Lipid layer thickness and tear meniscus height measurements for the differential diagnosis of evaporative dry eye subtypes
Xuan Sang 1 , Yan Li 2 , Liu Yang 1 , Jia-Hui Liu 1 , Xiao-Ran Wang 1 , Chao-Yang Li 1 , Ying Liu 1 , Chen-Jie Wang 1 ,Xiong-Jun He 2 , Shou-Bi Wang 1 , Zhi-Chong Wang 1
1 State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou 510060, Guangdong Province, China
2 Department of Ophthalmology, Zhujiang Hospital of Southern Medical University, Guangzhou 510060, Guangdong Province,China
· KEYWORDS: evaporative dry eye; lipid layer thickness; tear meniscus height
INTRODUCTION
Evaporative dry eye (EDE) is due to excessive water loss from the exposed ocular surface in the presence of normal lacrimal secretory function [1-2] , which is different from the aqueous tear-deficient dry eye (ADDE). EDE is much more common than ADDE, and obstructive meibomian gland dysfunction (MGD) is likely the most frequent cause of EDE [3] . Therefore, the diagnosis of EDE mainly represented by obstructive MGD in most clinical studies.The universally accepted diagnostic criteria for EDE are dry eye-related symptoms, abnormal break-up time (BUT) [4] ,and normal Schirmer I test (SIT) along with MGD [5-6] . EDE has been recently classified to distinguish those causes that are dependent on intrinsic conditions of the lids and ocular surfaces from those that arise from extrinsic influences,ignoring the role of tear film [1] . Recently, an increasing amount of evidence has indicated that a large number of EDE cases are caused by other factors other than MGD: the Asia Dry Eye Society proposed that short BUT-type dry eye with minimally decreased or normal tear production and minimal vital staining may be associated with severe symptoms [2,7] , which is not accompanied by obvious MGD, has become prevalent,especially in Asian populations. Recent research has also shown a form of nonobvious MGD may be the leading cause of EDE [8-10] .
The tear film lipid layer (TFLL) covers the outer surface of the eye and is an important component that stabilizes the tear film,as it can prevent evaporative water loss [11] . When the TFLL is completely deficient, the rate of tear evaporation increases four-fold [12] . Therefore, changes in lipid layer thickness(LLT) and tear volume may be useful tools in the diagnosis of EDE. However, the values of LLT, tear volume and their relationships in EDE as reported in previous studies were not unified [13-14] .
In this study, we classified EDE into obstructive MGD EDE and non-obstructive MGD EDE subtypes, depending on whether the patients had obstructive MGD. Furthermore, we compared and analyzed the clinical characteristics of these two EDE subtypes and attempted to find a new diagnostic index for differentiating them.
SUBJECTS AND METHODS
Subjects We performed a cross-sectional study of newly diagnosed EDE patients who consecutively visited the Zhongshan Ophthalmic Center of Sun Yat-sen University from May to November 2016. The diagnostic criteria for EDE included dry eye-related symptoms, abnormal BUT, and normal SIT [15] .Depending on whether signs were accompanied by obstructive MGD, the patients were classified as obstructive MGD EDE and non-obstructive MGD EDE. Obstructive MGD was assessed according to the following 4 clinical parameters:1) meibomian gland dropout; 2) altered meibomian gland secretion (meibum quality varied in appearance from a cloudy fluid to a viscous fluid containing particulate matter to a densely opaque, inspissated or toothpaste-like material;3) changes in lid morphology, including plugging of the meibomian orifices, anterior or retro-placement of the mucocutaneous junction itself, and the inflammation of the lid margin, as evidenced by a thickening of the lid margin,vascular engorgement, and telangiectasia of the posterior lid margin; 4) and poor meibum expression by digital compression. In combination with the Foulks-Bron scoring system [16] , we set items 2) to 4) as necessary for a diagnosis of obstructive MGD, whereas item 1) was not necessary for a diagnosis. Patients under 18 years old and those who wore contact lenses, had a history of ocular surgery (include the use of a punctal plug) within the previous 3mo, used topical ocular medications within the prior 2wk, with seriously exposure of the ocular surface, e.g. increased palpebral fissure width and lid deformity, with ocular infection, or seborrhea or an autoimmune disease were excluded. Patients whose meibomian gland had excessive lipid secretion were also excluded. This study was performed according to the tenets of the Declaration of Helsinki. The study protocol was approved by the Medical Ethics Committee of the Zhongshan Ophthalmic Center of Sun Yat-sen University and was registered at Chictr.org.cn(registration No.ChiCTR-IOR-17010483). Informed consent was obtained from all patients after an explanation of the purpose and possible consequences of the study.
Clinical Tests Clinical tests were performed on the right eye of each patient. If this eye was excluded from the study,data were collected for the left eye. Each examinations was performed by a single examiner. The tests were performed in the following sequence. 1) We administered a Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire to the patients. The scores on the SPEED questionnaire ranged from 0-28. 2) Average LLT measurements were obtained using a LipiView interferometer (TearScience Inc., Morrisville,North Carolina, USA). Briefly, the patients were instructed to maintain fixation on the camera, which recorded a 20-second video of an interferometry image of the tear film. The unit of measurement used was interferometry color units (ICU),which is an index of LLT, with 1 ICU corresponding to approximately 1 nm. The LipiView interferometer displays a maximum of 100 nm in any case with an LLT greater than 100 nm (Figure 1). 3) The lower TMH was measured using a keratograph 5M (K5M; Oculus, Optikgerate, Germany) in tear meniscus mode (Figure 2). 4) Tear break-up time (TBUT)was measured following the instillation of 5-10 μL of 1%fluorescein dye [17] . The mean of three measurements was recorded. After TBUT was measured, corneal and conjunctival epithelial staining was graded based on the Oxford scoring scheme [18] . The SIT was performed without topical anesthesia.The length of wetting was recorded after 5min abnormal tear film stability was determined according to the Japanese dry eye criteria, which involved using the TBUT (<5s), and abnormal tear production evaluated by using the SIT (<5 mm). 5) Lid margin abnormalities were scored as 0 (absent) or 1 (present)for the following parameters: vascular engorgement, plugged meibomian gland orifices, anterior or retro-placement of the mucocutaneous junction, and irregularity in the lid margin(range, 0-4) [19] . 6) The degree of expressible meibomian glands was quantified by the instrument of meibomian gland evaluator exerting a constant force of 0.8-1.2 g/mm 2 on five glands in the central third of the lower lid and was graded as follows: grade 0, all 5 glands were expressive; grade 1, 3 to 4 glands were expressive; grade 2, 1 or 2 glands were expressive; and grade 3,none of the glands were expressive [20] . 7) The meibum quality over the eight lower lid glands was graded as follows: grade 0, clear; grade 1, cloudy; grade 2, cloudy with granular debris;and grade 3, thick and toothpaste-like. Each of the central eight glands of the lower eyelid was graded on a scale from 0 to 3. The scores given to these eight glands were summed to obtain a total score (range, 0-24) [21] . 8) The examiner evaluated meibomian gland morphology using a tool provided in the meibography mode of the LipiView interferometer, and meibomian gland loss (MGL) was semi-quantitatively graded from grade 0 (no loss of meibomian glands) to grade 3 (the lost area included more than two-thirds of the total meibomian gland area) [22] . The room was maintained at 23℃ to 25℃ and 50%-60% humidity during the examinations.
