Eye reflux: an ocular extraesophageal manifestation of gastric reflux
Danilo Mazzacane 1 ,Valerio Damiani 2 , Michela Silvestri 3 , Giorgio Ciprandi 4 , Pierfranco Marino 5 ;G.O.A.L.-O.S.D. Study Group
1 Asst Pavia and Asst Melegnano and Martesana, Pavia 27100, Italy
2 DMG Medical Department, Pomezia 00071, Italy
3 Istituto Giannina Gaslini, Genoa 16147, Italy
4 Ospedale Policlinico San Martino, Genoa 16132, Italy
5 Dipartimento di Scienze della Salute, Università del Molise,Campobasso 86100, Italy
Abstract · AlM: To suspect laryngopharyngeal reflux (LPR) in patients with ocular surface disease (OSD).· METHODS: The present study evaluated a group of subjects with OSD assessing the Ocular Surface Disease lndex (OSDl) and the Reflux Symptom lndex (RSl) to detect patients with suspected LPR and define a possible relationship between tests.· RESULTS: Two hundred and ninety subjects (175 females,mean age: 60.41±15.68y) were consecutively visited at ophthalmologist offices. One hundred and one (34%)patients had pathological RSl (>13) and consequently a suspected LPR.· CONCLUSlON: The current study shows that suspected LPR may be common (34%) in patients with OSD and a suspected LPR may be considered in OSD patients when RSl score is >13 and OSDl score is >42.
· KEYWORDS: gastric reflux; eye reflux; ocular surface disease index; reflux symptom score.
INTRODUCTION
Gastroesophageal reflux disease (GERD) represents a widespread disorder as about 40% of US population suffers from it [1-2] . From a clinical point of view, GERD is characterized by two main symptoms, such as heartburn and regurgitation. Actually, it is well known that gastric reflux may stray the oesophagus. Thus, several extraesophageal symptoms have been identified as potential outcome of an underlying reflux. The Montreal Classification includes chronic cough, asthma, and laryngopharyngeal reflux (LPR)as extraesophageal manifestations of GERD [3] . LPR is the consequence of aggressive refluxate exposure on upper airways, namely larynx and pharynx [4] . LPR symptoms typically consist of hoarseness, sore throat, globus sensation,and throat clearing. LPR may be associated with GERD,but it may also occur as alone disorder without typical oesophageal symptoms: the so called “silent reflux”, such as a gastroduodenal reflux without perceived oesophageal and/or gastric symptoms [5-6] . For these reasons, LPR is difficult to diagnose as there is no pathognomonic symptoms or standard diagnostic criteria and there is conflicting thought between gastroenterologists and otolaryngologists. On the other hand,LPR is very common and represent a relevant burden both concerning social and personal costs and impaired quality of life [7] . Therefore, LPR management is an impellent and intriguing challenge for general practitioners and specialists.
Even though the pathophysiological mechanisms of gastric reflux are not completely defined, the pathogenic pathway causing mucosal damages evident. In fact, low pH of refluxate and pepsin play a major role in inducing chronic mucosal inflammation [8-10] . Pepsin is a proteolytic enzyme deriving from pepsinogen and activated by low pH (at least <4) that is produced only in the stomach. Therefore, pepsin detection outside gastric area may be considered without doubt a biomarker for gastric reflux [11] . Consistently with this postulate,several studies investigated the presence of pepsin in different organs, including larynx, pharynx, paranasal sinus, mouth, and internal ear [12-13] . In line with this research field, some studies addressed the investigation to support the hypothesis that also the eye could be a target organ of LPR. The first study considered the possibility that gastric reflux may contribute to the development of acquired nasolacrimal duct obstruction [14] .This suggestive speculation paved the way to other investigations.An Iranian study reported the Helicobacter pylori colonization in the lacrimal sac mucosa of patients with obstruction of the nasolacrimal sac: an indirect demonstration of LPR at eye level [15] . In addition, a recent review pointed out this issue highlighting the relevant association between H pilori infection and ocular involvement [16] . Further, it has been provided the direct evidence that pepsin may be present in the tears of subjects with LPR [17-18] . Consequently, the hypothesis to be tested in the current study was the possible suspect of LPR in patients with ocular surface disease (OSD). Thus, the present study evaluated a group of subjects with OSD, assessing the Ocular Surface Disease Index (OSDI) and the Reflux Symptom Index (RSI) to investigate the frequency of suspected LPR and define a possible relationship between these diagnostic tests.
