Chronic ocular GVHD: limbal and conjunctival stem cell allografts from the same hematopoietic stem cell donor
Elias F Jarade, Hala El Rami, Youssef Abdelmassih, Mazen Amro
Beirut Eye and ENT Specialist Hospital, Beirut 116-5311,Lebanon
Dear Editor,
I am Dr. Elias F Jarade from the Beirut Eye and ENT Specialist Hospital, Beirut, Lebanon. I write to describe a novel surgical technique in the management of chronic ocular graft-versus-host disease (GVHD).
Allogeneic hematopoietic stem cell transplantation (HSCT)has become the standard of care in many hematologic diseases [1] . In this procedure, hematopoietic progenitor cells derived from an HLA-matched donor, are transplanted into the receiver where they attack the host tumor cells. However,these immunocompetent T-cells can also recognize normal host antigens as foreign, resulting in a primary cause of morbidity known as GVHD [2-3] . Acute GVHD presents with maculo-papular rash, cholestatic jaundice, and gastro-intestinal symptoms [2] . Chronic GVHD is multisystemic affecting most commonly the skin, the oral mucosa, the liver, and the eyes [3] .Ocular findings are usually limited to conjunctival hyperhemia and dryness. However, vision-threatening complications may occur and include cicatricial conjunctivitis, corneal neovascularization, ulceration, and spontaneous perforation [4-5] .In this article, we introduce a novel surgical technique which consists of transplanting limbal and conjunctival stem cells dissected from the same allogeneic hematopoietic stem cell donor into an ocular surface suffering from severe chronic GVHD. In theory, this would promote healing through bypassing the alloreactivity component of GVHD.
A 35-year-old female patient was referred to our hospital for the evaluation of chronic ocular GVHD. She underwent HSCT 7 years ago, for acute lymphoblastic leukemia. The hematopoietic stem cell donor was the patient’s brother.Ocular disease started one year after HSCT with progressive deterioration of the ocular surface despite aggressive topical and systemic immunosuppression. Visual acuity was hand motion in the right eyes (RE) and counting fingers at one meter in the left eye (LE). Slit lamp examination revealed severe conjunctival injection, cicatrization of the bulbar conjunctiva,corneal neovascularization and lipid keratopathy, diffuse corneal thinning, three centrally-burried nylon sutures RE related to a history of spontaneous perforation, and inferior corneal scarring LE (Figure 1A, 1B). The ocular media were clear and the retina was attached on B-scan ultrasonography.
Donor keratolimbal and conjunctival allografts were obtained from the patient’s brother who was the hematopoietic stem cell donor. We opted to harvest stem cells from the donor’s both eyes to prevent stem cell deficiency. The goal was to transplant the allografts into the patient’s LE. The procedure and its experimental nature were explained to both the patient and her brother who signed an informed consent. All procedures were carried out under general anesthesia. The study complied with the principles of the Declaration of Helsinki.
We started our surgery by harvesting the donor tissues. The eye was rotated inferiorly using limbal 4-0 silk sutures. A calibrated diamond knife (Micra ® , USA), set at 250 µm depth, was used to create a 7 mm long superior arcuate corneal incision just central to the limbus (Figure 2A). Lamellar dissection using 57-blade knife was performed to undermine the limbus with limbal stem cells (Figure 2B). A caliper was used to mark the adjacent bulbar conjunctiva on both sides of the incision,10 mm posterior to the limbus with a distal width of 11 mm. A mixed solution of lidocain 1% and epinephrine 1:100 000 was used to separate the conjunctiva from the underlying Tenon’s capsule (Figure 2C). Westcott scissors were used to dissect the previously marked conjunctiva (Figure 2D). As a result,a 7×10×10×11 mm trapezoidal-shaped conjunctival tissue attached to a 7×1 mm lamellar keratolimbal tissue containing the limbal stem cells was obtained and preserved temporally in a balanced salt solution. In order to harvest the conjunctival stem cells from the lower fornix, the RE was rotated superiorly and the same mixed lidocaine-epinephrine solution was used to separate the conjunctiva from the underlying Tenon’s capsule (Figure 2E), then a 5 mm×10 mm conjunctival lenticule was shaped and cut using the Westcott scissors(Figure 2F). The same procedure was also performed on the LE of the donor in order to harvest identical keratolimbal and conjunctival lenticules. To replace the deficient conjunctival and keratolimbal tissues, an overlay of amniotic membranes was sutured to both donor’s eyes using 10-0 interrupted Vicryl sutures.