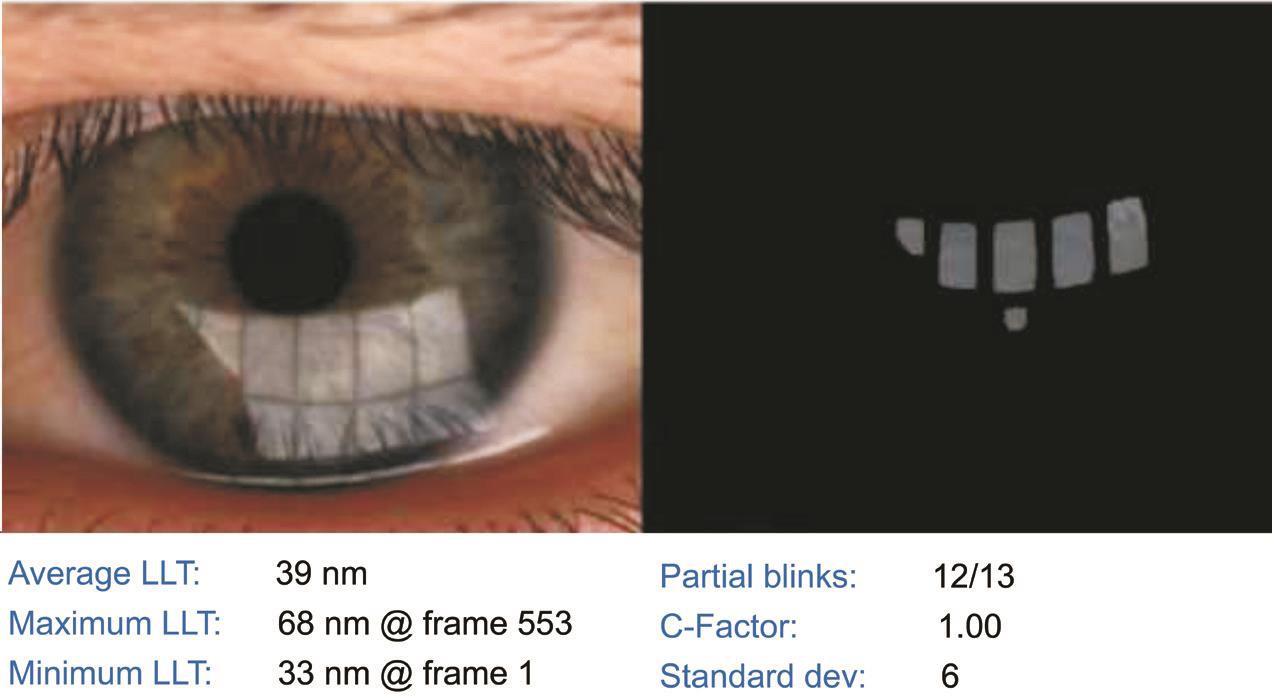
Figure 1 The top image of specular reflections on the tear film and the LLT is measured using an interferometry color assessment by specular reflection. The bottom data show the change of the average, maximum, and minimum LLT during 20s. C-Factor represents the reliability of the measurements LLT: Lipid layer thickness.
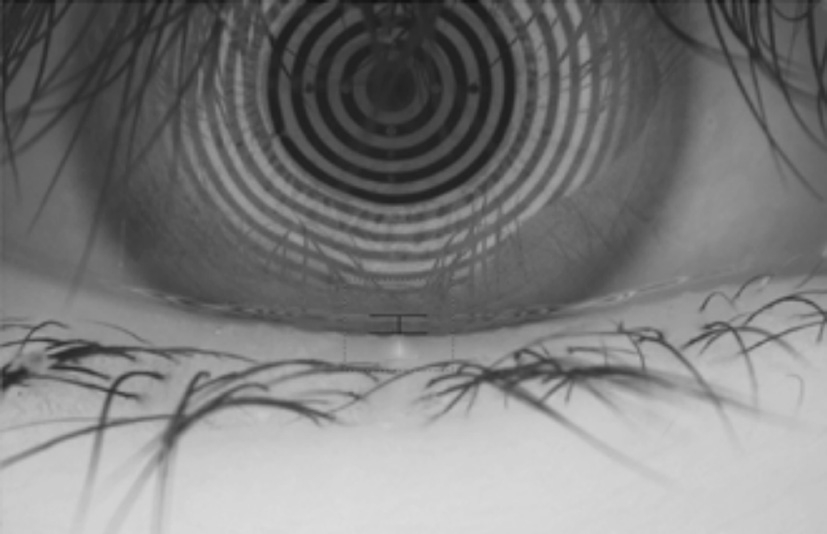
Figure 2 Measurement of lower TMH using Keratograph 5M TMH: Tear meniscus height measurements.
