Table 1 PCR: primer sequences and fragment lengths
bp: Base pair.
Miroslav Stamenkovic 1,2 , Vesna Lukic 1 , Sonja Suvakov 3,4 , Tatjana Simic 3,4 , Ivan Sencanic 1 , Marija Pljesa-Ercegovac 3,4 , Vesna Jaksic 1,4 , Sinisa Babovic 1 , Marija Matic 3,4 , Aleksandra Radosavljevic 4,5 ,Ana Savic-Radojevic 3,4 , Tatjana Djukic 3,4
1 University Eye Clinic, Medical Center Zvezdara, Belgrade 11000, Serbia
2 Faculty of Special Education and Rehabilitation, University of Belgrade, Belgrade 11000, Serbia
3 Institute of Medical and Clinical Biochemistry, Belgrade 11000, Serbia
4 Faculty of Medicine, University of Belgrade, Belgrade 11000,Serbia
5 Hospital for Eye Diseases, Clinical Center of Serbia, Belgrade 11000, Serbia
Abstract · AlM: To evaluate glutathione transferase theta 1 and mu 1 ( GSTT1 and GSTM1 ) polymorphisms as determinants of primary open angle glaucoma (POAG) risk, independently or in combination with cigarette smoking, hypertension and diabetes mellitus.· METHODS: A case-control study with 102 POAG patients and 202 age and gender-matched controls was carried out.Multiplex-polymerase chain reaction method was used for the analysis of GSTM1 and GSTT1 polymorphisms.The differences between two groups were tested by the t -test or χ 2 test. Logistic regression analysis was used for assessing the risk for disease development.· RESULTS: The presence of GSTM1-null genotype did not contribute independently towards the risk of POAG.However, individuals with GSTT1-active genotype were at almost two-fold increased risk to develop glaucoma( P =0.044) which increased up to 4.36 when combined with GSTM1-null carriers ( P =0.024). When glutathione transferase ( GST ) genotypes were analyzed in association with cigarette smoking, hypertension and diabetes,only carriers of GSTT1-active genotype had significantly increased risk of POAG development in comparison with GSTT1-null genotype individuals with no history of smoking, hypertension and diabetes, respectively(OR=3.52, P =0.003; OR=10.02, P <0.001; OR=4.53, P =0.002).· CONCLUSlON: The results obtained indicate that both GSTM1-null and GSTT1-active genotypes are associated with increased POAG risk among smokers, suggesting potential gene-environment interaction in glaucoma development.
· KEYWORDS: GSTM1 ; GSTT1 ; primary open angle glaucoma risk; smoking; hypertension; diabetes mellitus
Glaucoma, the second leading cause of blindness in the world, represents a group of diseases characterized by progressive degeneration of the retinal ganglion cells (RGC)and their axons [1] . Primary open angle glaucoma (POAG) is the most common form with multifactorial etiology, including genetic and environmental factors [2] . As for the genetic factors,until now, only three genes have been associated to POAG pathogenesis, optineurin, myocilin, and tryptophan-aspartic acid repeat containing protein 36 [2] . Beside positive family history, the recent study indicated elevated intraocular pressure(IOP), older age, sub-Saharan African ethnic origin and high myopia as main risk factors for POAG [3-4] . Furthermore,other important contributing factors influencing primarily the increase of IOP are related to atherosclerosis development,specifically cigarette smoking, hypercholesterolemia, diabetes mellitus and obesity [5] . What is more, it has been proposed that oxidative stress, as a consequence of elevated IOP,might contribute to disease progression by inducing outflow resistance through trabecular meshwork (TM) and the RGC damage [5-8] .
Glutathione transferases (GST) include a family of enzymes with distinct catalytic and non-catalytic functions [9] . Namely,GST catalyze the breakdown and the detoxification reactions of different xenobiotics and reactive oxygen species (ROS) [10] .Furthermore, literature date are indicating GST as modulators of cell proliferation and apoptosis, in terms of regulators of different protein kinases, one of them being apoptosis signal-regulating kinase 1 (ASK1) [11-12] . Almost all cytosolic GST classes exhibit genetic polymorphism [13] . Deletions polymorphisms occurring in glutathione transferase mu 1 ( GSTM1 ) and glutathione transferase theta 1 ( GSTT1 ) genes,resulting in a complete absence of enzyme activity [13] influence individual predisposition to environmental and oxidative stress [11,14] . Namely, due to a homozygous deletion of a 15-kb allele of the GSTM1 gene, about half of the Caucasians are carriers of GSTM1-null genotype and do not have an active GSTM1 enzyme [15-17] . As for the GSTT1 genotype, the lack of the enzyme activity is present in 20% of Caucasians carriers of the GSTT1-null genotype [18] .
Many studies have investigated the association of GSTM1 and GSTT1 polymorphism with the risk of POAG, however with inconsistent results. The lack of association and controversial results for both GSTM1 and GSTT1 genotypes in relation to POAG might be due to the specific genetic background present in different world populations, the environmental factors and their interactions [19-20] . Both polymorphisms were extensively studied in oxidative stress-related conditions, such as obesity,type 2 diabetes, coronary heart disease, neurodegenerative diseases or smoking habits [21-27] . Based on the important role of GSTM1 enzyme in detoxifying benzodiolepoxide,present in tobacco smoke and environmental pollution, GSTM1-null -genotype-smoking interplay in POAG could be hypothesized [13,27] . To our knowledge, the effect of those polymorphisms in conjunction with hypertension and diabetes in POAG was not investigated as yet.
Owing to the fact that oxidative damage caused by increased production of ROS might play an important role in the pathophysiology of POAG, the aim of this study was to determine the relative risk associated with GSTM1 and GSTT1 polymorphisms, as well as possible association of those polymorphisms with cigarette smoking, hypertension and diabetes mellitus, as possible modifying risk factors for POAG.
