and the fascia was set aside, the lateral rectus was pulled outward to unfold completely. The lateral rectus was clamped with a 12.5 cm needle holder for 15s, and the clamping strength was set as 2 kg (Groups A and D), 4 kg(Groups B and E) and 6 kg (Groups C and F) as previously described [11] . The clamping position is at the rectus belly,backward the sclera insertion of cat’s lateral rectus about 3-4 mm long, and the clamping plane is 7 mm in length and 4 mm in width. After clamping, the lateral rectus was placed in its original position, and the conjunctiva sac and fur were stitched.Erythromycin ointment (Shanghai General Pharmaceutical Co., Ltd., China) was applied to the surgical incision. Over the subsequent 3d, chloramphenicol eye drops (Shijiazhuang Gerui Pharmaceutical Co., Ltd., China) were administered 4 times daily, and erythromycin ointment was applied to the left eye once nightly for infection prophylaxis.
Measurement of Ocular Alignment To assess ocular alignment,the general methods used in this study were based on those described by previous studies [12-15] . Thirty minutes before measurement, compound tropicamide eye drops (Shenyang Sinqi Pharmaceutical Co., Ltd., China) were administered to both eyes. After the administration of 1% phenobarbital sodium(30 mg/kg) anesthesia, cats were placed in a fixed position. The cat’s two eyes were maintained in a parallel position to obtain fundus photographs using the fundus angiography instrument(TRC-50EX, Topcon, Japan).
First, the photographic lens was moved slowly and carefully until the optic disc was on the nasal side and the edge of the fundus photographs. The angle between the Fundus angiography instrument and the median line was formed by projecting onto the horizontal plane (Figure 1). Then,the angle was measured before and after the injury. The difference between these two angles (angle α) was determined.Eventually, the sum of the binocular degrees of angles α was obtained to determine the angle degree that was only the value of the ocular alignment after injury.

Figure 1 Measurement of ocular alignment R: Right eye; L: Left eye; A: Position of eye before injury; A’: Position of eye after injury.OD: Optic disc; C: Cornea; FAI: Fundus angiography instrument.
Light Microscope Study On the 4 th and the 7 th days after the operation, the left lateral recti of 6 cats in each group were excised (Figure 2), suspended and fixed with 4%paraformaldehyde solution. The samples were embedded in paraffin and sectioned at a 4 µm thickness. Then, the sections were rehydrated and stained with hematoxylin eosin (HE). The morphological character of the samples was examined using a light microscope (Olympus, Japan).
Immunohistochemical Examination Paraffin sections (6 animals per group) were cut at a 4 µm thickness, deparaffinized with xylene and rehydrated. After endogenous peroxidase activity was blocked with 1% H 2 O 2 for 30min at room temperature, the sections were treated with 0.01 mol/L citrate(pH=6.0) in a 500 W microwave oven for 15min for antigen retrieval. Then, the sections were incubated with animal (goat)serum for 30min. After being rinsed in phosphate buffered saline (PBS), the sections were incubated with rabbit anticat polyclonal primary antibodies (proliferating cell nuclear antigen, PCNA; Shanghai Xiangsheng Biotechnology Co.,Ltd., China) at a dilution of 1:100 in a moist chamber at 4℃overnight. After being incubated in a water bath for 45min and washed in PBS, the sections were incubated with goat anti-rabbit IgG (Beijing 4A Biotech Co., Ltd., China). After the immunohistochemical reaction was examined by light microscopy, the sections were hydrated in a graded series of ethanol. A coverslip was placed over the samples before observation under light microscopy.