Statistical Analysis We used SPSS19.0 for Windows for statistical calculations. The normal distribution of the data was first confirmed using the Shapiro-Wilk test. A comparative analysis of clinical parameters between two EDE subtypes was performed using a nonparametric analysis of variance test(Mann-Whitney U ), and categorical data were analyzed using the χ 2 test. Correlations among average LLT, TMH, and other clinical parameters were estimated using Spearman’s rank correlation coefficient.
We used receiver operating characteristic (ROC) curves and a logistic regression model to evaluate the efficiency of using single and combined average LLT and TMH measurements to differentiate these two subtypes. All results are expressed as medians (ranges), and P values less than 0.05 were considered to indicate statistical significance.
Table 1 Demographics of the study groups n (%)
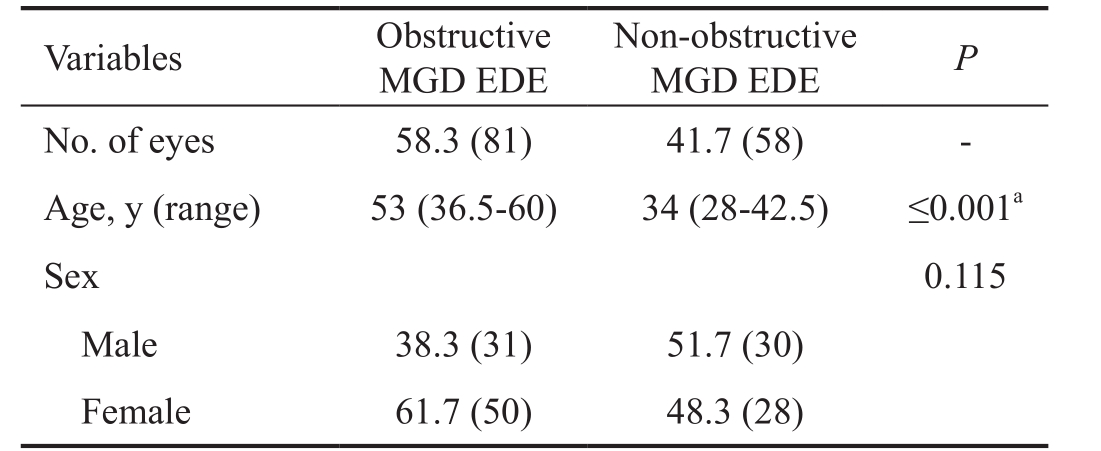
a In comparisons between the non-obstructive MGD EDE and obstructive MGD EDE groups, P <0.05 indicates a statistically significant difference. For age, we used the Mann-Whitney U test. For the numbers of eyes and sex, we used the χ 2 test. MGD: Meibomian gland dysfunction; EDE: Evaporative dry eye; LLT: Lipid layer thickness.
Variables Obstructive MGD EDE Non-obstructive MGD EDE P No. of eyes 58.3 (81) 41.7 (58) -Age, y (range) 53 (36.5-60) 34 (28-42.5) ≤0.001 a Sex 0.115 Male 38.3 (31) 51.7 (30)Female 61.7 (50) 48.3 (28)
RESULTS
Demographics of Evaporative Dry Eye A total of 139 eyes in 139 EDE patients were enrolled in this study, including 81 patients with obstructive MGD EDE and 58 patients with non-obstructive MGD EDE. The non-obstructive MGD EDE patients had a median age of 34 years old and were younger than those in the obstructive MGD EDE group, whose median age was 53 years old ( P ≤0.001). In the obstructive MGD EDE and non-obstructive MGD EDE groups, 61.7% and 48.3%,respectively, of the patients were women, with no significant difference between the groups (Table 1).
Clinical Features of Evaporative Dry Eye Subtypes Median average LLT was thicker in the obstructive MGD EDE group than in the non-obstructive MGD EDE group [75 nm (58.5-98.5) vs 58 nm (53.25-67.75); P ≤0.001], and median TMH was higher in the obstructive MGD EDE group than in the non-obstructive MGD EDE group [0.34 nm (0.24-0.44) vs 0.23 nm (0.16-0.31), P ≤0.001]. TBUT was slightly higher in the obstructive MGD EDE group than in the non-obstructive MGD EDE group ( P =0.03).
Table 2 Comparison of clinical characteristics between obstructive MGD EDE and non-obstructive MGD EDE median (range)
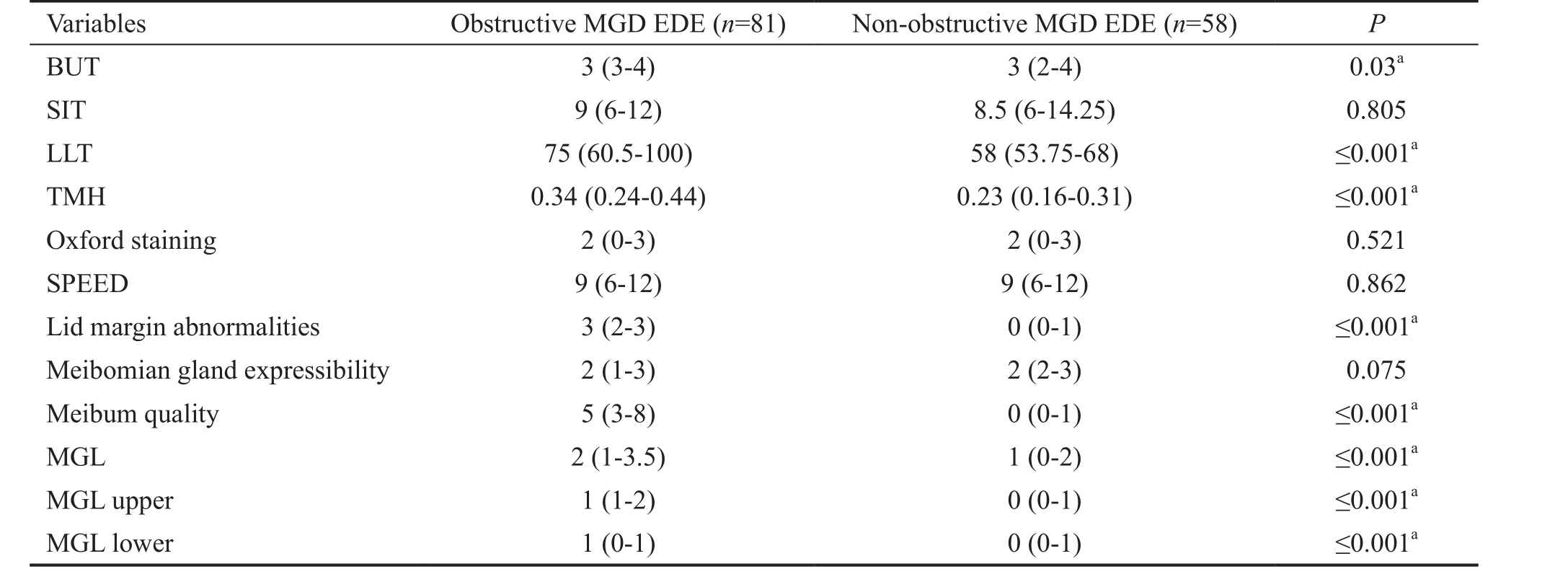
a P <0.05 indicates a statistically significant difference. For LLT, TMH, BUT, ST, FL, SPEED, and meibomian gland characteristics, we used the Mann-Whitney U test. MGD: Meibomian gland dysfunction; EDE: Evaporative dry eye; LLT: Lipid layer thickness; BUT: Break-up time; SIT:Schirmer I test; TMH: Tear meniscus height measurements; SPEED: Standard patient evaluation of eye dryness; MGL: Meibomian gland loss.