Figure 1 Photographs of the ocular surface from the preoperative time, 1, 2 and 6mo after the surgery Preoperative slit-lamp photograph of the patient’s RE (A) and LE (B). LE at the one month’ (C-D), two months’ (E), and six months’ (F) postoperative visits.
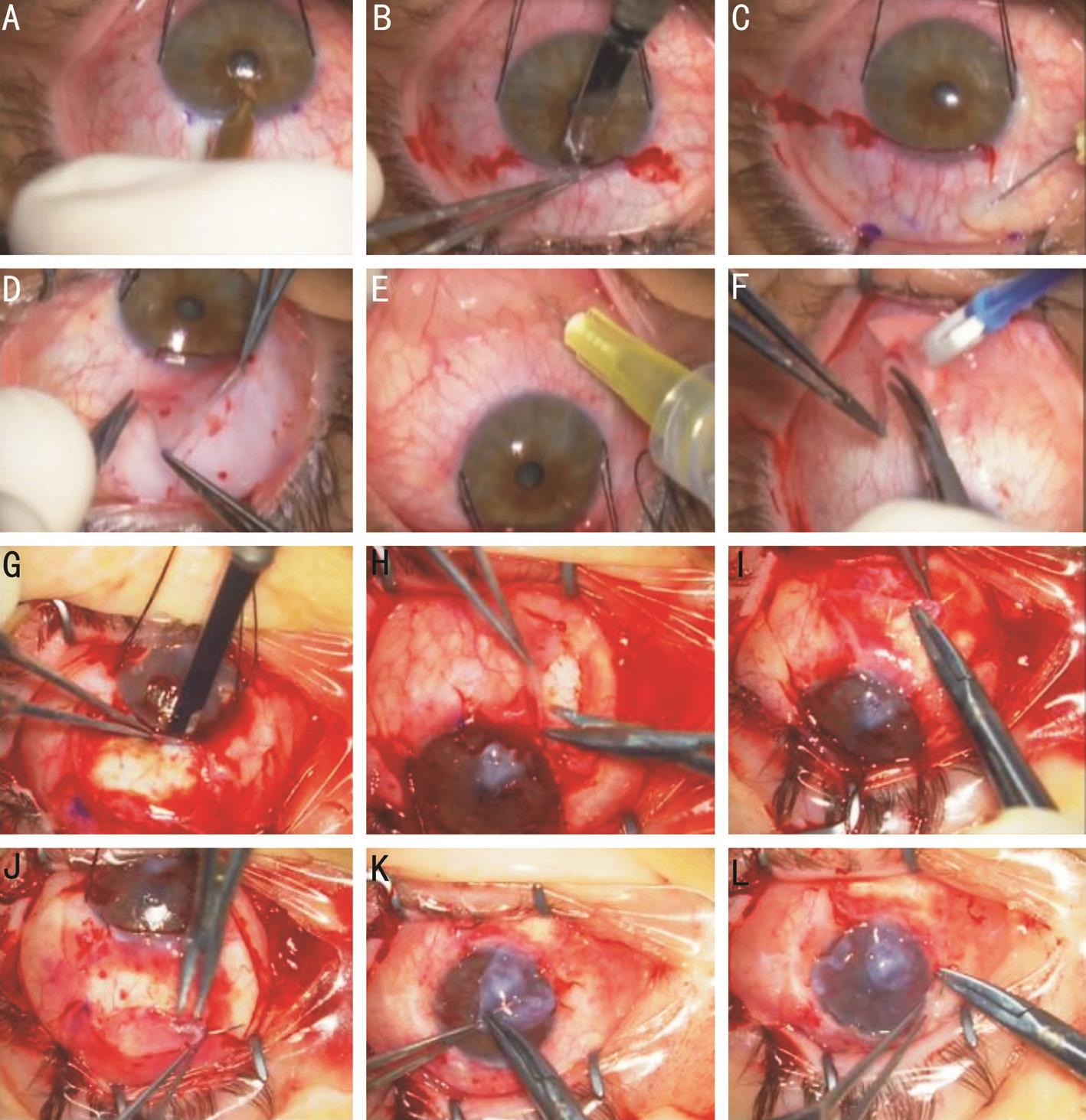
Figure 2 Depiction of the surgical steps performed to transplant keratolimbal and conjunctival allografts form the donor’s both eyes to the patient’s left eye A-F: Donor’s surgery. A: Creation of a 7 mm long, 250 µm depth, arcuate corneal incision; B: Limbal lamellar dissection with a 57-blade knife; C: Dissection of the bulbar conjunctiva with a lidocaine-epinephrine mixture; D: Removal of the conjunctival-keratolimbal lenticule; E: Dissection of the forniceal conjunctiva with a lidocaine-epinephrine mixture; F: Removal of a 5×10 mm conjunctival lenticule from the inferior fornix. G-L: Patient’s surgery. G: Dissection and removal of a superior keratolimbal and conjunctival lenticule; H: Dissection and removal of an inferior keratolimbal and conjunctival lenticule; I-J: Suturing of the donor’s conjunctival and keratolimbal allografts to the recipient’s bed; K: Amniotic membrane patch over the inferior perforation; L: Amniotic membrane overlay.