SUBJECTS AND METHODS
The current cross-sectional study enrolled 290 consecutive outpatients (175 females, mean age: 60.41±15.68y) with OSD.These subjects were visited at ocular offices between February and June 2017. The study was conducted according to the Declaration of Helsinki and all patients signed an informed consent. All subjects were visited by an ophthalmologist belonging to the Scientific Association Gruppo Oculisti Ambulatoriali Liberi (GOAL) and completed two diagnostic questionnaires: the OSDI and the RSI.
OSDI is a 12-item questionnaire to investigate ocular symptoms [19] . Further, reliability and validity were confirmed by two studies [20-21] . OSDI questionnaire is of low burden to the patient, as it takes approximately 5min to be completed, and was successfully used by researchers and clinicians in patients with different diseases, including connective disorders [22] .The OSDI scoring was performed according to the reference guidelines [23-24] .
RSI is a self-administered nine-item questionnaire developed by Belafsky et al [25] and colleagues for assessing symptoms in patients with reflux disease. It is so simple that can be completed in less than 1min. The scale for each individual item ranges from 0 (no problem) to 5 (severe problems), with a maximum score of 45. It has been concluded that RSI has high reproducibility and validity for the diagnosis of reflux if an RSI score >13 is defined as abnormal [25] . Therefore, RSI may be considered a pragmatic tool in the approach of patients with suspected LPR [26] .
Statistical Analysis Demographic and clinical characteristics were described using means with Standard Deviation (SDs)for normally-distributed continuous data ( i.e. age) or medians with lower and upper quartiles for not normally-distributed data ( i.e. for the score of each item of OSDI questionnaire)or as absolute frequency and percentages for categorical data( i.e. frequency of patients who refers sensitivity to light).Any statistically significant difference in the mean values or in the median values of each continuous variable between patients with normal or pathologic RSI (as defined at the RSI questionnaire) was evaluated with the unpaired t -test or Mann-Whitney U test, respectively.
Comparison of frequency distributions was made by means of the Chi-square test or the Fisher’s exact test in case of expected frequencies <5. Odds ratios (OR) were reported with 95% confidence intervals (CI). Correlation between the RSI and the total OSDI score was evaluated with Spearman rankorder correlation coefficient. We labelled the strength of the association as follows: for absolute values of r , 0 to 0.19 is regarded as very weak, 0.2 to 0.39 as weak, 0.40 to 0.59 as moderate, 0.6 to 0.79 as strong and 0.8 to 1 as very strong correlation [27] .
The best cut-off point of total OSDI score able to discriminate between patients with or without LRP was estimated on the basis of the receiver operator characteristic (ROC) curve analysis. Statistical significance was set at P <0.05, and the analyses were performed using GraphPad Prism software(GraphPad Software Inc, CA, USA) and MedCalc (Ostend,Belgium).
RESULTS
The present study analysed the reports of 290 outpatients.Patients were subdivided in two sub-groups on the basis of the RSI score: patients with normal RSI (≤13, RSI negative group)and patients with abnormal RSI (>13, RSI positive group). RSI negative group consisted of 189 (65.2%) subjects (114 females,mean age: 51.4±14.74y); RSI positive group consisted of 101(34.8%) subjects (61 females, mean age: 63.88±14.62y).
The OSDI outcomes were analysed considering both the 3 individual items, such as ocular symptoms, vision-related function, and environmental triggers, and the global score.The distribution of the median scores is reported according to the RSI outcomes in Table 1. Noteworthy, all single items and global scores were significantly higher in patients with abnormal RSI ( P <0.0001, each comparison). A moderate( r =0.58) correlation between total OSDI score and RSI scores was detected, as reported in Figure 1.
We also estimated the frequency of ocular symptoms, vision related functioning, and environmental triggers evaluated by the OSDI questionnaire in patients with pathologic or normal RSI: for each question concerning different eye issues,patients rating their symptom/problem equal to zero were classified as not having symptoms/problems whereas those rating their symptom/problem ≥1 were classified as having symptoms/problems. As shown in Table 2, ocular symptoms,vision related functioning, and environmental triggers were significantly more frequently reported by RSI positive patients.Particularly pain, poor vision, and problems when using a computer or watching TV were almost 5-fold more frequent in RSI positive patients than in RSI negative patients. RSI positive patients showed an almost 7-fold more frequent gritty feeling or problems when reading or having problems if low humidity. More frequent problems if windy conditions or in air conditioned spaces were reported 10-to-20-fold more frequently by RSI positive patients.