Variables Obstructive MGD EDE ( n =81) Non-obstructive MGD EDE ( n =58) P BUT 3 (3-4) 3 (2-4) 0.03 a SIT 9 (6-12) 8.5 (6-14.25) 0.805 LLT 75 (60.5-100) 58 (53.75-68) ≤0.001 a TMH 0.34 (0.24-0.44) 0.23 (0.16-0.31) ≤0.001 a Oxford staining 2 (0-3) 2 (0-3) 0.521 SPEED 9 (6-12) 9 (6-12) 0.862 Lid margin abnormalities 3 (2-3) 0 (0-1) ≤0.001 a Meibomian gland expressibility 2 (1-3) 2 (2-3) 0.075 Meibum quality 5 (3-8) 0 (0-1) ≤0.001 a MGL 2 (1-3.5) 1 (0-2) ≤0.001 a MGL upper 1 (1-2) 0 (0-1) ≤0.001 a MGL lower 1 (0-1) 0 (0-1) ≤0.001 a
The scores for lid margin abnormalities, meibum quality,and MGL were significantly higher in the obstructive MGD EDE group than in the non-obstructive MGD EDE group(all P ≤0.001). The meibomian gland expressibility were slightly lower in the non-obstructive MGD EDE group than in the obstructive MGD EDE group, but this difference was marginally non-significant ( P =0.075). Patients with each EDE subtype were similar in SIT, SPEED, and ocular surface staining. These results were shown in Table 2.
Correlations Among Average LLT, TMH, and Clinical Parameters In the obstructive MGD EDE group, average LLT was remarkably negatively correlated with meibomian expressibility ( r =-0.541, P ≤0.001) and positively correlated with lid margin abnormalities, although this relationship was marginally non-significant ( r =0.197; P =0.077). TMH was positively correlated with MGL (total MGL: r =0.552, P ≤0.001; upper MGL: r =0.438, P ≤0.001; lower MGL: r =0.407, P ≤0.001).
In non-obstructive MGD EDE, average LLT was negatively correlated with Oxford staining and meibomian expressibility( r =-0.461, P ≤0.001; r =-0.396, P =0.002). TMH was not correlated with other parameters (Table 3).
Efficiency of Using Average LLT and TMH Measurements to Differentiate the EDE Subtypes Average LLT and TMH were evaluated as test variables, and obstructive MGD EDE and non-obstructive MGD EDE were used as state variables in the ROC curve analysis. Average LLT and TMH significantly discriminated between these two EDE subtypes[LLT test: area under the curve (AUC) =0.754, P ≤0.001,cutoff value=69 nm, sensitivity=63% and specificity=77.6%;TMH test: AUC=0.774, P ≤0.001, cutoff value=0.3 mm,sensitivity=66.7% and specificity=74.1%]. Average LLT and TMH were used as a single variable, with average LLT representing the variable X1 and TMH representing thevariable X2 in a logistic regression model, resulting in the following regression equation: Y=-6.156+0.055×average LLT+9.813×TMH. In addition, the new variable Y was taken as a test variable for the ROC curve. Combining average LLT and TMH was more effective than either single test fordiscriminating EDE subtypes: AUC=0.856, sensitivity=81.5%,and specificity=74.1% (Figure 3; Table 4).
Table 3 Correlations among average LLT, TMH, and clinical parameters

MGD: Meibomian gland dysfunction; EDE: Evaporative dry eye;LLT: Lipid layer thickness; BUT: Break-up time; SIT: Schirmer I test; TMH:Tear meniscus height measurements; MGL: Meibomian gland loss.