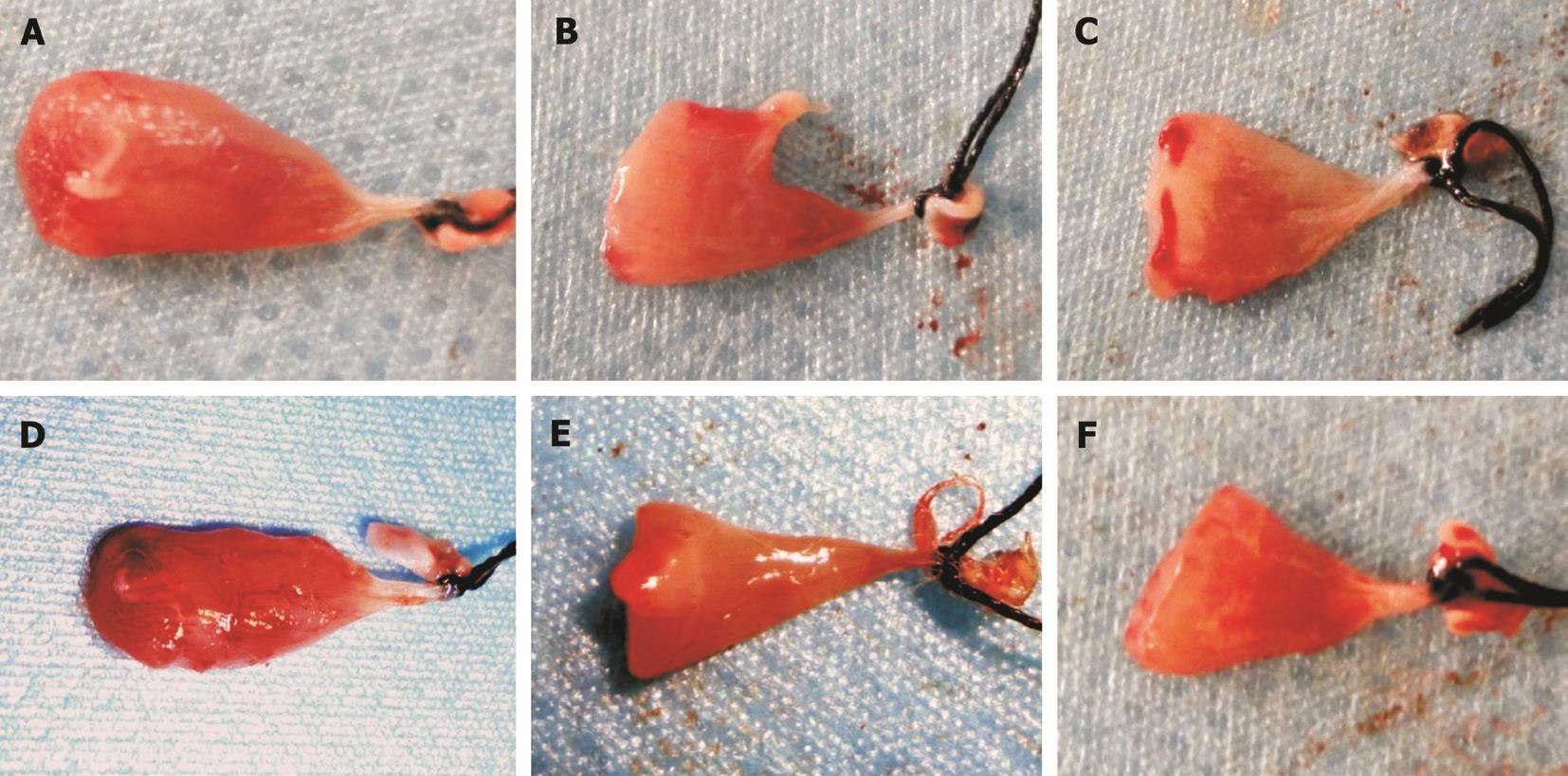
Figure 2 Lateral recti after injury A, B, C: With a clamping strength of 2, 4 and 6 kg on the 4 th day; D, E, F: With a clamping strength of 2, 4 and 6 kg on the 7 th day, respectively. The clamping position can be easily observed.
Muscle Force Measurement Muscle force measurements were performed as previously described with modification [16-19] .On day 4 (Groups A, B and C) and day 7 (Groups D, E and F)after the operation, the cats were anesthetized with 1%phenobarbital sodium. Lateral recti (36 left lateral rectus and 36 right lateral rectus, respectively) were snipped at the sclera insertion, tied with 4-0 silk thread, suspended and connected to the force transducer. The rectus was positioned vertically with the cat’s head in a fixed position. Stimulating electrodes were inserted into the rectus, and paraffin oil was applied for moisture and isolation. The force generated by the rectus was recorded in grams using the BL-420/820 organism function experiment system (Chengdu TME Technology Co., Ltd., China). The supramaximum stimulation intensity was determined by increasing the voltage (V) until maximal contraction was achieved. The primary stimulus intensity was set at 0 V, and the stimulus frequency was set at 100 Hz. The stimulus times were 100 times, with increments of 0.5 V. The muscle forces of both eyes were measured. The animals were euthanatized after measurements were obtained.
Statistical Analysis All data were analyzed using the SPSS version 16.0 software (SPSS Inc., Chicago, IL, USA). The comparison of multiple means with analysis of variance,equality of variance tests, and the pairwise comparison among the means were performed using the LSD method. All tests were two-tailed. P -values <0.05 were considered significant.
In our previous study, 1, 2, 3, 4, 5 and 6 kg strengths were applied for clamping of lateral rectus and to observe the pathology of injured muscles by HE staining from the 1 st to 14 th day. We observed the morphological changes on the 4 th day and 7 th day given that inflammatory reactions were almost distinct on the 4 th day, whereas fibrous repair peaked on the 7 th day. Based on these preliminary results, the following experiments were set all through the study.
Hematoxylin Eosin Staining Muscle fibers in the control group appeared in a regular arrangement (Figure 3A and 3B).Injured specimens obtained 4d after injury revealed structural deterioration. Hemorrhage and distended capillaries were noted between myofibers. Neutrophil infiltration, microvascular dilatation, vascular structure distortion, and vacuolization were also observed in the injured area. In samples obtained 7d after injury, edema was relieved compared with samples obtained 4d after injury. Chronic inflammatory cell infiltration was noted in the injured area.
Muscle injury can be graded as mild, moderate and severe. In moderate injuries, this grade can be further classified as lowdegree, in which muscle fibers were damaged (degeneration or broken) in less than 1/3 of the sample (Figure 3C and 3D, Figure 4A and 4B); mid-degree, in which muscle fibers were damaged in less than 2/3 of the sample (Figure 3E and 3F, Figure 4C and 4D); and heavy-degree, in which muscle fibers were damaged in greater than 2/3 of the sample but not throughout the entire sample (Figure 3G and 3H, Figure 4E and 4F). Limitation and dysfunction are also noted in this class [11,20] .
Immunohistochemical Staining Positive staining of PCNA was indicated by brown granules in the nucleus. Abundant positive nuclei were noted in the sections of the injured muscle group, whereas minimal positively stained granules were identified in the sections of the normal muscle group (Figure 5).