Patients One hundred and two patients with POAG (45 men and 57 women, mean age 72.47±8.30y) were recruited from Eye Clinic, Zvezdara University Medical Centre, Belgrade and a group of 202 controls (91 men and 111 women, mean age 71.73±6.89y). Before the enrollment in the study, all patients were clinically examined and the diagnosed of POAG by an ophthalmologists. The following criteria were used for the diagnosis of POAG: IOP greater than 21 mm Hg before treatment with IOP lowering drugs, characteristic cupping of the optic disc, clinically present open angle of the anterior chamber on the gonioscopy and the typical glaucoma visual field changes detected by Humphrey’s visual field analyzer.
Only glaucoma patients who fulfilled the necessary criteria were included in our study. The exclusion criteria were: IOP bellow 21 mm Hg, the presence of the clinically open angle without any visual field and optic disc abnormalities, history of uveitis, trauma, primary angle closure glaucoma or secondary glaucoma. The demographic data together with the information about diabetes mellitus, hypertension and smoking status were gathered from each study subject using standard questionnaire at the moment of blood collection. In our study, smokers were delineated as individuals who smoked every day for a minimum of 60d period before their enrolment in the study.Furthermore, they gave the information how many cigarettes they smoked daily and the duration of smoking, as well. All the study participant have signed the informed consent before they were enrolled in the study. The study was approved by the Ethical Committee of the University Medical Centre Zvezdara,Belgrade and the research was carried out in a compliance with the Declaration of Helsinki.
Sample Collection and DNA Analysis Commercially available DNA kit (Qiagen, USA) was used to isolate DNA from blood leucocytes. The analysis of GSTM1 and GSTT1 polymorphisms was carried out by polymerase chain reaction(PCR) using the multiplex PCR method [28] . CYP1A1 was used as a housekeeping gene (Table 1). PCR protocol was as followed: denaturation at 94℃ for 4min followed by 94℃ for 30s; annealing: 59℃ for 30s; extension: 72℃ for 45s; number of cycles: 30; final extension: 72℃ for 5min.The blinded samples for quality control were included to ensure the validation of procedures and steps in the genotype identification and the investigators who performed the multiplex PCR analysis were uninformed of the case-control status. The similarity of the results for the blinded samples was 100%.
Statistical Analysis The data were analyzed using the Statistical Package for the Social Sciences (SPSS, version 17.0; SPSS, Chicago, IL, USA). Categorical variables were presented using frequency [ n (%)] counts and parametric variables were presented as mean±standard deviation. The t -test for continuous variables with normal distribution and χ 2 test for categorical variables were used to assess the differences between two groups. The logistic regression analysis was used for assessing the risk for disease development and the results were adjusted by age and gender. P <0.05 was considered statistically significant.
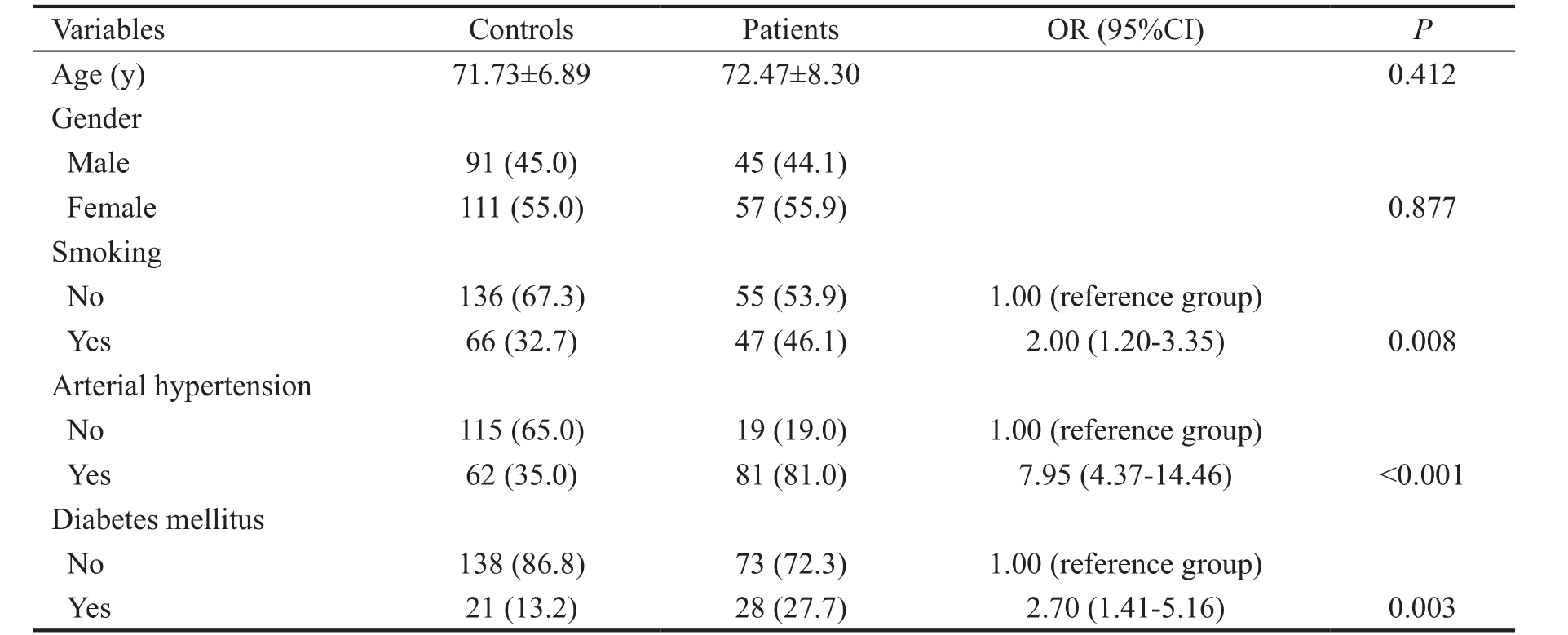
The clinical characteristics of POAG patients and controls are presented in Table 2. A total of 102 patients (45 males and 57 females) and 202 controls (91 males and 111 females) were included in the study. We found no statistical significance regarding age ( P =0.412) and gender ( P =0.877) between the glaucoma patients and controls. As expected, the smokingprevalence among POAG patients was higher (46.1%) than in the control group (32.7%). After adjustment for age and gender we found that the history of cigarette smoking was associated with significantly increased POAG risk of 2.0 (95%CI 1.20-3.35, P =0.008).