Non-obstructive MGD EDE r P r P LLT BUT 0.181 0.106 0.237 0.073 SIT -0.152 0.175 -0.184 0.168 TMH 0.137 0.222 0.034 0.802 Oxford staining -0.048 0.671 -0.461 ≤0.001 Meibomian expressibility -0.541 0.000 -0.396 0.002 Meibum quality -0.133 0.236 -0.039 0.770 Lid margin abnormalities 0.197 0.077 -0.133 0.318 MGL -0.061 0.590 -0.049 0.716 MGL upper -0.056 0.621 -0.032 0.812 MGL lower -0.078 0.490 -0.098 0.463 TMH BUT -0.004 0.975 -0.105 0.432 SIT -0.009 0.934 0.039 0.770 Oxford staining -0.07 0.536 0.047 0.725 Meibomian expressibility 0.032 0.774 -0.127 0.342 Meibum quality -0.115 0.308 0.034 0.8 Lid margin abnormalities -0.103 0.360 0.117 0.382 MGL 0.552 0.000 0.053 0.693 MGL upper 0.438 0.000 0.153 0.253 MGL lower 0.407 0.000 -0.038 0.775 Variables Obstructive MGD EDE
Table 4 Efficiency of using average LLT, TMH, and a combination of both measurements to differentiate obstructive MGD EDE and non-obstructive MGD EDE
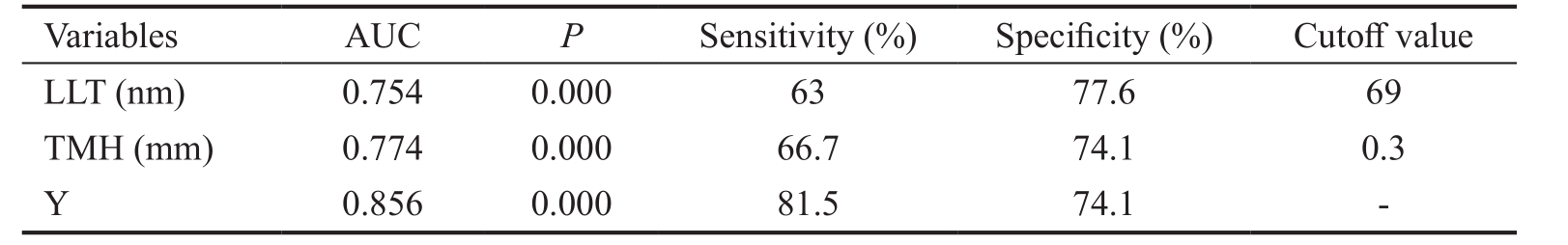
MGD: Meibomian gland dysfunction; EDE: Evaporative dry eye; LLT: Lipid layer thickness; TMH: Tear meniscus height; AUC: Area under the curve.
Variables AUC P Sensitivity (%) Specificity (%) Cutoff value LLT (nm) 0.754 0.000 63 77.6 69 TMH (mm) 0.774 0.000 66.7 74.1 0.3 Y 0.856 0.000 81.5 74.1 -
Differences in Criteria Associated with the Combination of Average LLT and TMH in Obstructive MGD EDE and Non-obstructive MGD EDE Patients Based on the ROC curve for the average LLT single test, the average LLT cutoffvalue was set at 69 nm. The TMH cutoff value of 0.25 mm was obtained using the following regression equation: Y=-6.156+0.055× average LLT+9.813×TMH. Therefore, when average LLT and TMH were combined, the cases were divided into four categories, as shown in Table 5. The majority of obstructive MGD EDE patients had average LLT≥69 nm and TMH≥0.25 mm, whereas the majority of non-obstructive MGD EDE had average LLT<69 nm and TMH<0.25 mm.
DISCUSSION
In this study, we used the universally accepted diagnostic criteria for obstructive MGD proposed by the International Workshop on MGD [23] , to classify EDE patients into obstructive MGD EDE and non-obstructive MGD EDE groups. We found that 58.3% and 41.7% of the patients had obstructive MGD EDE and non-obstructive MGD EDE,respectively. The results of a recent clinic-based patient cohort found that 45.1% of the EDE patients were without obstructive MGD [24] , supporting our findings.
We compared and analyzed the average LLT, TMH, and other clinical parameters between obstructive MGD EDE and nonobstructive MGD EDE. There was a significant difference in average LLT between these two EDE subtypes. The median average LLT value was 58 nm in non-obstructive MGD EDE and was inversely correlated with meibomian expressibility:having fewer expressing meibomian glands was associated with having a thinner LLT. This result is in agreement with a theory suggesting that meibomian glands secrete lipids to the ocular surface, which results in the formation of the TFLL. The median average LLT value was 75 nm in obstructive MGD EDE. This result was similar to the results reported by Jung et al [19] , who reported that the median LLT values in dry eye syndrome with obstructive MGD or hypersecretory MGD were 79 nm and 100 nm, respectively. Both of these LLT values were higher than the value in the normal group (67 nm).And they reported that increased age was significantly related to increased LLT. As what we found that the older patients in obstructive MGD EDE with the thicker LLT than those in the non-obstructive MGD EDE.
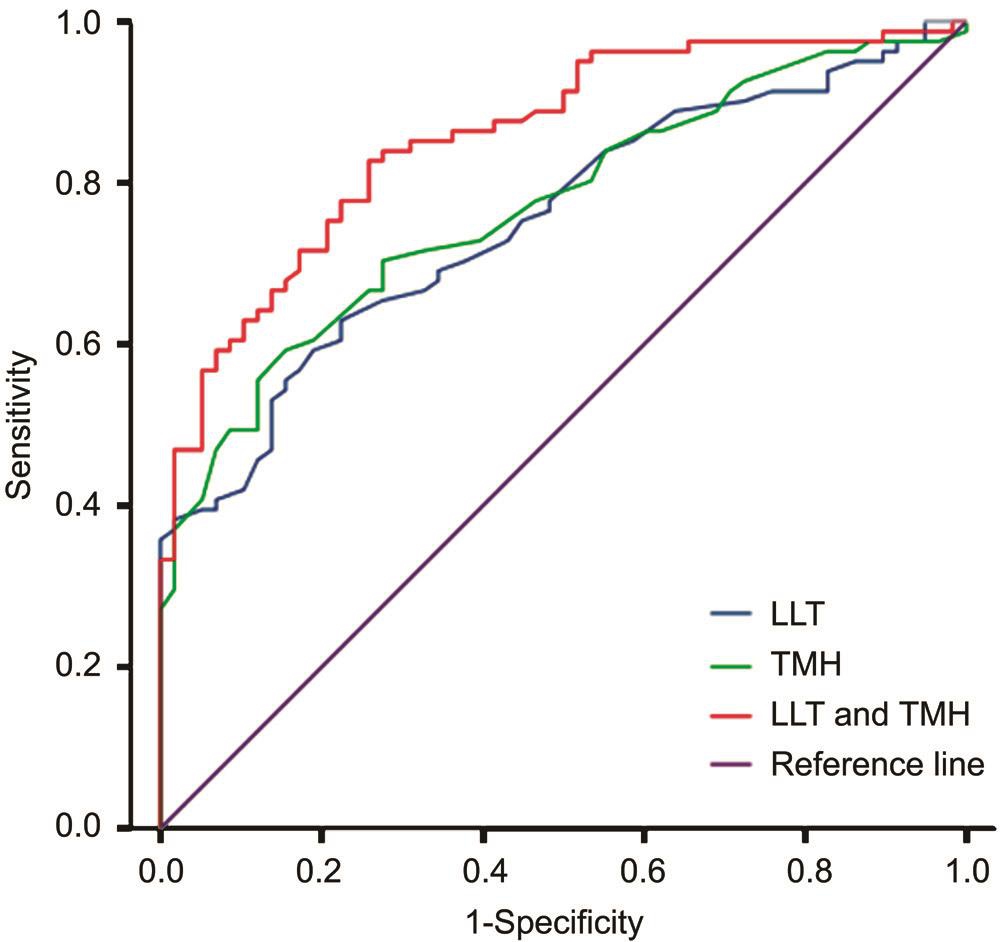
Figure 3 Discriminating EDE subtypes using LLT, TMH, or a combination of both.