Measurement of Muscle Force The threshold of the electric stimulation was remarkably elevated in the injured lateral muscle (Figure 6). Stimulus thresholds of the injured lateral rectus muscles were significantly increased compared with the control lateral rectus muscles in all animals ( P A =0.004, P B =0.002, P C =0.002, P D =0.037, P E =0.014, P F =0.002; Figure 6). Changes in the threshold of electric stimulation suggested that the electrical conduction of injured muscles was influenced by clamping.
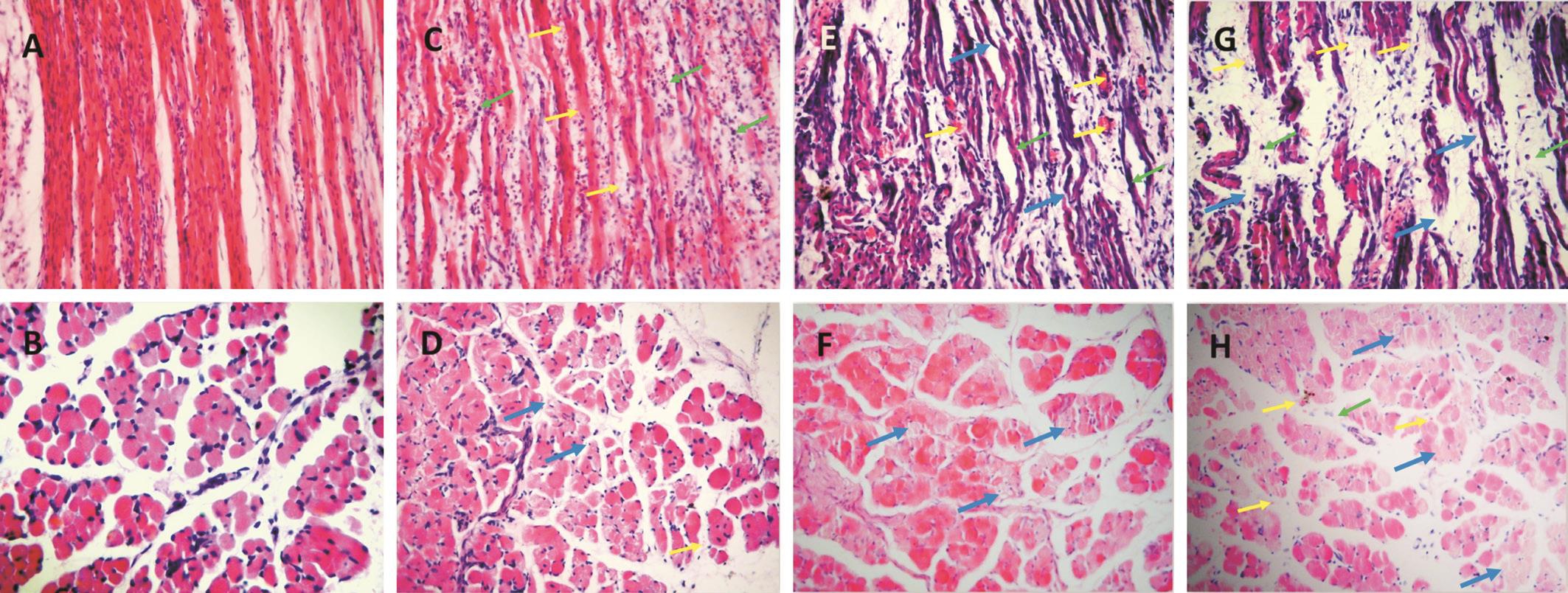
Figure 3 HE staining of lateral rectus in cat after clamping at 4 th day (200×) A, B: Normal myofibers; C, D: Mild groups (2 kg); E, F:Moderate groups (4 kg); G, H: Severe groups (6 kg). The yellow arrows indicate red blood cells, the green arrows indicate inflammatory cells,and the blue arrows indicate broken fibers.