Table 1 PCR: primer sequences and fragment lengths

bp: Base pair.
Table 2 Baseline characteristic of patients with POAG and respective controls mean±SD, n (%)
OR: Odds ratio adjusted for age and gender.
Glaucoma patients and controls were further stratified according to the presence of either arterial hypertension or diabetes mellitus and the risk of POAG development was assessed afterwards. Indeed, almost eight-fold increased risk to develop glaucoma was found in individuals with the history of arterial hypertension (OR=7.95, 95%CI=4.37-14.46, P <0.001)in comparison with the healthy ones. As for diabetes mellitus,diabetics were in 2.7-fold increased risk to develop POAG during life compared to the individuals with no history of diabetes (OR=2.70, 95%CI=1.41-5.16, P =0.003).
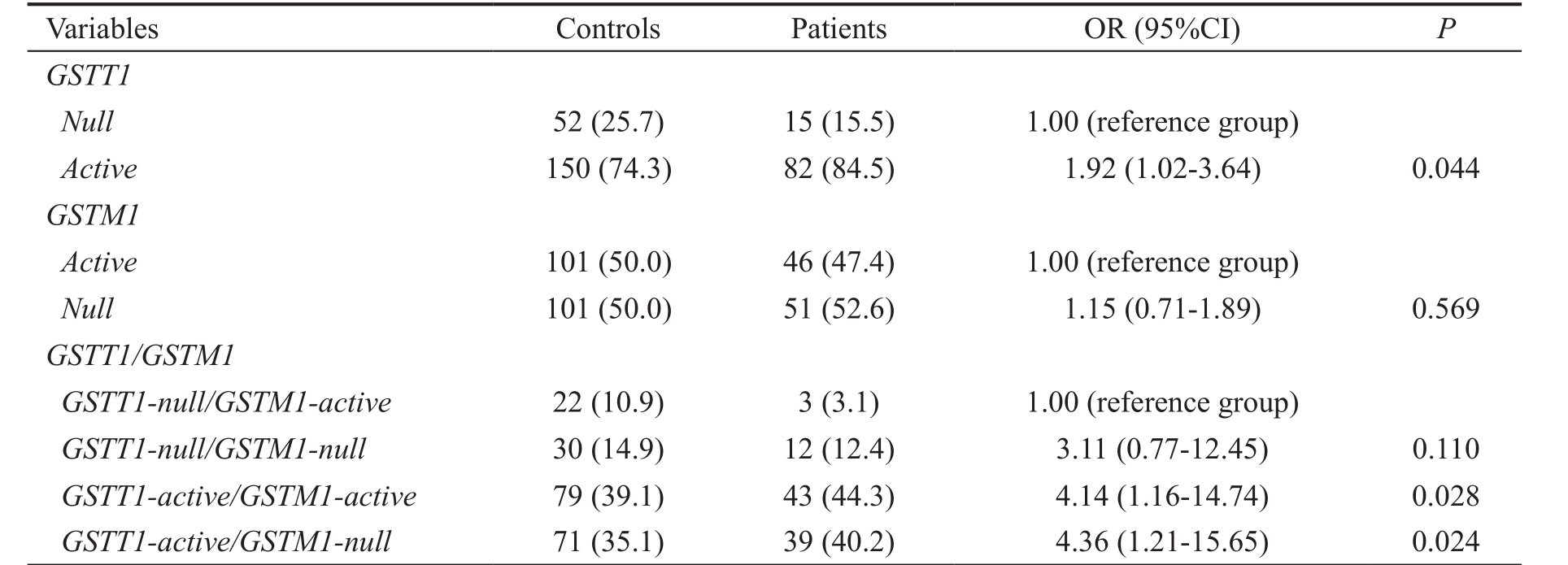
Glutathione Transferase Genotypes and Primary Open Angle Glaucoma Risk The frequency of GSTM1-null genotype was slightly higher in glaucoma patients (52.6%)than in controls (50.0%), hence without statistical significance(OR=1.15, 95%CI=0.71-1.89, P =0.569). The GSTT1-active genotype was significantly more common in patients with POAG (84.5%) than in healthy controls (74.3%). Furthermore,the individuals with GSTT1-active genotype were at almost two-fold increased risk to develop glaucoma than carriers of GSTT1-null genotype (OR=1.92, 95%CI=1.02-3.64, P =0.044)while the risk was even higher when combined with GSTM1-null genotype (OR=4.36, 95%CI=1.21-15.65, P =0.024) (Table 3).