Table 5 Comparison of different LLT&TMH criteria across EDE subtypes n
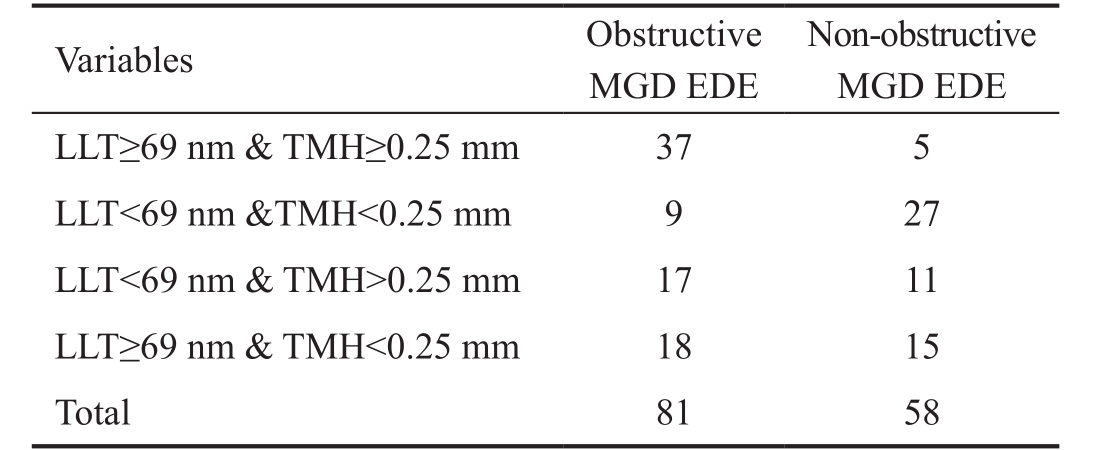
MGD: Meibomian gland dysfunction; EDE: Evaporative dry eye; LLT:Lipid layer thickness; TMH: Tear meniscus height measurements.
Non-obstructive MGD EDE LLT≥69 nm & TMH≥0.25 mm 37 5 LLT<69 nm &TMH<0.25 mm 9 27 LLT<69 nm & TMH>0.25 mm 17 11 LLT≥69 nm & TMH<0.25 mm 18 15 Total 81 58 Variables Obstructive MGD EDE
However, in obstructive MGD EDE group, LLT was higher,while tear film was unstable. King-Smith et al [25] found that whereas tear film break-up was often accompanied by thin lipids, in some cases, the affected lipid region was surprisingly thicker than the surrounding lipid region. Thus, tear film evaporation may not necessarily be correlated with LLT, which is thought to be related to interactions between the lipids and mucins [26] or the changes in lipid components of ocular surface.Previous studies have demonstrated that in MGD patients,commensal bacteria produce bacterial lipolytic exoenzymes that can break down normal lipid components, which might cause a defective TFLL and induce inflammation in the lid and ocular surface. Moreover, we found that lid abnormalities,especially lid inflammation, were more serious in obstructive MGD EDE than in non-obstructive MGD EDE patients and lid inflammation was slightly positively associated with LLT. This result further supports the notion that lid inflammation may be caused by bacteria and may affect lipid components of TFLL and LLT in obstructive MGD EDE, even though a normal or thicker LLT might be connected with excessive evaporation and an unstable tear film. Therefore, it requires us to detect the changes in lipid components of TFLL of these two EDE subtypes in future studies.
In this study, we evaluated tear volume by measuring TMH.The median value TMH in the obstructive MGD EDE group(0.34 mm) was higher than the value in the non-obstructive MGD EDE group (0.23 mm), but there was no significant difference in SIT between the two subtypes, and this value was not associated with TMH. This result indicated the following. 1)The results of SIT and TMH measurements were inconsistent.Although the SIT is considered the traditional method of evaluating tear volume, it has poor diagnostic sensitivity and repeatability and has produced fluctuating data [27] . Therefore,we viewed the results of TMH measurements as more precise.2) The higher TMH in the obstructive MGD EDE group might be related to inflammation that resulted in reflective tear secretion. Moreover, the value was significantly related to meibomian gland loss (MGL), suggesting that an increase in tear fluid production likely compensates for MGL in these patients. A multicenter study supported our result [13] . 3) TMH was lower in the non-obstructive MGD EDE group. Ring et al [14] proposed that a low TMH implied a low tear film thickness and that a low tear film thickness was responsible for slow spreading of the TFLL which was related to an unstable tear film. Therefore, either the low TMH or the slow TFLL spreading rate could be used as an index of low aqueous volume. So we thought that the significant lower TMH in the non-obstructive MGD EDE could be a sign of starting ADDE and should be discussed further.