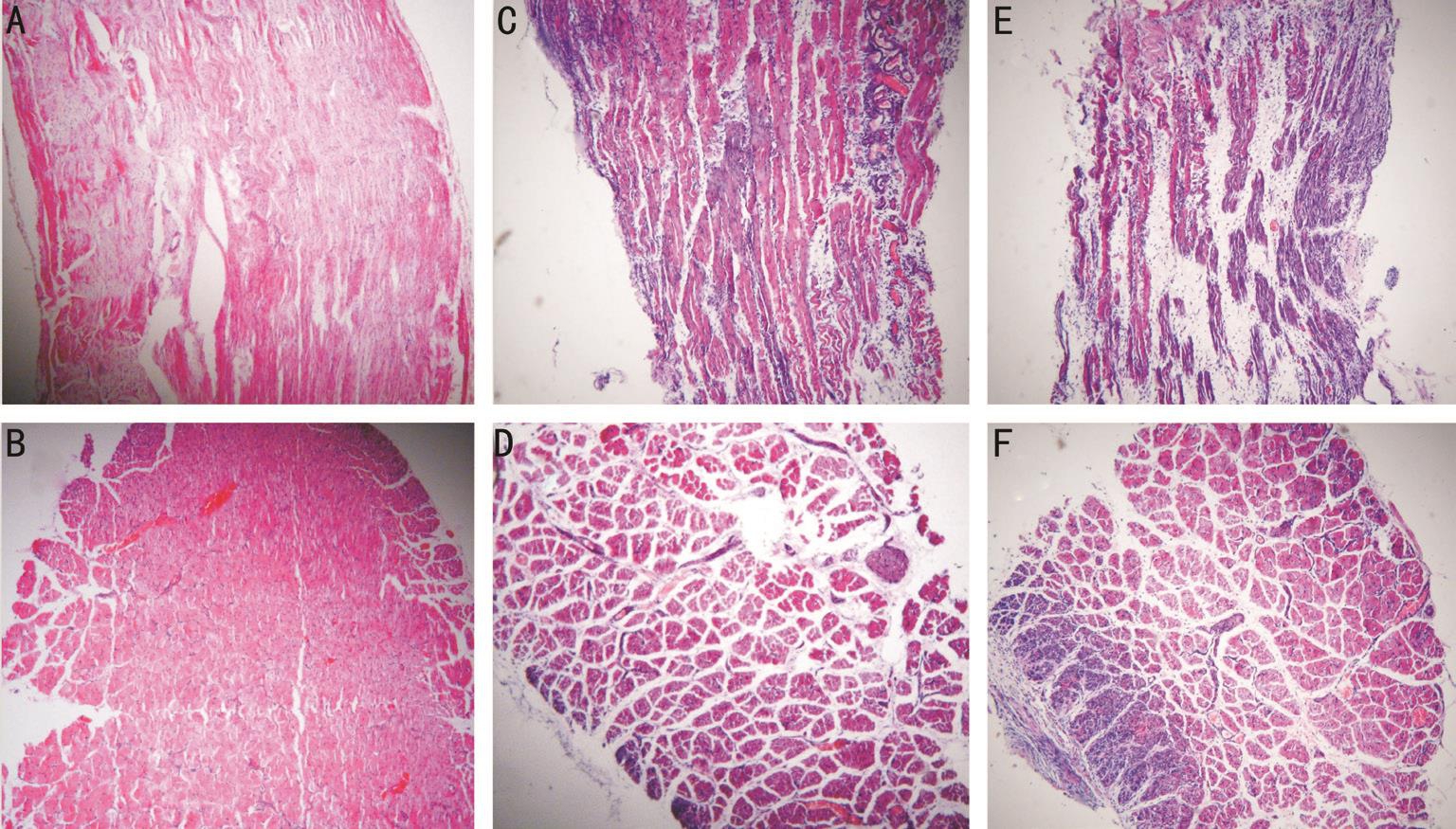
Figure 4 HE staining of lateral rectus in cat after clamping on 7 th day (100×) A, B: Mild groups (2 kg). C, D: Moderate groups (4 kg); E, F:Severe groups (6 kg).
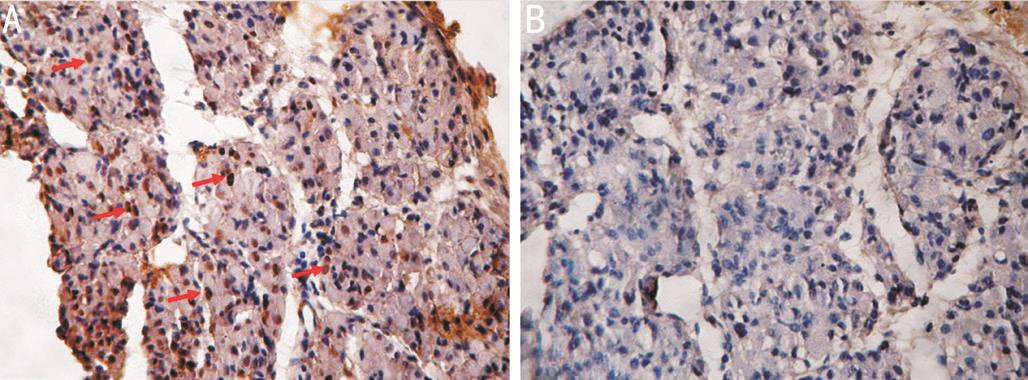
Figure 5 PCNA immunohistochemical staining after injury at 4 th day(200×) A: Positive staining. Red arrows: Positive stained nucleus; B:Controls.
The comparison of muscle force of the bilateral lateral rectus muscles in each group after injury at 4 V is shown in Figure 7. On the 4 th and 7 th days, the muscle force of the left lateral rectus was significantly reduced compared with that of the right lateral rectus in each group ( P A =0.003, P B =0.001, P C =0.001, P D =0.001, P E =0.001, P F =0.017). At 4 V, the muscle forces of left lateral recti were compared in a pairwise fashion among groups 2, 4 and 6 kg injury at 4d (Groups A, B and C), respectively. Group A exhibited significant differences from Groups B and C ( P A-B =0.021, P A-C =0.002). However, no significant differences were noted between Groups B and C( P B-C =0.053). No significant differences were noted among groups 2, 4 and 6 kg injury at 7d (Groups D, E and F)( P D-E =0.693, P D-F =0.324, P E-F =0.413), respectively.