The patient’s LE was prepped and draped in a sterile manner.Two 4-0 silk sutures were used to retract the LE inferiorly.Superiorly and inferiorly, a quadrant of conjunctival tissue (of the same size of the donated conjunctival tissue) was dissected and an arcuate 7 mm limbectomy was performed at the same depth of 250 µm (Figure 2G, 2H). A superficial lamellar keratectomy was then carried out to dissect the irregular epithelium and the superficial stromal opacities using a 57-blade knife. While performing the lamellar keratectomy, the inferior cornea at 6 o’clock was extremely thin and presented a micro-perforation self-sealed with iris tissue. Viscoelastic material was used to reposition the iris tissue and a double layered amniotic membrane patch was later performed to seal the area of perforation (Figure 2K). The 2 quadrants of the donor’s trapezoidal kerato-limbal-conjunctival tissues were transplanted into the recipient’s LE inferiorly and superiorly and the 2 quadrants of the donor’s forniceal conjunctival tissues were transplanted superiorly and inferiorly peripheral to the transplanted kerato-limbal-conjunctival quadrants (Figure 2I, 2J). At the end of the surgery, the cornea was covered with a single layer amniotic membrane that was sutured in place with interrupted 10-0 vicryl sutures (Figure 2L). At the end of the procedure, a bandage contact lens was placed over the eye.Two weeks after the LE surgery, the RE was operated with a superficial lamellar keratectomy to remove the lipid deposits and an amniotic membrane was sutured over the ocular surface with several interrupted 10-0 vicryl sutures.
The procedure was well-tolerated by the donor with no permanent alteration of the ocular surface. After the recipient’s LE surgery, topical steroids, cyclosporine 0.05%, antibiotics,and lubricants were prescribed. At the 8-weeks’ followup, the visual acuity was 20/200 LE. The cornea was clear superiorly, nasally and temporally with no epithelial defect.Fibrotic scarring was observed in the area of perforation.However, the disease course was complicated at 10wk by LE endophthalmitis. There was no corneal abscess or leakage and it was found that the endophthalmitis was endogenous in nature, related to a focus of acute pneumonia, and unrelated to the surgery itself. Vitreous cultures yielded streptococcus oralis and the patient was treated with core vitrectomy and oral/intravitreal antibiotics. A total retinal detachment occurred one month later. At that time, the cornea was clear enough to allow a lensectomy-vitrectomy with intravitreal silicone oil injection. Over the next 3mo, the LE became quiet. The progressive improvement of the LE ocular surface is depicted in Figure 1C-1F. However, the vision remained poor due to retinal fibrosis and injected silicone oil. The right cornea demonstrated minimal improvement.
Although our case lacked a diagnostic pathology specimen, the clinical picture was typical of GVHD-related ocular morbidity with corneal neovascularization, and spontaneous perforations.One possible mechanism for corneal neovascularization is an immune-mediated limbitis resulting in limbal stem cell insufficienc [4] . An immune-mediated inflammation of the bulbar conjunctiva is also reported, leading to destruction of goblet cells and dry eyes [6] . Together with limbal insufficiency,severe dry eye leads to sterile corneal ulcers, melting and spontaneous perforations. At this stage, there is no chance of visual rehabilitation through classical management. Penetrating keratoplasty and keratoprosthesis would have a high failure rate. Based on the concept of alloreactivity in GVHD, where the donor’s T-cells attack the host’s normal tissues, we decided to implant limbal and conjunctival stem cells dissected from the same allogeneic hematopoietic stem cell donor. The goal was to regenerate an ocular surface that would not be recognized as foreign by the donor’s immune system which was now forming a major part of the immunity of the receiver.Within less than 6mo, the conjunctival surface improved with mild residual injection, the corneal neovascularization regressed and the corneal surface resumed significant clarity.We assumed that the prominent regression of corneal neovascularization was most likely related to the resumption of healthy limbal population and barrier function. The 2 quadrants of transplanted limbal stem cells were able to regenerate a new corneal epithelium over the amniotic membrane scaffold that was not recognized as foreign by the new adopted immune system. Conjunctival stem cells reside in part in the inferior fornix [7] . The 2 quadrants of forniceal cells could have helped regenerate a non-inflamed conjunctiva with relatively normal goblet cell function that could have contributed to the fast healing process of the ocular surface. The amniotic membrane could have played a role as well but not to the remarkable extent that was observed in our case. The RE which was only treated with superficial keratectomy and amniotic membrane transplantation, demonstrated minimal improvement in corneal clarity. Therefore, we assume that the transplantation of stem cells played the major role in the recovery of the LE.Successful surface reconstruction in chronic ocular GVHD was also reported by Meller et al [8] who transplanted limbal epithelial cells derived from the same hematopoietic stem cell donor. These cells were harvested from a small biopsy and cultivated on amniotic membrane prior to transplantation,whereas in our case, direct anatomical translocation was carried out from the donor’s to the receiver’s ocular surface.This treatment strategy might also be a promising option in the treatment of other organ manifestations of GVHD by preserving the stem cells of the donor for potential use in the future. One major limitation lies in the pathogenesis of chronic GVHD which is poorly understood and involves complex pathways comprising both autoimmune and alloreactive mechanisms [9-10] . Thus, our technique would theoretically address only part of the pathophysiological process and longer follow-up is needed to assess its safety and efficacy.