Table 1 Median scores in ocular symptoms, vision related functioning, and environmental triggers evaluated by the OSDI questionnaire in patients with pathologic or normal RSI
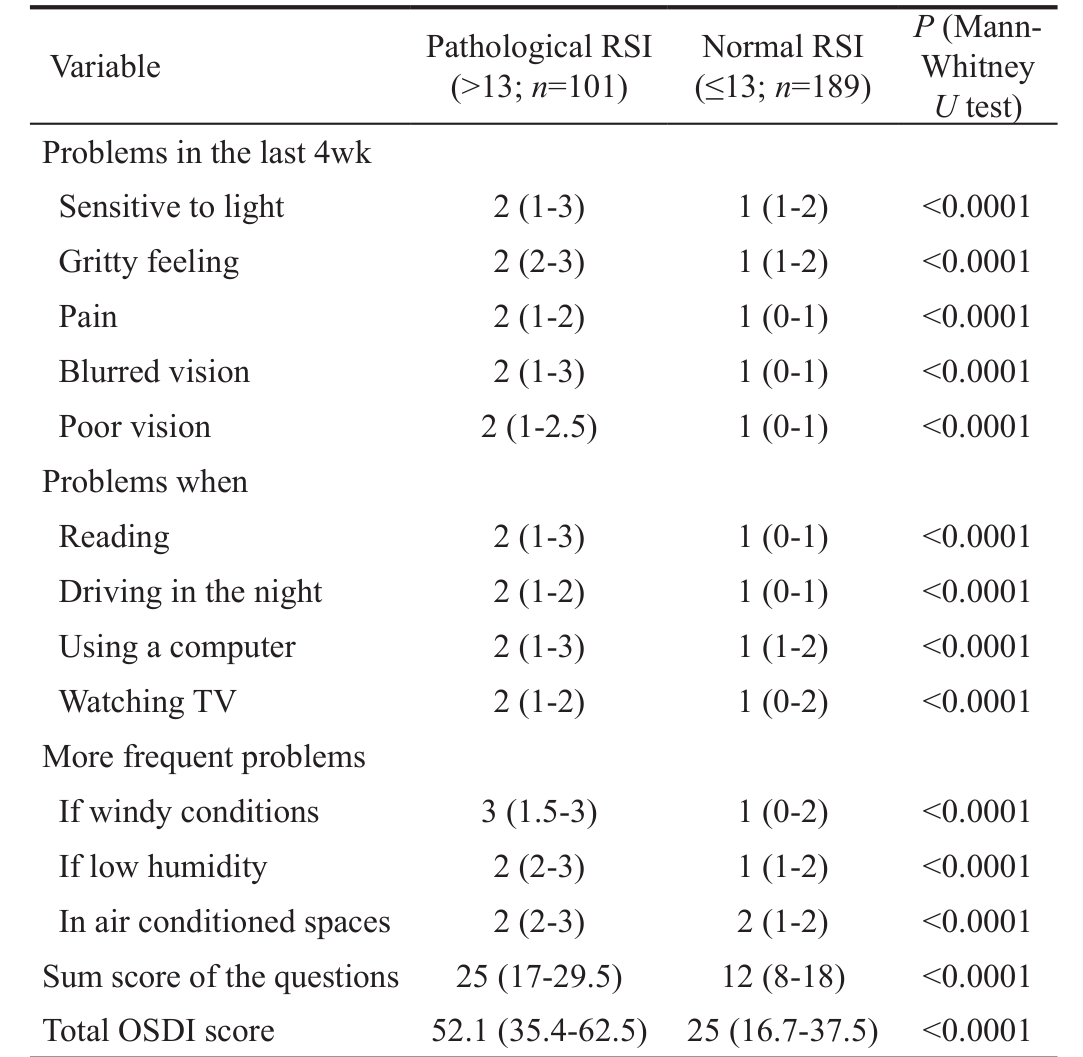
OSDI: Ocular Surface Disease Index; RSI: Reflux Symptom Index.All data are reported as medians with lower and upper quartiles in parentheses.