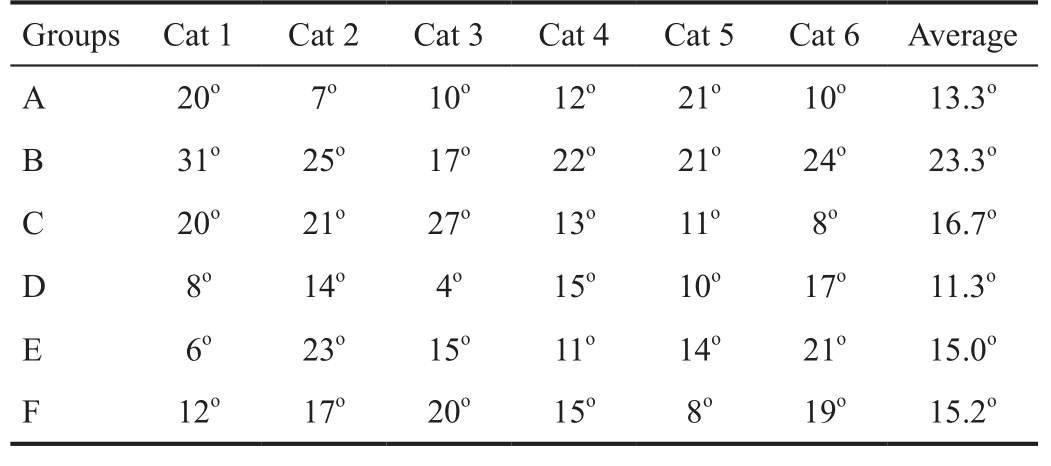
Interocular Alignment The eye position deviation of all groups is displayed in Table 1. The eye positions of cats in each group revealed varying degrees of medial deviation on either the 4 th day (Groups A, B and C) or the 7 th day (Groups D, E and F). The degree of deviation in Group A was less than that of Group B ( P A-B =0.011), and no significant differences were noted among the other groups ( P A-C =0.461, P B-C =0.130, P D-E =0.188, P D-F =0.199, P E-F =0.944). Thus, no apparent difference was noted in the extent of deviation among all three degrees of injury.
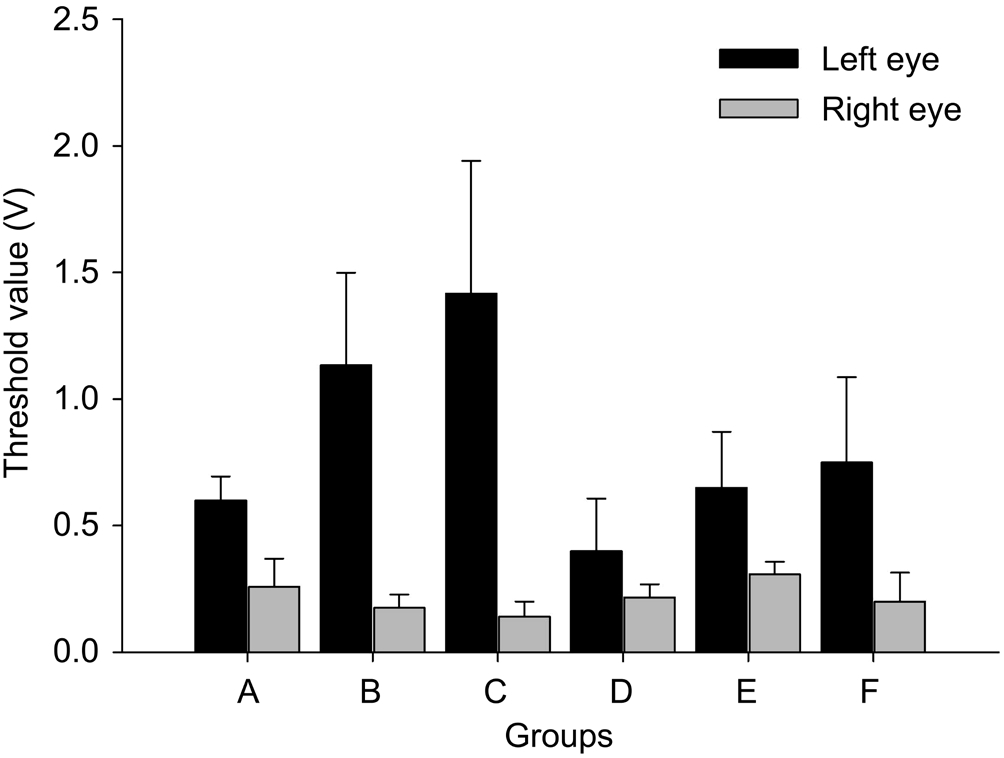
Figure 6 Comparison of stimulus thresholds between the bilateral lateral rectus muscles of cats after injury The threshold of electric stimulation was remarkably increased in the injured left lateral muscle. Stimulus thresholds of injured eyes were significantly increased compared with the control eyes in all groups ( P <0.05).
Multiple animal models of acute skeletal muscle injury have been constructed via multiple physical, chemical and biological means as above-mentioned. Establishing an acute extraocular muscle injury model can refer to that of acute skeletal muscle injury and nerve injury. However, the injuries may be excessive or variant [21-22] . Actually, the extraocular muscles are tiny, located deep in the orbital cavity, and difficult to expose, thereby rendering the methods cited above, such as blunting, unsuitable for establishing a model of extraocular muscle injury. Our previous study reported injury of the extraocular muscle by clamping with a needle holder and adjustable degrees in a rabbit model, indicating that clamping might serve as a suitable method for modeling [11] .