ACKNOWLEDGEMENTS
Conflicts of Interest: Jarade EF, None; El Rami H, None; Abdelmassih Y, None; Amro M, None.
REFERENCES
1 Pasquini MC, Wang Z, Horowitz MM, Gale RP. 2010 report from the Center for International Blood and Marrow Transplant Research(CIBMTR): current uses and outcomes of hematopoietic cell transplants for blood and bone marrow disorders. Clin Transpl 2010:87-105.
2 Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J,Thomas ED. 1994 Consensus Conference on acute GVHD grading. Bone Marrow Transplant 1995;15(6):825-828.
3 Lee SJ, Flowers ME. Recognizing and managing chronic graft-versushost disease. Hematology Am Soc Hematol Educ Program 2008:134-141.
4 Tabbara KF, Al-Ghamdi A, Al-Mohareb F, Ayas M, Chaudhri N, Al-Sharif F, Al-Zahrani H, Mohammed SY, Nassar A, Aljurf M. Ocular findings after allogeneic hematopoietic stem cell transplantation. Ophthalmology 2009;116(9):1624-1629.
5 Mohammadpour M, Maleki S, Hashemi H, Beheshtnejad AH. Recurrent corneal perforation due to chronic graft versus host disease; a clinicopathologic report. J Ophthalmic Vis Res 2016;11(1):108-111.
6 Hosseini H, Kumar PV, Geramizadeh B, Nowroozizadeh B, Ramzi M. Conjunctival scrape cytology findings in patients with chronic graftversus-host disease following allogeneic bone marrow transplantation. Acta Cytol 2010;54(3):272-276.
7 Stewart RM, Sheridan CM, Hiscott PS, Czanner G, Kaye SB. Human conjunctival stem cells are predominantly located in the medial canthal and inferior forniceal areas. Invest Ophthalmol Vis Sci 2015;56(3):2021-2030.
8 Meller D, Fuchsluger T, Pauklin M, Steuhl KP. Ocular surface reconstruction in graft-versus-host disease with HLA-identical livingrelated allogeneic cultivated limbal epithelium after hematopoietic stem cell transplantation from the same donor. Cornea 2009;28(2):233-236.
9 Shimada M, Onizuka M, Machida S, Suzuki R, Kojima M, Miyamura K, Kodera Y, Inoko H, Ando K. Association of autoimmune diseaserelated gene polymorphisms with chronic graft-versus-host disease. Br J Haematol 2007;139(3):458-463.
10 Patriarca F, Skert C, Sperotto A, Zaja F, Falleti E, Mestroni R, Kikic F, Calistri E, Filì C, Geromin A, Cerno M, Fanin R. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol 2006;34(3):389-396.
Citation: Jarade EF, El Rami H, Abdelmassih Y, Amro M. Chronic ocular GVHD: limbal and conjunctival stem cell allografts from the same hematopoietic stem cell donor. Int J Ophthalmol 2018;11(9):1569-1572
Received: 2017-10-24 Accepted: 2018-04-09
DOl: 10.18240/ijo.2018.09.24
Correspondence to: Elias F Jarade. Beirut Eye and ENT Specialist Hospital, Al-Mathaf Square, Beirut 116-5311,Lebanon. ejarade@yahoo.com