P (Mann-Whitney U test)Problems in the last 4wk Sensitive to light 2 (1-3) 1 (1-2) <0.0001 Gritty feeling 2 (2-3) 1 (1-2) <0.0001 Pain 2 (1-2) 1 (0-1) <0.0001 Blurred vision 2 (1-3) 1 (0-1) <0.0001 Poor vision 2 (1-2.5) 1 (0-1) <0.0001 Problems when Reading 2 (1-3) 1 (0-1) <0.0001 Driving in the night 2 (1-2) 1 (0-1) <0.0001 Using a computer 2 (1-3) 1 (1-2) <0.0001 Watching TV 2 (1-2) 1 (0-2) <0.0001 More frequent problems If windy conditions 3 (1.5-3) 1 (0-2) <0.0001 If low humidity 2 (2-3) 1 (1-2) <0.0001 In air conditioned spaces 2 (2-3) 2 (1-2) <0.0001 Sum score of the questions 25 (17-29.5) 12 (8-18) <0.0001 Total OSDI score 52.1 (35.4-62.5) 25 (16.7-37.5) <0.0001 Variable Pathological RSI(>13; n =101)Normal RSI(≤13; n =189)
ROC analysis allowed to define as >41.67 the best total OSDI score cut-off able to discriminate patients with abnormal RSI: area under the curve (AUC) was 0.812 (0.762-0.855), sensitivity 65.35%, specificity 85.19%, and Youden index J 0.51, as reported in Figure 2.
The best cut-off point of overall OSDI score able to discriminate between patients with or without LPR was estimated on the basis of the ROC curve analysis. Patients with an overall OSDI score >41.67 were considered as “disease positive” patients(patients with suspected LPR), whereas patients with an overall OSDI score ≤41.67 were considered “disease negative”patients (patients without LPR).
DISCUSSION
GERD is a common and well recognized disorder that may be easily diagnosed mainly on a clinical ground. However,LPR diagnosis is debated and there is no agreement about its real clinical relevance. Anyway, there is convincing evidence demonstrating an LPR role in airways inflammation involving some organs, such as larynx, pharynx, paranasal sinus, and middle ear [27-31] . In particular, it has been demonstrated the pathogenic role of pepsin in otitis media [32-33] . These studies are crucial as they paved the way to investigate also a possible LPR impact also on eye. First studies addressed this topic and evidence of pepsin presence in the tears has been documented [18] . The possible explanation of this way could depend on a peculiar mechanism. Pepsin can move to lacrimal film passing through the nasal cavity, the inferior meatus, and the nasolacrimal duct.
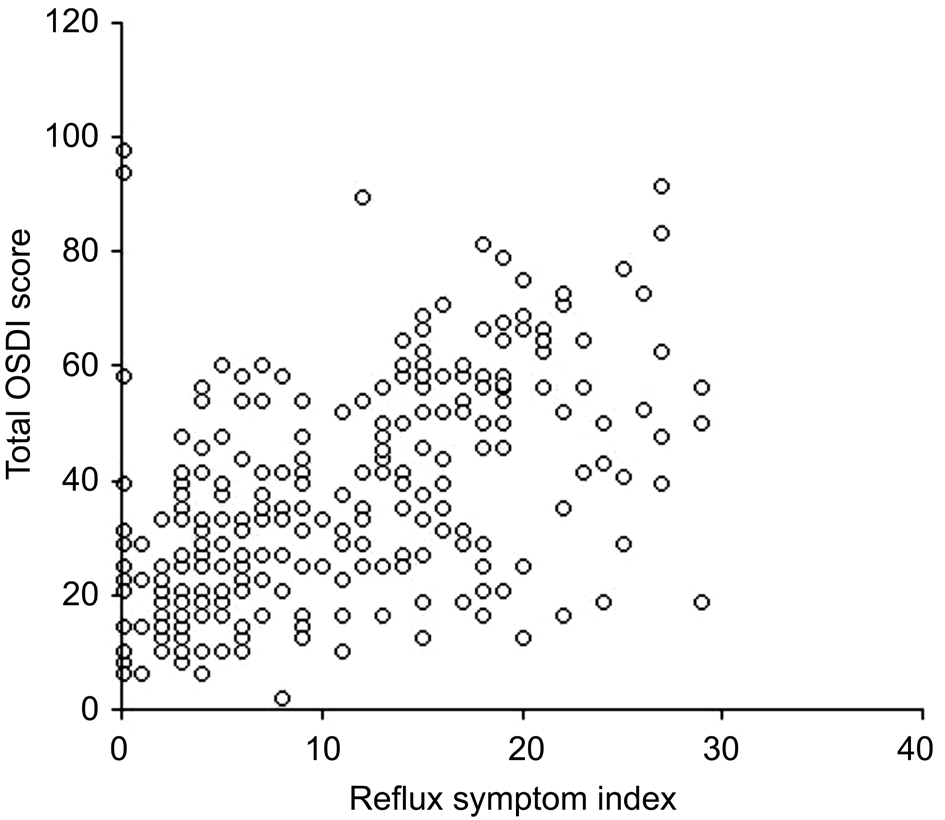
Figure 1 Correlation between total OSDI score and RSI score.