The rabbit model we previously established is useful for investigating the pathology and treatment after injury to a certain degree. However, clamping that depends on the length of time, is similar to the damage caused by blunt trauma and ischemia/reperfusion; this method is not completely consistent with traumatic muscle injury [4,23] . Therefore, adjusting the strength applied to the clamp muscles rather than the length of time is more suitable for introducing different degrees of injury in an acute extraocular muscle injury model.
Human beings have binocular vision given that the visual axis of the two eyes are parallel or approximately parallel, thus generating overlapping visual fields and depth perception [24] .Cats have cone-like orbital structures that are similar to humans. With regard to binocular visual function, cats also share more in common with humans than do rabbits, making cats a more suitable candidate for eye position observations.
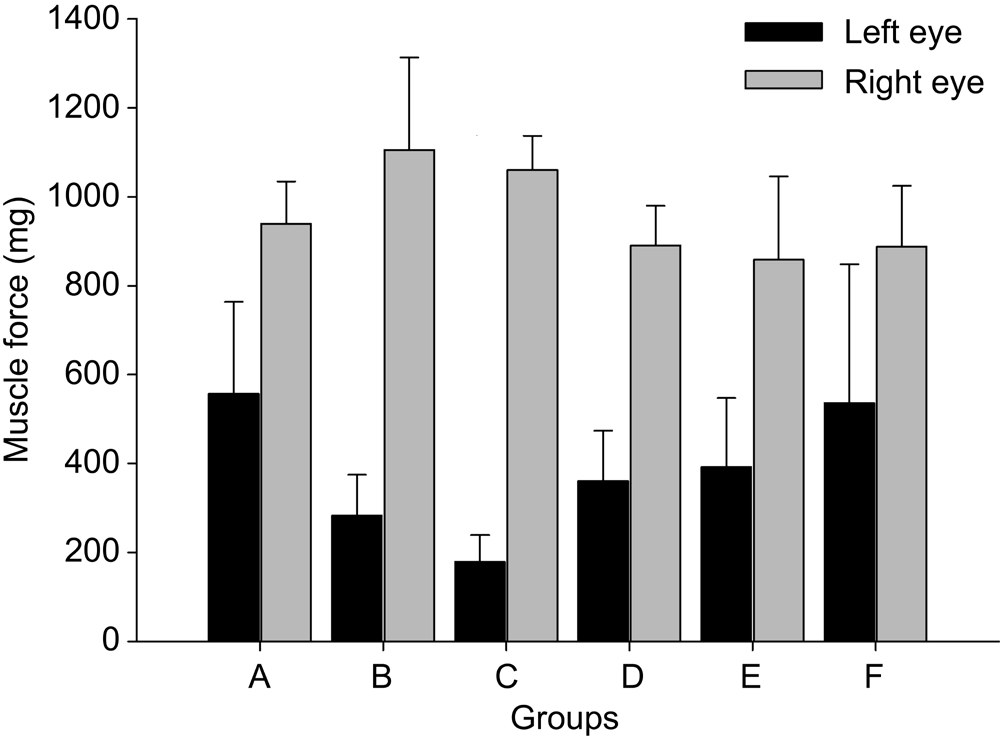
Figure 7 Comparison of muscle forces between the bilateral rectus muscles of cats at 4 V after injury At 4 V, the muscle forces of left lateral recti were significantly reduced compared with the right lateral recti in each group ( P <0.05).
Table 1 Interocular alignment of cats in the six groups after operation
Based on our previous studies, 2, 4 and 6 kg clamping strengths were selected to establish the injury model [11] . The degree of damage is correlated with the area of clamping [25] .Using the same clamping strength, a smaller area resulted in greater damage. Therefore, a universal area of clamping should be applied to all groups. An even distribution of the clamping force is also vital to achieve a similar extent of injury within a group. As showed in Figure 2, the clamping position can be easily observed on the gross specimen. The clamping area should best cover most of the ventral part of the muscle.
The inflammatory reaction was apparent in the damaged area of all injury groups during the injury process. In the 2 kg group, the myofibers were swollen and inflammatory infiltration. In the 6 kg group, the myofiber structure exhibited marked disarray broken fibers. Clamping with different forces caused different degrees of injury in lateral recti. It is worth noting that morphological observations do not quantify the degree of damage well. This non-strict quantitative damage scale selected out of the clamp force is only relatively approximate. This is also associated with subsequent muscle force measurement and eye position changes, which can quantify the degree of injury. A proper damage scale is more helpful for the preparation of a perfect model. Therefore, it is necessary to further subdivide the clamp force and increase the sample size.
PCNA, also referred to as cyclin, is an acid intranuclear protein that is regarded as marker of cell proliferation [26] . In the present observation of injured muscles, the positive granularities of PCNA were observed in the nuclei. These positive-stained nuclei with clearly identified nucleoli were relatively larger than negatively stained nuclei. The results indicated selfregeneration and repair, likely through activation, proliferation and ultimately merging into the muscle fiber of satellite cells.However, PCNA staining is indeed nonspecific, it may indicate proliferation of satellite cells, fibroblasts, neutrophils, which induced by muscles injury. And in some severe injury groups and areas, PCNA-positive granularities were not markedly increased. Myocytes were likely broken off and not effectively repaired through regeneration. In this case, repair was mainly achieved through fibrous repair. The expression of PCNA suggests the proliferation of myocytes.