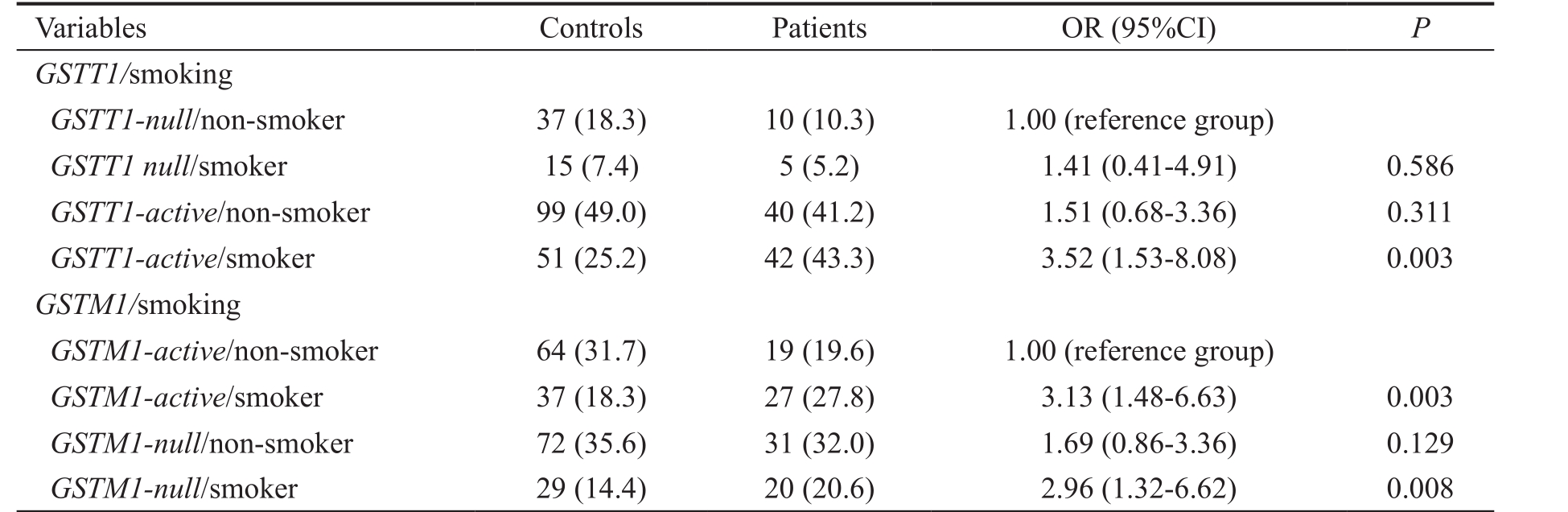
Modifying Effect of Smoking, Hypertension and Diabetes Mellitus on Glutathione Transferases Genotypes in Relation to Primary Open Angle Glaucoma Risk The significant association was found for both GST genotypes and the risk of POAG in smokers. However, modifying effect of smoking was observed only among smokers with GSTT1-active genotype who were at 3.5-fold higher risk to develop POAG (OR=3.52, 95%CI=1.53-8.08, P =0.003) (Table 4).
When modifying effect of hypertension was analyzed in relation to GST genotypes, it was observed that GSTT1-active hypertensive carriers were at 10-fold increased risk to develop glaucoma (OR=10.02, 95%CI=3.26-30.73, P <0.001). Namely,the overall risk was higher in combination, than when the risk of GSTT1 or hypertension was assessed alone. Although hypertensive GSTM1-null carriers were at increased risk to develop glaucoma (OR=7.12, 95%CI=3.06-16.56, P <0.001),this can be attributed to independent effect of hypertension(Table 5).
Further, the aim of our attention was POAG patients with diabetes mellitus (Table 6). We found that diabetic carriers of GSTT1-active genotype were at 4.5-times increased risk to develop glaucoma (OR=4.53, 95%CI=1.76-11.66, P =0.002).The risk was higher than the risk of GSTT1 or diabetes when assessed alone. Similarly to hypertension, increased risk in GSTM1-null carriers with diabetes was probably the consequence of independent effect of diabetes (OR=2.85,95%CI=1.09-7.41, P =0.032).
Table 3 GSTT1 and GSTM1 genotypes in relation to the risk of POAG n (%)
OR: Odds ratio adjusted for age and gender.
Table 4 Modifying effect of smoking on GST genotypes in relation to the risk of POAG n (%)
OR: Odds ratio adjusted for age and gender.