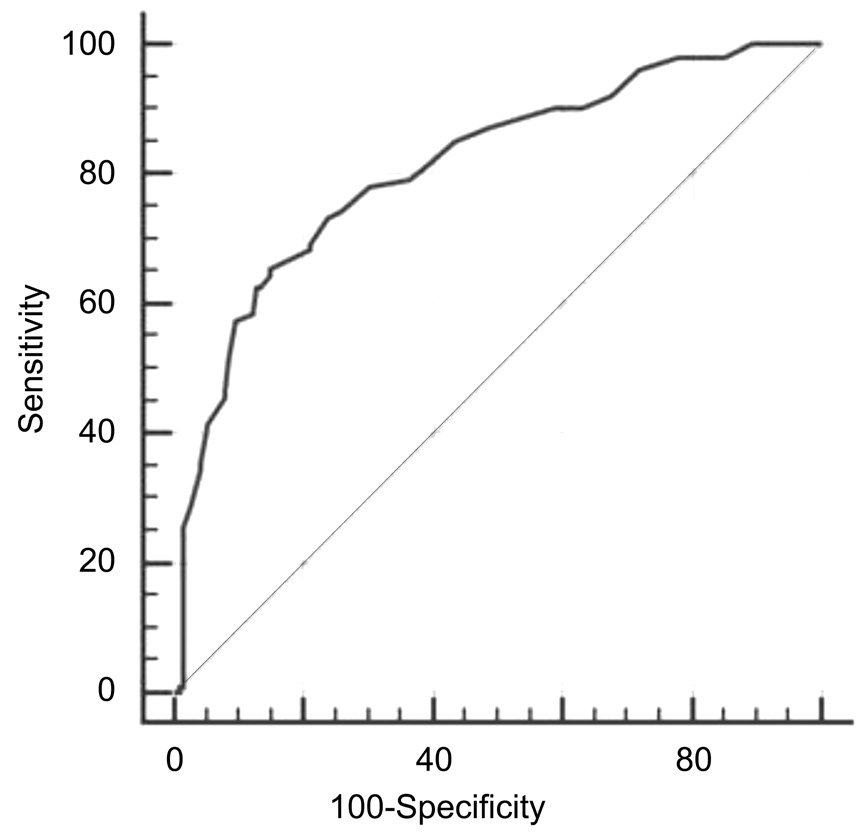
Figure 2 ROC curve to define the best total OSDI score cut-off able to discriminate patients with or without LPR on the basis of normal/abnormal RSI.
On the other hand, LPR diagnosis has been proposed on the basis of clinical insight and using a specific questionnaire, i.e. RSI [25] . On the basis of this background, the current study tested the hypothesis that LPR could affect eye, mainly concerning anterior segment area and function.
The present outcomes demonstrate that suspected LPR is common in a selected population as more than one third of patients have a positive RSI questionnaire suggestive for LPR.Patients with suspected LPR frequently may present impaired ocular function and symptoms. In particular, patients with suspected LPR show a significant association between severity of digestive symptoms and ocular complaints. This outcome underlines the close association between suspected LPR and ocular involvement as the severity of reflux symptoms well correlated with ocular symptom severity. Noteworthy, an OSDI cut-off has been defined as reliable predictor for LPR: 41.67.Another, clinically relevant outcome is the age of patients:usually old patients more easily may have LPR [34] . This aspectis rather understandable as it is well known that GERD is common in people aging more than 50y.