The electric stimulation thresholds of injured lateral muscles were increased on either the 4 th or 7 th day after injury. Thus,injured muscles require greater electric stimulation to generate detectable contractions compared with the normal extraocular muscles. And under the identical intensity of electric stimulation, the injured external recti showed a decrease in muscle force compared with the normal ones.The extent of decline corresponded to the extent of injury.On the 7 th day, some injured muscles in the mild injury group exhibited equivalent myodynamia compared with the normal group, indicating recovery and restoration of myodynamia in the injured muscles as repair and regeneration proceeded.Anderson et al [16] demonstrated that muscle forces were related to stimulation intensity and frequency. Furthermore,changes in muscle forces are dependent upon the position and region of injured muscles [27] . Moreover, the “normal muscles” (contralateral muscles) also had an adaptation after injury, and the muscle forces of them might got change to some extent. But the contralateral muscles used as a control is only a better choice than none. According to the Hering’s law,yoke muscle (right medial rectus) of the injured muscle will receive equal augmented exciting pulses correspondingly and become acting excessively; and according to the Sherrington’s law, antagonistic muscle (right lateral rectus) will receive more inhibitory pulses, thus leading to weakened muscle force. In another word, the muscle force of the contralateral lateral rectus should decrease. There are lacks of data to show that how much the change of muscle force, but this kind of“adaptation” might be tiny. We choose the contralateral lateral rectus for contrast because: firstly, it functions as the clamping muscle, and it’s a good self-contrast; secondly, if it takes the lateral rectus of another cat as contrast, there will be obvious differences in sizes of muscles and strength of contraction,and so on. All of the factors described above might cause differences in muscle forces.
To measure the ocular alignment, we observed the extent of deviation using a fundus angiography instrument. Some scholars attempted to obtain orthophoria pictures of cats during waking states to determine their eye positions [12] . We have also tried this method; however, it was hard to keep the cats in a stationary position during the procedure. As an alternative, we obtained pictures when the cats were anaesthetized. However,when a cat is paralyzed, each eye can adopt an angle of torsion.Thus, Berman et al [14] photographed the slit-shaped entrance pupils first in alert cats and then after paralysis; the amount of rotation for each eye to correct the error was measured [15] .Accurate measurement of the eye position of animals is more difficult than that of humans, and many details need to be explored.
After the left lateral rectus is injured, the eye position of cat becomes inwardly deviated compared with pre-operation eye. However, no significant differences with regard to the extent of deviation were noted among the three groups with different extents of injury on the 4 th day or 7 th day after injury(Table 1). These results may have occurred because clamping only destroyed one muscle. The change in eye position after extraocular muscles injury is complicated. The change is influenced by the balance and antagonism of each extraocular muscle as well as the function of each extraocular muscle [28] .Furthermore, inflammation, fibrosis and changes of extraocular muscle pulley can influence the eye alignment after injury [29] .In addition, the global layer or orbital layer of the extraocular muscle influences eye movement [30] . Therefore, although the eye positions of cat got significant changes after injury,the presented clamping injury failed to cause remarkable differences among the six groups. In other words, the change in eye position is not entirely commensurate with the degree of injury, that is, the change in grading. As mentioned above,the methods may require some improvements, such as better precision of the degree of muscle force, improvements in clamping appliances, exposure of the clamped muscle,precise injury of the belly of the muscle, and reduction of the anesthetic effect on eye position. The above factors need to be taken into account in the improvement of animal models.
In summary, we establish a cat model of acute extraocular muscle injury induced by clamping, with assessment by observing the morphological changes and evaluating the changes in interocular alignment and muscle forces. The cat model is easily reproduced and can be considered for experimental studies of extraocular muscle injury, especially mechanical injury. The suitability of this animal model has yet to be tested in scientific practice. At least, this cat model is an improvement over our previous rabbit model. The treatment of extraocular muscle injury using this cat model is currently being investigated by our group. The method of making and evaluating the model of acute extraocular muscle injury can provide ideas for further modeling. Therefore, the establishment of an extraocular muscle injury model in cats may be an efficient means to investigate pathological changes,disease progression and treatment of acute extraocular muscle injury.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge Professor Tian-Bing Ding, Department of Medical Microbiology and Parasitology,the Fourth Military Medical University, for the revision and pathological advises.
Foundation: Supported by the Natural Science Research Foundation of Shaanxi Province, China (No.SJ08C241).
Conflicts of Interest: Zhi JJ, None; Yan H, None; Sun LH, None.
REFERENCES
1 Baek SH, Lee EY. Clinical analysis of internal orbital fractures in children. Korean J Ophthalmol 2003;17(1):44-49.
2 Larian B, Wong B, Crumley RL, Moeinolmolki B, Muranaka E, Keates RH. Facial trauma and ocular/orbital injury. J Craniomaxillofac Trauma 1999;5(4):15-24.
3 Liu SR, Song XF, Li ZK, Shen Q, Fan XQ. Postoperative improvement of diplopia and extraocular muscle movement in patients with reconstructive surgeries for orbital floor fracters. J Craniofac Surg 2016;27(8):2043-2049.
4 Kwon JH, Moon JH, Kwon MS, Cho JH. The differences of blowout fracture of the inferior orbital wall between children and adults. Arch Otolaryngol Head Neck Surg 2005;131(8):723-727.
5 Gosau M, Schöneich M, Draenert FG, Ettl T, Driemel O, Reichert TE.Retrospective analysis of orbital floor fractures-complications, outcome,and review of literature. Clin Oral Investig 2011;15(3):305-313.