Compared with the obstructive MGD EDE, the non-obstructive MGD EDE were not involve obvious lid inflammation, altered meibum quality, and obvious MGL but involve reduced meibomian gland expressibility. Therefore, we hypothesize that it could be classified as hyposecretory MGD which is characterized by decreased meibomian lipid secretion without glandular duct obstruction. Although there is no published and verified evidence of primary hyposecretion and some researchers reported that this condition may be associated with contact lens wear [28] . Or it may also be a nonobvious MGD EDE which is a precursor to obstructive MGD EDE [8-10] .Therefore, obtaining an early diagnosis and treatment for nonobstructive MGD EDE is likely to decrease the severity of EDE.
As previously mentioned, we found that there were significant differences in LLT and TMH between the obstructive MGD EDE and non-obstructive MGD EDE groups. Even though lid abnormalities, meibum quality, and MGL were also significantly different, they are all semi-quantitative values that are associated with signs of MGD. LLT and TMH measurements are quantitative and serve as an index of tear fluid. Hence, we attempted to use average LLT and TMH measurements to differentiate the two EDE subtypes. We found that the efficiency of combining measurements of LLT and TMH to differentiate the two EDE subtypes was optimal and had a sensitivity of 81.5% and a specificity of 74.1%. The obstructive MGD EDE patients had LLT≥69 nm and TMH≥0.25 mm, while the non-obstructive MGD EDE patients had LLT<69 nm and TMH<0.25 mm. Therefore, the treatment of non-obstructive MGD EDE is to supplement with tear substitutes that include lipids for increasing LLT and the artificial tears for increasing tear volume. While the treatment of obstructive MGD EDE is to reduce the lid inflammation,focus on the ocular surface inflammation and changes in lipid components of TFLL which could due to lid inflammation or bacteria, instead of supplementing with tear substitutes.
However, we would like to emphasize the potential limitations of our study: First, there were no healthy controls. It would be ideal to include healthy controls to assess if there is a significant difference in LLT and TMH between healthy and disease states. Secondly, we researched on the average LLT.Based on the tear interference images from the LipiView interferometer, blinking is important for the formation and distribution of the lipid layer and we have observed that LLT is changeable between blinks [29] . Therefore, we should evaluate the overall profile of the lipid layer including LLTmax and LLTmin in future research.
In summary, we found that age, average LLT, TMH, and meibomian gland parameters were different between obstructive MGD EDE and non-obstructive MGD EDE. We hypothesize that the non-obstructive MGD EDE may belong to hyposecretory MGD or also be a precursors of obstructive MGD-EDE which is non-inflamed. The further step is to pursue the etiology and pathogenesis of non-obstructive MGD EDE and our data also should be followed up to determine whether non-obstructive MGD EDE is a precursor to obstructive MGD EDE or whether there is a dynamic pattern of transformation between obstructive MGD EDE and non-obstructive MGD EDE patients. We also propose that using a combination of LLT and TMH measurements could help to differentiate these two EDE subtypes, which may result in the availability of more precise treatments for EDE patients in clinical practice.
ACKNOWLEDGEMENTS
The authors thank Ying Xu (Kidney Department, Shandong Provincial Hospital, Shandong, China) for her assistance with the statistical analyses.
Foundations: Supported by the Provincial Frontier and Key Technology Innovation Special Fund of Guangdong Province(No.2015B020227001); the Guangzhou Science and Technology Plan Scientific Research Projects (No.201504010023).
Conflicts of Interest: Sang X, None; Li Y, None; Yang L, None; Liu JH, None; Wang XR, None; Li CY, None; Liu Y, None; Wang CJ, None; He XJ, None; Wang SB, None; Wang ZC, None.
REFERENCES
1 Lemp MA, Baudouin C, Baum J, Dogru M, Foulks GN, Kinoshita S, Laibson P, McCulley J, Murube J, Pflugfelder SC, Rolando M, Toda I. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2007;5(2SI):75-92.
2 Tsubota K, Yokoi N, Shimazaki J, Watanabe H, Dogru M, Yamada M,Kinoshita S, Kim HM, Tchah HW, Hyon JY, Yoon KC, Seo KY, Sun X,Chen W, Liang L, Li M, Liu Z. New perspectives on dry eye definition and diagnosis: a consensus report by the asia dry eye society. Ocul Surf 2017;15(1):65-76.
3 Chhadva P, Goldhardt R, Galor A. Meibomian gland disease the role of gland dysfunction in dry eye disease. Ophthalmology 2017;124(11S): S20-S26.
4 Chen M, Miki M, Lin S, Yung Choi S. Sodium fluorescein staining of the cornea for the diagnosis of dry eye: a comparison of three eye solutions. Med Hypothesis Discov Innov Ophthalmol 2017;6(4):105-109.
5 Mian SI. Evaporative dry eye disease: promising new approaches for diagnosis and treatment. Curr Opin Ophthalmol 2015;26(4):288.
6 Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int 2015;112(5):71-81;quiz 82.
7 Kawashima M, Yamada M, Suwaki K, Shigeyasu C, Uchino M, Hiratsuka Y, Yokoi N, Tsubota K. A clinic-based survey of clinical characteristics and practice pattern of dry eye in Japan. Adv Ther 2017;34(3):732-743.
8 Blackie CA, Korb DR, Knop E, Bedi R, Knop N, Holland EJ. Nonobvious obstructive meibomian gland dysfunction. Cornea 2010;29(12):1333-1345.
9 Goto E, Monden Y, Takano Y, Mori A, Shimmura S, Shimazaki J,Tsubota K. Treatment of non-inflamed obstructive meibomian gland dysfunction by an infrared warm compression device. Br J Ophthalmol 2002;86(12):1403-1407.
10 Goto E, Dogru M, Fukagawa K, Uchino M, Matsumoto Y, Saiki M,Tsubota K. Successful tear lipid layer treatment for refractory dry eye in office workers by low-dose lipid application on the full-length eyelid margin. Am J Ophthalmol 2006;142(2):264-270.
11 Millar TJ, Schuett BS. The real reason for having a meibomian lipid layer covering the outer surface of the tear film - a review. Exp Eye Res 2015;137:125-138.
12 Nichols KK, Foulks GN, Bron AJ, Glasgow BJ, Dogru M, Tsubota K,Lemp MA, Sullivan DA. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci 2011;52(4):1922-1929.