Table 2 Frequency of ocular symptoms, vision related functioning, and environmental triggers evaluated by the OSDI questionnaire in patients with pathologic or normal RSI
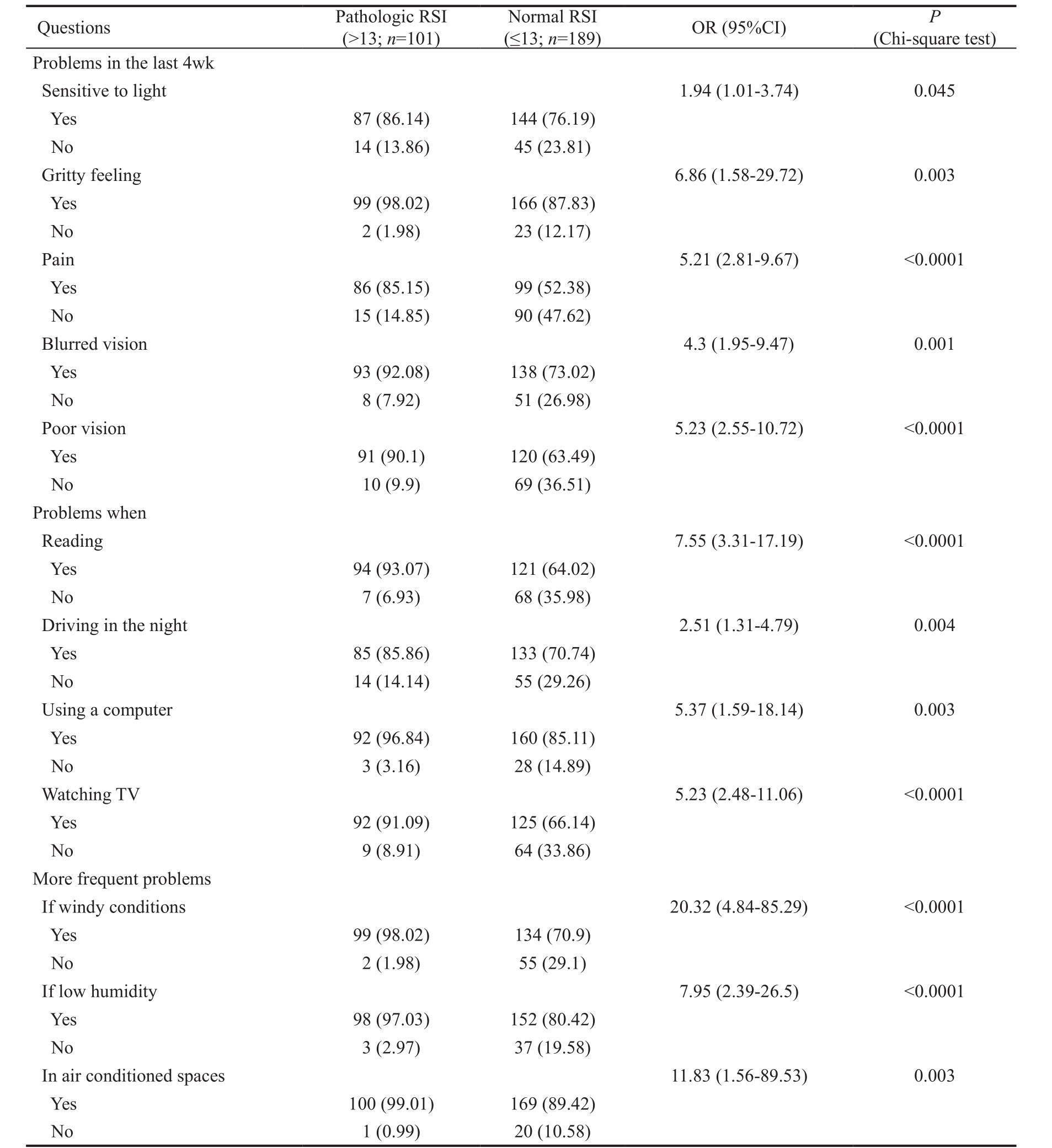
OSDI: Ocular Surface Disease Index; RSI: Reflux Symptom Index; OR: Odds ratio; CI: Confidence intervals. Data are reported as absolute frequency (number) and percentages in parentheses.
(≤13; n =189) OR (95%CI) P (Chi-square test)Problems in the last 4wk Sensitive to light 1.94 (1.01-3.74) 0.045 Yes 87 (86.14) 144 (76.19)No 14 (13.86) 45 (23.81)Gritty feeling 6.86 (1.58-29.72) 0.003 Yes 99 (98.02) 166 (87.83)No 2 (1.98) 23 (12.17)Pain 5.21 (2.81-9.67) <0.0001 Yes 86 (85.15) 99 (52.38)No 15 (14.85) 90 (47.62)Blurred vision 4.3 (1.95-9.47) 0.001 Yes 93 (92.08) 138 (73.02)No 8 (7.92) 51 (26.98)Poor vision 5.23 (2.55-10.72) <0.0001 Yes 91 (90.1) 120 (63.49)No 10 (9.9) 69 (36.51)Problems when Reading 7.55 (3.31-17.19) <0.0001 Yes 94 (93.07) 121 (64.02)No 7 (6.93) 68 (35.98)Driving in the night 2.51 (1.31-4.79) 0.004 Yes 85 (85.86) 133 (70.74)No 14 (14.14) 55 (29.26)Using a computer 5.37 (1.59-18.14) 0.003 Yes 92 (96.84) 160 (85.11)No 3 (3.16) 28 (14.89)Watching TV 5.23 (2.48-11.06) <0.0001 Yes 92 (91.09) 125 (66.14)No 9 (8.91) 64 (33.86)More frequent problems If windy conditions 20.32 (4.84-85.29) <0.0001 Yes 99 (98.02) 134 (70.9)No 2 (1.98) 55 (29.1)If low humidity 7.95 (2.39-26.5) <0.0001 Yes 98 (97.03) 152 (80.42)No 3 (2.97) 37 (19.58)In air conditioned spaces 11.83 (1.56-89.53) 0.003 Yes 100 (99.01) 169 (89.42)No 1 (0.99) 20 (10.58)Questions Pathologic RSI(>13; n =101)Normal RSI
However, this study has some relevant limitations, including the cross-sectional design, the lack of functional and macroscopic investigation of the upper digestive and respiratory tract,the lack of pepsin assessment in the tears, and the lack of a follow-up. Moreover, the degree of the relationship could be not enough to define the real link betwwen reflux and eye involvement.