Table 5 Modifying effect of hypertension on GST genotypes in relation to the risk of POAG n (%)
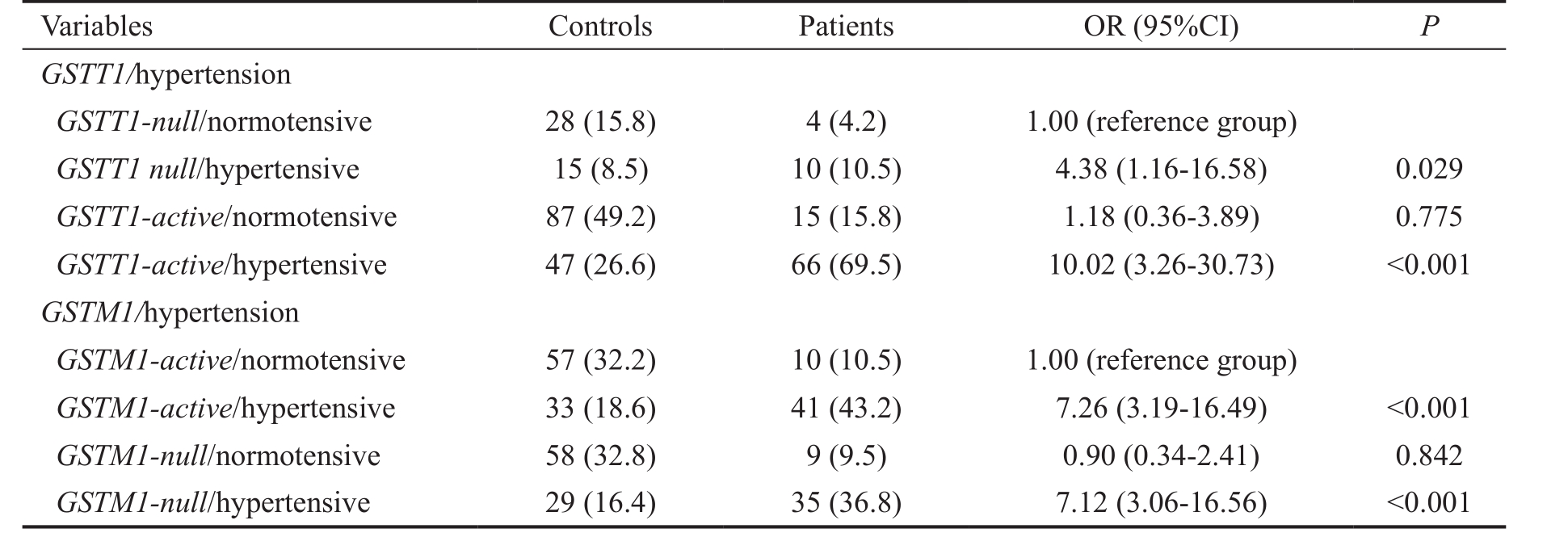
OR: Odds ratio adjusted for age and gender.
Based on relatively high prevalence of hypertensive smokers among POAG patients in our study, we further stratified them according to both GST genotypes, as well as smoking and hypertension status. We found that hypertensive smokers,carriers of the GSTT1-active or GSTM1-null genotype,were at increased risk of developing glaucoma compared to normotensive non-smokers, carriers of GSTT1-null or GSTM1-active genotype ( P <0.001, data not shown). However, due to the limited number of study participants in each subgroup, the observed joint effect of hypertension and smoking on GST genotypes in relation to POAG risk requires further statistical analysis on a larger population.
In the present study, independent GSTT1-active genotype was associated with almost 2-fold increased risk to develop POAG.The risk was even higher when this genotype was combined with GSTM1-null genotype. Furthermore, we have shown that these genotypes in conjunction with cigarette smoking,hypertension and diabetes increase the risk of POAG.
The research data underline the important role of ROS andoxidative stress in pathophysiology and progression of POAG [5-8] . Oxidative stress, due to an imbalance between the generation of ROS and the antioxidant defense, can result in cell membrane lipid peroxidation and damage to DNA and proteins affecting different eye structures. Specifically, it has been proposed that oxidative stress provides a setting for RGC damage and along these lines directly participates in optic nerve neuropathy [29] . Moreover, ROS may compromise TM integrity facilitating the increase of IOP which is considered to be the most important risk factor for POAG [6] .
Table 6 Modifying effect of diabetes mellitus on GST genotypes in relation to the risk of POAG n (%)
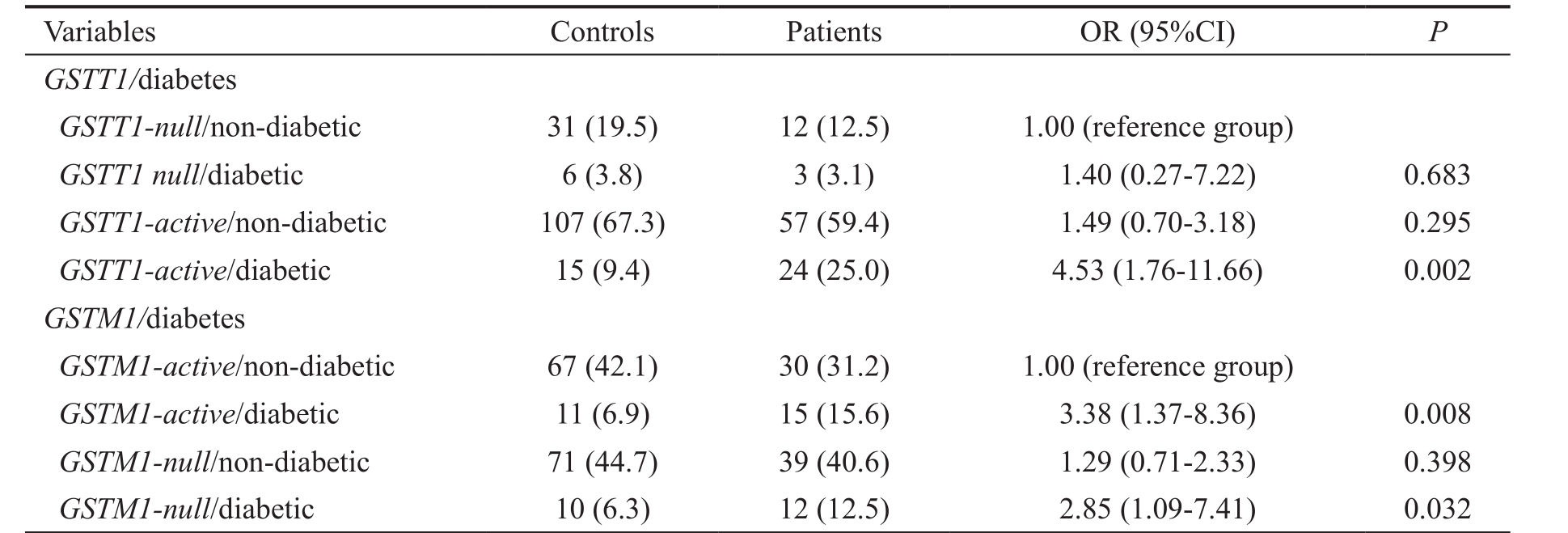
OR: Odds ratio adjusted for age and gender.