6 Delos D, Leineweber MJ, Chaudhury S, Alzoobaee S, Gao Y, Rodeo SA. The effect of platelet-rich plasma on muscle contusion healing in a rat model. Am J Sports Med 2014;42(9):2067-2074.
7 Ljubicic V, Adhihetty PJ, Hood DA. Application of animal models:chronic electrical stimulation-induced contractile activity. Can J Appl Physiol 2005;30(5):625-643.
8 Armstrong RB, Ogilvie RW, Schwane JA. Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol 1983;54(1):80-93.
9 Ogasawara R, Fujita S, Hornberger TA, Kitaoka Y, Makanae Y,Nakazato K, Naokata I. The role of mTOR signalling in the regulation of skeletal muscle mass in a rodent model of resistance exercise. Sci Rep 2016;6:31142.
10 Lindeström LM, Ekblad E. Structural and neuronal changes in rat ileum after ischemia with reperfusion. Dig Dis Sci 2004;49(7-8):1212-1222.
11 Sun LH, Yan H. Establishment of an animal model for acute extraocular muscle injury with clamping. Guo Ji Yan Ke Za Zhi 2009;9(6):1069-1071.
12 Cynader M. Interocular alignment following visual deprivation in the cat. Invest Ophthalmol Vis Sci 1979;18(7):726-741.
13 Sherman SM. Development of interocular alignment in cats. Brain Res 1972;37(2):187-203.
14 Berman N, Blakemore C, Cynader M. Binocular interaction in the cat’s superior colliculus. J Physiol (Lond) 1975;246(3):595-615.
15 Combes RD, Shah AB. The use of in vivo, ex vivo, in vitro,computational models and volunteer studies in vision research and therapy, and their contribution to the Three Rs. Altern Lab Anim 2016;44(3):187-238.
16 Anderson BC, Christiansen SP, McLoon LK. Myogenic growth factors can decrease extraocular muscle force generation: a potential biological approach to the treatment of strabismus. Invest Ophthalmol Vis Sci 2008;49(1):221-229.
17 Bilgin B, Gursoy H, Basmak H, Ozkurt M, Tuncel N, Canaz F, Isiksoy S, Colak E. The effects of bupivacaine injection and oral nitric oxide on extraocular muscle in the rabbit. Graefes Arch Clin Exp Ophthalmol 2013;251(9):2227-2233.
18 Nelson KR, Stevens SM, McLoon LK. Prolongation of relaxation time in extraocular muscles with brain derived neurotrophic factor in adult rabbit. Invest Ophthalmol Vis Sci 2016;57(13):5834-5842.
19 Guo H, Gao Z, Chen W. Contractile force of human extraocular muscle: a theoretical analysis. Appl Bionics Biomech 2016;2016:1-8.
20 Boutin RD, Fritz RC, Steinbach LS. Imaging of sports-related muscle injuries. Radiol Clin North Am 2002;40(2):333-362.
21 Huang QM, Ye G, Zhao ZY, Lv JJ, Tang L. Myoelectrical activity and muscle morphology in a rat model of myofascial trigger points induced by blunt trauma to the vastus medialis. Acupunct Med 2013;31(1):65-73.
22 Funk K, Scheerer N, Verhaegh R, Pütter C, Fandrey J, de Groot H.Severe blunt muscle trauma in rats: only marginal hypoxia in the injured area. PLoS One 2014;9(10):e111151.
23 Warren GL, Call JA, Farthing AK, Baadom-Piaro B. Minimal evidence for a secondary loss of strength after an acute muscle injury: a systematic review and meta-analysis. Sports Med 2017;47(1):41-59.
24 Huang CB, Zhou J, Zhou Y, Lu ZL. Contrast and phase combination in binocular vision. PLoS One 2010;5(12):e15075.
25 Speer KP, Lohnes J, Garrett WE Jr. Radiographic imaging of muscle strain injury. Am J Sports Med 1993;21(1):89-95; discussion 96.
26 Hall PA, Levison DA. Review: assessment of cell proliferation in histological material. J Clin Pathol 1990;43(3):184-192.
27 Chen J, Kang Y, Deng D, Shen T, Yan J. Isolated total rupture of extraocular muscles. Medicine (Baltimore) 2015;94(39):e1351.
28 Porter JD, Baker RS, Ragusa RJ, Brueckner JK. Extraocular muscles:basic and clinical aspects of structure and function. Surv Ophthalmol 1995;39(6):451-484.
29 Dimitrova DM, Shall MS, Goldberg SJ. Stimulation-evoked eye movements with and without the lateral rectus muscle pulley. J Neurophysiol 2003;90:3809-3815.
30 Miller JM. Understanding and misunderstanding extraocular muscle pulleys. J Vis 2007;7(11):10-11.
Citation: Zhi JJ, Yan H, Sun LH. Establishment of an acute extraocular muscle injury model in cats. Int J Ophthalmol 2018;11(9):1475-1481
Received: 2017-07-24 Accepted: 2018-05-07
DOl: 10.18240/ijo.2018.09.08
Correspondence to: Hong Yan. Department of Ophthalmology,Xi’an No. 4 Hospital, Shaanxi Eye Hospital, Affiliated Guangren Hospital School of Medicine, Xi’an Jiaotong University, Xi’an 710004, Shaanxi Province, China; Department of Ophthalmology,Tangdu Hospital, Fourth Military Medical University, Xi’an 710038, Shaanxi Province, China. yhongb@fmmu.edu.cn