Anyway, a strength of the current study is the number of evaluated patients and the contemporary evaluation of digestive and ocular symptoms using validated instruments.
In conclusion, a suspected LPR may be common in patients with OSD and an OSDI score >42 may be predictive for positive RSI.
ACKNOWLEDGEMENTS
Conflicts of Interest: Mazzacane D, None; Damiani V, Employee at DMG, Italy; Silvestri M, None; Ciprandi G, None; Marino PF, None; G.O.A.L.-O.S.D. Study Group, None.
REFERENCES
1 Gyawali CP, Kahrilas PJ, Savarino E, Zerbib F, Mion F, Smout AJPM,Vaezi M, Sifrim D, Fox MR, Vela MF, Tutuian R, Tack J, Bredenoord AJ,Pandolfino J, Roman S. Modern diagnosis of GERD: the Lyon Consensus. Gut 2018;67(7):1351-1362.
2 Gelardi M, Silvestri M, Ciprandi G, et al . Relieving laryngopharingeral reflux (RELIEF) survey in otolaryngology-II the viewpoint of the patient. J Biol Regul Homeost Agents 2018;32(1 Suppl. 2):21-28.
3 Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006;101(8):1900-1920
4 Vaezi MF, Hicks DM, Abelson TI, Richter JE. Laryngeal signs and symptoms and gastroesophageal reflux disease (GERD): a critical assessment of cause and effect association. Clin Gastroenterol Hepatol 2003;1(5):333-344.
5 Kahrilas PJ, Shaheen NJ, Vaezi MF; American Gastroenterological Association Institute; Clinical Practice and Quality Management Committee.American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology 2008;135(4):1392-1413
6 Nehra AK, Alexander JA, Loftus CG, Nehra V. Proton pump inhibitors:review of emerging concerns. Mayo Clin Proc 2018;93(2):240-246.
7 Francis DO, Rymer JA, Slaughter JC, Choksi Y, Jiramongkolchai P,Ogbeide E, Tran C, Goutte M, Garrett CG, Hagaman D, Vaezi MF. High economic burden of caring for patients with suspected extraesophageal reflux. Am J Gastroenterol 2013;108(6):905-911.
8 Bulmer DM, Ali MS, Brownlee IA, Dettmar PW, Pearson JP. Laryngeal mucosa: its susceptibility to damage by acid and pepsin. Laryngoscope 2010;120(4):777-782.
9 Adhami T, Goldblum JR, Richter JE, et al . Role of gastric and duodenal ingredients in laryngeal tissue injury: an experimental study in dogs. Am J Gastroenterol 2004;99:2098-2106.
10 Loughlin CJ, Koufman JA, Averill DB, Cummins MM, Kim YJ, Little JP, Miller IJ Jr, Meredith JW. Acid-induced laryngospasm in a canine model. Laryngoscope 1996;106(12 Pt 1):1506-1509.
11 Johnston N, Wells CW, Samuels TL, Blumin JH. Pepsin in non-acid refluxate can damage hypopharyngeal epithelial cells. Ann Otol Rhinol Laryngol 2009;118:677-685.
12 Saber H, Ghanei M. Extra-esophageal manifestations of gastroesophageal reflux disease: controversies between epidemiology and clinic. Open Respir Med J 2012;6:121-126.
13 Kung YM, Hsu WH, Wu MC, Wang JW, Liu CJ, Su YC, Kuo CH,Kuo FC, Wu DC, Wang YK. Recent advances in the pharmacological management of gastroesophageal reflux disease. Dig Dis Sci 2017;62(12):3298-3316.
14 Owji N, Abtahi SM. Does gastroesophageal reflux contribute to development of acquired nasolacrimal duct obstruction? Med Hypotheses 2010;74(3):455-456.