GST are polymorphic enzymes serving as cellular guardians against the oxidative stress. In our study GSTM1-null genotype was more frequent in the group of glaucoma patients but without statistical significance in comparison with healthy controls which is in agreement with several other investigations [30-33] .On the other hand, the association of either GSTM1-null genotype, described in Brazilian [34-35] , Italian [36] , Turkish [37] and Greek [38] population or GSTM1-active genotype described in an Estonian [33] and Turkish population [39] supported the notion of GSTM1 as a risk factor of POAG. Regarding GSTT1 deletion polymorphism, we found that carriers of GSTT1-active genotype were at significantly increased risk to develop POAG, which is in concordance with the results of a research conducted in the Brazilian population [34] , while, in contrast to this, several others studies found association for GSTT1-null genotype [39-40] . In the studies of Chinese [31] , Mexican [41] , Estonian [33] and Turkish population [37] no association was found of GSTT1 genotype and the risk for POAG.
Regarding recognized risk factors for POAG development in this study, an increase in POAG risk was observed among individuals with smoking habits, hypertension and diabetes, which is in agreement with previous findings [42-46] .Beside more than 60 carcinogens, cigarette smoke is also an abundant source of the free radicals. Both polycyclic aromatic hydrocarbon (PAH) metabolites and free radicals are detoxified by GST [13] . We found that GSTT1-active smokers were at 3.5-fold increased risk in comparison with non-smoker GSTT1-null carriers. These results are biologically plausible since GSTT1 mediated detoxification of various xenobiotics sometimes produces even more toxic products [13] . Such a mechanism could give a possible explanation for the increased risk of POAG among carriers of GSTT1-active genotype especially if they are smokers. Moreover, hypertension and diabetes contributed to genotype-associated POAG risk in the analyzed polymorphisms, especially in GSTT1-active genotype carriers,thus emphasizing the important role of gene-environmental interactions in POAG development.
The results of our study showing more than 4-fold risk of POAG in carriers of GSTM1-null/GSTT1-active genotype are in agreement with the recent study by Rocha et al [34] . In their study, they have found a powerful association of GSTM1-null genotype with higher levels of IOP, more drastic optic nerve and visual field damage. On the grounds that prolonged oxidative stress may contribute to the increase of IOP [6] ,our results on increased risk in GSTM1-null / GSTT1-active genotype indicate that oxidative damage might significantly affects the pathophysiology of POAG.
Recent findings strongly suggested the apoptosis of the RGC as one of the main events in both pathogenesis and progression of POAG, with special emphasis on activation of ASK1-mediated apoptotic pathway [47] . On the other hand, deletion of ASK1 gene prevents RGC death, including retinal ischemia and optic nerve injury as shown in various experimental glaucoma models [47] . The probable molecular mechanism underlying the role of GSTM1 in disease deterioration might be the GSTM1 non-catalytic regulatory role in apoptotic ASK1-MAPK (mitogen-activated protein kinase) signaling cascade. Several studies have shown that, independently of its transferase activity, GSTM1 protein can regulate ASK1 activity by protein-protein interaction. Thus, under non-stimulated conditions, GSTM1-1 suppresses ASK1-mediated activation of c-Jun NH2-terminal kinase/stress-activated protein kinase(JNK/SAPK) signaling cascade, and inhibits the apoptotic cell death dependent of ASK1. On the contrary, different stressors,such as ROS, can induce dissociation of GSTM1:ASK1 protein complex, consequently initiating ASK1-mediated apoptotic signaling pathway [48] . In that way, it can be speculated that in GSTM1-null POAG patients, apoptosis is more intense in RGC, even after antioxidant treatment. Thus, determination of GSTM1 and GSTT1 genotypes might be the valuable indicator in order to assess the risk for POAG and also as potential therapeutic targets.
Certain limitations are recognized in our study. Relatively small number of the study participants might be the source of potential biases and decrease the power of the study which may influence the study findings. In our study, all the participants were Caucasians, therefore the possible effect of ethnicity could not be assessed. Future studies with detailed patient information and rigorous designs are needed to increase the power of the study and to confirm the findings of our research.Even though, the present study justifies the assumption that GSTM1 and GSTT1 polymorphisms modulate the risk of POAG, with special emphasis on GSTT1-active genotypes.Since POAG is a condition where early diagnosis and treatment are of a great importance, diagnostic tests to determine those who are at risk to develop glaucoma can be very valuable.The final purpose of the research would be the identification of a full set of genes eligible to contribute to glaucoma and to develop not just diagnostic but prognostic tests. Such a set of genes would potentially provide a way to identify those more susceptible to develop POAG and introduce early treatment before permanent optic nerve degeneration and blindness develop.
ACKNOWLEDGEMENTS
Foundations: Supported by the Serbian Ministry of Education,Science and Technological Development (No.175052;No.450009).
Conflicts of Interest: Stamenkovic M, None; Lukic V, None; Suvakov S, None; Simic T, None; Sencanic I, None; Pljesa-Ercegovac M, None; Jaksic V, None; Babovic S, None; Matic M, None; Radosavljevic A, None; Savic-Radojevic A, None; Djukic T, None.
REFERENCES
1 Weinreb RN, Khaw PT. Primary open angle glaucoma. Lancet 2004;363(9422):1711-1720.
2 Wiggs JL. Genetic etiologies of glaucoma. Arch Ophthalmol 2007;125(1):30-37.
3 Renard JP, Rouland JF, Bron A, et al . Nutritional, lifestyle and environmental factors in ocular hypertension and primary open angle glaucoma: an exploratory case-control study. Acta Ophthalmol 2013;91(6):505-513.