13 Arita R, Morishige N, Koh S, Shirakawa R, Kawashima M, Sakimoto T, Suzuki T, Tsubota K. Increased tear fluid production as a compensatory response to meibomian gland loss: a multicenter cross-sectional study. Ophthalmology 2015;122(5):925-933.
14 Ring MH, Rabensteiner DF, Horwath-Winter J, Boldin I, Horantner R,Haslwanter T. Introducing a new parameter for the assessment of the tear film lipid layer. Invest Ophthalmol Vis Sci 2012;53(10):6638-6644.
15 Cuevas M, González-García MJ, Castellanos E, Quispaya R, Parra Pde L, Fernández I, Calonge M. Correlations among symptoms, signs, and clinical tests in evaporative-type dry eye disease caused by meibomian gland dysfunction (MGD). Curr Eye Res 2012;37(10):855-863.
16 Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. Ocul Surf 2003;1(3):107-126.
17 Toda I, Fujishima H, Tsubota K. Ocular fatigue is the major symptom of dry eye. Acta Ophthalmol 1993;71(3):347-352.
18 Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003;22(7):640-650.
19 Jung JW, Park SY, Kim JS, Kim EK, Seo KY, Kim TI. Analysis of factors associated with the tear film lipid layer thickness in normal eyes and patients with dry eye syndrome. Invest Ophthalmol Vis Sci 2016;57(10):4076-4083.
20 Hykin PG, Bron AJ. Age-related morphological changes in lid margin and meibomian gland anatomy. Cornea 1992;11(4):334-342.
21 Korb DR, Blackie CA. Meibomian gland diagnostic expressibility:correlation with dry eye symptoms and gland location. Cornea 2008;27(10):1142-1147.
22 Arita R, Itoh K, Inoue K, Amano S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology 2008;115(5):911-915.
23 Tomlinson A, Bron AJ, Korb DR, Amano S, Paugh JR, Pearce EI, Yee R, Yokoi N, Arita R, Dogru M. The international workshop on meibomian gland dysfunction: rieport of the diagnosis subcommittee. Invest Ophthalmol Vis Sci 2011;52(4):2006-2049.
24 Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea 2012;31(5):472-478.
25 King-Smith PE, Reuter KS, Braun RJ, Nichols JJ, Nichols KK. Tear film breakup and structure studied by simultaneous video recording of fluorescence and tear film lipid layer images. Invest Ophthalmol Vis Sci 2013;54(7):4900-4909.
26 Willcox MDP, Argueso P, Georgiev GA, Holopainen JM, Laurie GW, Millar TJ, Papas EB, Rolland JP, Schmidt TA, Stahl U, Suarez T,Subbaraman LN, Ucakhan OO, Jones L. TFOS DEWS II tear film report. Ocul Surf 2017;15(3):366-403.
27 Savini G, Prabhawasat P, Kojima T, Grueterich M, Espana E, Goto E.The challenge of dry eye diagnosis. Clin Ophthalmol 2008;2(1):31-55.
28 Arita R, Itoh K, Inoue K, Kuchiba A, Yamaguchi T, Amano S.Contact lens wear is associated with decrease of meibomian glands. Ophthalmology 2009;116(3):379-384.
29 Huang J, Hindman HB, Rolland JP. In vivo thickness dynamics measurement of tear film lipid and aqueous layers with optical coherence tomography and maximum-likelihood estimation. Opt Lett 2016;41(9):1981-1984.
Citation: Sang X, Li Y, Yang L, Liu JH, Wang XR, Li CY, Liu Y, Wang CJ,He XJ, Wang SB, Wang ZC. Lipid layer thickness and tear meniscus height measurements for the differential diagnosis of evaporative dry eye subtypes. Int J Ophthalmol 2018;11(9):1496-1502
Received: 2018-04-20 Accepted: 2018-06-05
DOl: 10.18240/ijo.2018.09.11
Abstract · AlM: To explore a new diagnostic index for differentiating the evaporative dry eye (EDE) subtypes by analysis of their respective clinical characteristics.· METHODS: A cross-sectional study of 139 patients(139 eyes) with EDE who were enrolled and classified as obstructive meibomian gland dysfunction (MGD) ( n =81) and non-obstructive MGD ( n =58) EDE. All patients completed a Standard Patient Evaluation of Eye Dryness (SPEED)questionnaire and were evaluated for average lipid layer thickness (LLT), tear meniscus height measurements(TMH), tear break-up time (TBUT), ocular surface staining score, Schirmer l test (SlT), lid margin abnormalities, and meibomian gland function and morphology.· RESULTS: Age, average LLT, TMH, scores of lid margin abnormalities, meibum quality, meibomian gland loss (MGL)(all P ≤0.001), and TBUT ( P =0.03) were all significantly different between obstructive MGD EDE patients and nonobstructive MGD EDE patients. Average LLT in obstructive MGD EDE was correlated with meibomian expressibility ( r =-0.541, P ≤0.001), lid margin abnormalities were marginally not significant ( r =0.197, P =0.077), and TMH was correlated with MGL (total MGL: r =0.552, P ≤0.001; upper MGL: r =0.438, P ≤0.001; lower MGL: r =0.407, P ≤0.001). Average LLT in non-obstructive MGD EDE, was correlated with meibomian expressibility and Oxford staining ( r =-0.396, P =0.002; r =-0.461, P ≤0.001). The efficiency of combining average LLT and TMH was optimal, with a sensitivity of 80.2% and a specificity of 74.1%. Obstructive MGD EDE patients had an average LLT≥69 nm and TMH≥0.25 mm,while non-obstructive MGD EDE patients had an average LLT<69 nm and TMH<0.25 mm.· CONCLUSlON: Obstructive MGD EDE and nonobstructive MGD EDE have significantly different clinical characteristics. Combining average LLT and TMH measurements enhanced their reliability for differentiating these two subtypes and provided guidance for offering more precise treatments for EDE subtypes.
Correspondence to: Zhi-Chong Wang. State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yatsen University, Guangzhou 510060, Guangdong Province,China. wangzhichong@gzzoc.com.
Co-first authors: Xuan Sang and Yan Li