15 Owji N, Abtahi SMB, Azarpira N. Detection of Helicobacter pylori in the lacrimal sac mucosa of the patients with primary acquired nasolacrimal duct obstruction. Eye Reports 2011;1:e141.
16 Bagheri M, Rashe Z, Ahoor MH, Somi MH. Prevalence of helicobacter pylori infection in patients with central serous chorioretinopathy: a review. Med Hypothesis Discov Innov Ophthalmol 2017;6(4):118-124.
17 Magliulo G, Plateroti R, Plateroti AM. Gastroesophageal reflux disease and the presence of pepsin in the tears. Med Hypotheses 2013;80(2):129-130.
18 Iannella G, Di Nardo G, Plateroti R, Rossi P, Plateroti AM, Mariani P,Magliulo G. Investigation of pepsin in tears of children with laryngopharyngeal reflux disease. Int J Pediatr Otorhinolaryngol 2015;79(12):2312-2315.
19 Walt JG, Rowe MM, Stern KL. Evaluating the functional impact of dry eye: the Ocular Surface Disease Index. Drug Int J 1997;31:1436.
20 Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol 2000;118(5):615-621.
21 Pakdel F, Gohari MR, Jazayeri AS, Amani A, Pirmarzdashti N, Aghaee H. Validation of farsi translation of the Ocular Surface Disease Index. J Ophthalmic Vis Res 2017;12(3):301-304.
22 Versura P, Frigato M, Bernabini B, Mulè R, Malavolta N, Campos EC. Ocular surface analysis in patients affected with rheumatic diseases. Reumatismo 2004;56(4):262-271.
23 Miller KL, Walt JG, Mink DR, et al . Minimally clinically important difference fort he Ocular Surface Disease Index. Arch Ophthalmol 2010;128:94-101.
24 O’Brien PD, Collum LM. Dry eye: diagnosis and current treatment strategies. Curr Allergy Asthma Rep 2004;4(4):314-319.
25 Belafsky PC, Postma GN, Koufman JA. Validity and reliability of the reflux symptom index (RSI). J Voice 2002;16(2):274-277.
26 Campagnolo AM, Priston J, Thoen RH, Medeiros T, Assunção AR.Laryngopharyngeal reflux: diagnosis, treatment, and latest research. Int Arch Otorhinolaryngol 2014;18(2):184-191.
27 Swinscow TDV. Correlation and regression. In: Swinscow TDV, Campbell MJ. eds. Statistics at square one. 9 th edn. Southampton: BMJ Publishing Group 1997:75-84.
28 O’Reilly RC, Soundar S, Tonb D, Bolling L, Yoo E, Nadal T, Grindle C, Field E, He Z. The role of gastric pepsin in the inflammatory cascade of pediatric otitis media. JAMA Otolaryngol Head Neck Surg 2015;141(4):350-357.
29 Abd El-Fattah AM, Abdul Maksoud GA, Ramadan AS, Abdalla AF,Abdel Aziz MM. Pepsin assay: a marker for reflux in pediatric glue ear. Otolaryngol Head Neck Surg 2007;136(3):464-470.
30 He Z, O’Reilly RC, Mehta D. Gastric pepsin in middle ear fluid of children with otitis media: clinical implications. Curr Allergy Asthma Rep 2008;8(6):513-518.
31 Samuels TL, Johnston N. Pepsin as a marker of extraesophageal reflux. Ann Otol Rhinol Laryngol 2010;119(3):203-208.
32 Tasker A, Dettmar PW, Panetti M, Koufman JA, P Birchall J, Pearson JP. Is gastric reflux a cause of otitis media with effusion in children? Laryngoscope 2002;112(11):1930-1934.
33 Lieu JE, Muthappan PG, Uppaluri R. Association of reflux with otitis media in children. Otolaryngol Head Neck Surg 2005;133(3):357-361.
34 Ciprandi G, Gelardi M. Gastric reflux: comparison between the gastroenterologist and the otorhinolaryngologist’s approach. Pragmatic conclusive remarks. J Biol Regul Homeost Agents 2018;32(1 Suppl. 2):33-38.
Citation: Mazzacane D, Damiani V, Silvestri M, Ciprandi G,Marino PF; G.O.A.L.-O.S.D. Study Group. Eye reflux: an ocular extraesophageal manifestation of gastric reflux. Int J Ophthalmol 2018;11(9):1503-1507
Received: 2018-02-28 Accepted: 2018-03-23
DOl: 10.18240/ijo.2018.09.12
Correspondence to: Giorgio Ciprandi. L.go R, Benzi 10,Genoa 16132, Italy. gio.cip@libero.it