4 Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S.Glaucoma. Lancet 2017;390(10108):2183-2193.
5 Wójcik-Gryciuk A, Skup M, Waleszczyk WJ. Glaucoma-state of the art and perspectives on treatment. Restor Neurol Neurosci 2015;34(1):107-123.
6 Saccà SC, Izzotti A, Rossi P, Traverso C. Glaucomatous outflow pathway and oxidative stress. Exp Eye Res 2007;84(3):389-399.
7 Aslan M, Cort A, Yucel I. Oxidative and nitrative stress markers in glaucoma. Free Radic Biol Med 2008;45(4):367-376.
8 Ghanem AA, Arafa LF, El-Baz A. Oxidative stress markers in patients with primary open angle glaucoma. Curr Eye Res 2010;35(4):295-301.
9 Wu B, Dong D. Human cytosolic glutathione transferases: structure,function, and drug discovery. Trends Pharmacol Sci 2012;33(12):656-668.
10 Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol 2005;45:51-88.
11 Board PG, Menon D. Glutathione transferases, regulators of cellular metabolism and physiology. Biochim Biophys Acta 2013;1830(5):3267-3288.
12 Tew KD, Townsend DM. Glutathione-s-transferases as determinants of cell survival and death. Antioxid Redox Signal 2012;17(12):1728-1737.
13 Hayes JD, Strange RC. Glutathione S-transferase polymorphisms and their biological consequences. Pharmacology 2000;61(3):154-166.
14 Hollman AL, Tchounwou PB, Huang HC. The association between geneenvironment interactions and diseases involving the human GST superfamily with SNP variants. Int J Environ Res Public Health 2016;13(4):379.
15 Benhamou S, Lee WJ, Alexandrie AK, et al . Meta- and pooled analyses of the effects of glutathione S-transferase M1 polymorphisms and smoking on lung cancer risk. Carcinogenesis 2002;23(8):1343-1350.
16 Tibaut M, Petrovič D. Oxidative stress genes, antioxidants and coronary artery disease in type 2 diabetes mellitus. Cardiovasc Hematol Agents Med Chem 2016;14(1):23-38.
17 Makuc J, Petrovič D. A review of oxidative stress related genes and new antioxidant therapy in diabetic nephropathy. Cardiovasc Hematol Agents Med Chem 2011;9(4):253-261.
18 Libetta C, Sepe V, Esposito P, Galli F, Dal Canton A. Oxidative stress and inflammation: implications in uremia and hemodialysis. Clin Biochem 2011;44(14-15):1189-1198.
19 Lu Y, Shi Y, Yin J, Huang Z. Are glutathione S-transferase polymorphisms(GSTM1, GSTT1) associated with primary open angle glaucoma? A meta-analysis. Gene 2013;527(1):311-315.
20 Huang W, Wang W, Zhou M, Chen S, Zhang X. Association of glutathione S-transferase polymorphisms (GSTM1 and GSTT1) with primary open angle glaucoma: an evidence-based meta-analysis. Gene 2013;526(2):80-86.
21 Almoshabek HA, Mustafa M, Al-Asmari MM, Alajmi TK, Al-Asmari AK. Association of glutathione S-transferase GSTM1 and GSTT1 deletion polymorphisms with obesity and their relationship with body mass index,lipoprotein and hypertension among young age Saudis. JRSM Cardiovasc Dis 2016;5:2048004016669645.
22 Raza S, Abbas S, Ahmad A, Ahmed F, Zaidi Z, Mahdi F. Association of glutathione-S-transferase (GSTM1 and GSTT1) and FTO gene polymorphisms with type 2 diabetes mellitus cases in northern India. Balk J Med Genet 2014;17(1):47-54.
23 Pinheiro DS, Rocha Filho CR, Mundim CA, Júnior P de M, Ulhoa CJ,Reis AAS, Ghedini PC. Evaluation of glutathione S-transferase GSTM1 and GSTT1 deletion polymorphisms on type-2 diabetes mellitus risk. PLoS One 2013;8(10):e76262.
24 Zhou D, Hu W, Wang Q, Jin Y. Glutathione S-transferase M1 polymorphism and coronary heart disease susceptibility: a meta-analysis involving 47596 subjects. Heart Lung Circ 2014;23(6):578-585.
25 Song Y, Shan Z, Luo C, Kang C, Yang Y, He P, Li S, Chen L, Jiang X, Liu L. Glutathione S-transferase T1 (GSTT1) null polymorphism,smoking, and their interaction in coronary heart disease: a comprehensive meta-analysis. Heart Lung Circ 2017;26(4):362-370.
26 Cerliani MB, Pavicic W, Gili JA, Klein G, Saba S, Richard S. Cigarette smoking, dietary habits and genetic polymorphisms in GSTT1, GSTM1 and CYP1A1 metabolic genes: a case-control study in oncohematological diseases. World J Clin Oncol 2016;7(5):395-405.
27 Matic M, Pekmezovic T, Djukic T, Mimic-Oka J, Dragicevic D, Krivic B, Suvakov S, Savic-Radojevic A, Pljesa-Ercegovac M, Tulic C, Coric V, Simic T. GSTA1, GSTM1, GSTP1, and GSTT1 polymorphisms and susceptibility to smoking-related bladder cancer: a case-control study. Urol Oncol 2013;31(7):1184-1192.
28 Abdel-Rahman SZ, el-Zein RA, Anwar WA, Au WW. A multiplex PCR procedure for polymorphic analysis of GSTM1 and GSTT1 genes in population studies. Cancer Lett 1996;107(2):229-233.
29 Nita M, Grzybowski A. The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid Med Cell Longev 2016;2016:3164734.
30 Jansson M, Rada A, Tomic L, Larsson LI, Wadelius C. Analysis of the glutathione S-transferase M1 gene using pyrosequencing and multiplex PCR-no evidence of association to glaucoma. Exp Eye Res 2003;77(2):239-243.
31 Fan BJ, Liu K, Wang DY, Tham CC, Tam PO, Lam DS, Pang CP.Association of polymorphisms of tumor necrosis factor and tumor protein p53 with primary open angle glaucoma. Invest Ophthalmol Vis Sci 2010;51(8):4110-4116.
32 Barbosa AM, Frare AB, Costa NB, Silva RE, Moura KK. GSTM1 polymorphism in patients with primary open angle glaucoma. Genet Mol Res 2012;11(3):3256-3262.
33 Juronen E, Tasa G, Veromann S, Parts L, Tiidla A, Pulges R, Panov A,Soovere L, Koka K, Mikelsaar AV. Polymorphic glutathione S-transferase M1 is a risk factor of primary open angle glaucoma among Estonians. Exp Eye Res 2000;71(5):447-452.
34 Rocha AV, Talbot T, Magalhães da Silva T, Almeida MC, Menezes CA,Di Pietro G, Rios-Santos F. Is the GSTM1 null polymorphism a risk factor in primary open angle glaucoma? Mol Vis 2011;17:1679-1686.
35 Silva CT, Costa NB, Silva KS, Silva RE, Moura KK. Association between primary open angle glaucoma and genetic polymorphisms GSTM1/GSTT1 in patients from Goiânia Central-West Region of Brazil. Genet Mol Res 2014;13(4):8870-8875.
36 Izzotti A, Saccà SC, Longobardi M, Cartiglia C. Mitochondrial damage in the trabecular meshwork of patients with glaucoma. Arch Ophthalmol 2010;128(6):724-730.
37 Yildirim O, Ateş NA, Tamer L, Oz O, Yilmaz A, Atik U, Camdeviren H. May glutathione S-transferase M1 positive genotype afford protection against primary open angle glaucoma? Graefes Arch Clin Exp Ophthalmol 2005;243(4):327-333.
38 Lavaris A, Gazouli M, Brouzas D, Moschos MM. Polymorphism analysis of GSTM1 and OPA1 genes in Greek patients with primary open angle glaucoma. In Vivo 2016;30(4):473-477.
39 Unal M, Güven M, Devranoğlu K, Ozaydin A, Batar B, Tamçelik N, Görgün EE, Uçar D, Sarici A. Glutathione S transferase M1 and T1 genetic polymorphisms are related to the risk of primary open angle glaucoma: a study in a Turkish population. Br J Ophthalmol 2007;91(4):527-530.
40 Abu-Amero KK, Morales J, Mohamed GH, Osman MN, Bosley TM.Glutathione S-transferase M1 and T1 polymorphisms in Arab glaucoma patients. Mol Vis 2008;14:425-430.
41 Buentello-Volante B, Elizondo-Olascoaga C, Miranda-Duarte A,Guadarrama-Vallejo D, Cabral-Macias J, Zenteno JC. Association study of multiple gene polymorphisms with the risk of adult-onset primary open angle glaucoma in a Mexican population. Exp Eye Res 2013;107:59-64.
42 Mitchell P, Lee AJ, Rochtchina E, Wang JJ. Open angle glaucoma and systemic hypertension: the blue mountains eye study. J Glaucoma 2004;13(4):319-326.
43 Bae HW, Lee N, Lee HS, Hong S, Seong GJ, Kim CY. Systemic hypertension as a risk factor for open angle glaucoma: a meta-analysis of population-based studies. PLoS One 2014;9(9):e108226.
44 Bonovas S, Filioussi K, Tsantes A, Peponis V. Epidemiological association between cigarette smoking and primary open angle glaucoma:a meta-analysis. Public Health 2004;118(4):256-261.
45 Zhou M, Wang W, Huang W, Zhang X. Diabetes mellitus as a risk factor for open angle glaucoma: a systematic review and meta-analysis. PLoS One 2014;9(8):e102972.
46 Shen L, Walter S, Melles RB, Glymour MM, Jorgenson E. Diabetes pathology and risk of primary open angle glaucoma: evaluating causal mechanisms by using genetic information. Am J Epidemiol 2016;183(2):147-155.
47 Katome T, Namekata K, Guo X, Semba K, Kittaka D, Kawamura K, Kimura A, Harada C, Ichijo H, Mitamura Y, Harada T. Inhibition of ASK1-p38 pathway prevents neural cell death following optic nerve injury. Cell Death Differ 2013;20(2):270-280.
48 Cho SG, Lee YH, Park HS, et al . Glutathione S-transferase mu modulates the stress-activated signals by suppressing apoptosis signalregulating kinase 1. J Biol Chem 2001;276(16):12749-12755.
Citation: Stamenkovic M, Lukic V, Suvakov S, Simic T, Sencanic I, Pljesa-Ercegovac M, Jaksic V, Babovic S, Matic M, Radosavljevic A, Savic-Radojevic A, Djukic T. GSTM1 -null and GSTT1-active genotypes as risk determinants of primary open angle glaucoma among smokers. Int J Ophthalmol 2018;11(9):1514-1520
Received: 2017-12-25 Accepted: 2018-03-23
DOl: 10.18240/ijo.2018.09.14
Correspondence to: Tatjana Djukic. Institute of Medical and Clinical Biochemistry, Faculty of Medicine, University of Belgrade, Pasterova 2, Belgrade 11000, Serbia. tatjana.djukic@med.bg.ac.rs