5-aza-2’-deoxycytidine in the regulation of antioxidant enzymes in retinal endothelial cells and rat diabetic retina
Man-Yun Xie1,2, Yan Yang1,2, Ping Liu1,2, Yan Luo3, Shi-Bo Tang4, 5
1Department of Ophthalmology, the Second Xiangya Hospital,Central South University, Changsha 410011, Hunan Province,China
2Hunan Clinical Research Center of Ophthalmic Disease,Changsha 410011, Hunan Province, China
3The State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou 510060, Guangdong Province, China
4Aier School of Ophthalmology, Central South University,Changsha 410015, Hunan Province, China
5Aier Research Institute of Ophthalmology, Changsha 410015,Hunan Province, China
Abstract● AlM: To investigate the roles of a DNA methyltransferase(DNMT) inhibitor 5-aza-2’-deoxycytidine (5-aza-dC) in the regulation of antioxidant enzymes in diabetic retinopathy(DR) models.● METHODS: DNMTs expressions and activity, and changes of two key antioxidant enzymes in DR, MnSOD (encoded by SOD2 gene) and glutathione S-transferase theta 1 (GSTT1),were quantified in the isolated human retinal endothelial cells (HRECs) exposed to high glucose (HG) with or without 5-aza-dC treatment. The downstream exacerbating factors including vascular endothelial growth factor(VEGF), intercellular adhesion molecule 1 (lCAM-1) and matrix metalloproteinase 2 (MMP2), which are implicated in the pathogenesis of DR and closely related to oxidative stress were also analyzed. The key parameters were confirmed in the retina from streptozotocin (STZ) diabetic rats.● RESULTS: DNMTs expression and DNMT activity was induced in HRECs exposed to HG. Hyperglycemia decreased MnSOD and GSTT1 expression. 5-aza-dC administration effectively suppressed DNMTs expression and activity and reversed the MnSOD and GSTT1 expression under HG condition. VEGF, lCAM-1 and MMP2 induced by HG were also suppressed by 5-aza-dC treatment. Similar results were observed in the retina from STZ diabetic rats.● CONCLUSlON: Our findings suggest that DNA methylation may serves as one of the mechanisms of antioxidant defense system disruption in DR progression. Modulation of DNA methylation using pharmaceutic means such as DNMT inhibitors could help maintain redox homeostasis and prevent further progression of DR.
INTRODUCTION
The retina is a tissue rich in polyunsaturated fatty acid and is very susceptible to damage due to its high oxygen demand. Oxidative stress has been reported to increase in the retina in diabetic condition and can trigger a series of pathologic pathways and thus contribute to the progression of diabetic retinopathy (DR)[1].
Impaired antioxidant defense system is insufficient to remove reactive oxygen species (ROS) in the cell and results in oxidative stress accumulation. In diabetes, suppression of antioxidant defense system is implicated in the pathogenesis of DR. Intracellular antioxidant levels are decreased in the diabetic retina, and the enzymes responsible for its metabolism are compromised[2-3]. Recent studies has been focusing on the vital roles of DNA methylation in the regulation of gene expression within the retinal cells and tissues in the progression of DR[4-6]. 5-aza-2’-deoxycytidine (5-aza-dC), which serves as one of the DNA methylation inhibitors and can result in demethylation of the tumor suppressor genes and thus cause their re-expression, has been shown to be protective in a series of pathological conditions[7-11]. While the effects of 5-aza-dC on DR has not been explored, the present study was aiming to test whether 5-aza-dC interfere in antioxidant enzymes expressions in DR.
SUBJECTS AND METHODS
Ethical Approval All experiments involved human eyes were performed according to the tenets of the Declaration of Helsinki for research involving human subjects andapproved by the Ethics Committee of the Second Xiangya Hospital, Central South University, Changsha, China. All procedures with animals were conducted in accordance with the Association for Research in Vision and Ophthalmology(ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research, approved by the Institutional Animal Care and Use Committee and the Ethics Committee in Animal and Human Experimentation of the Second Xiangya Hospital,Central South University, Changsha, China.
Table 1 Primers used in methylation-specific PCR and real-time PCR analysis
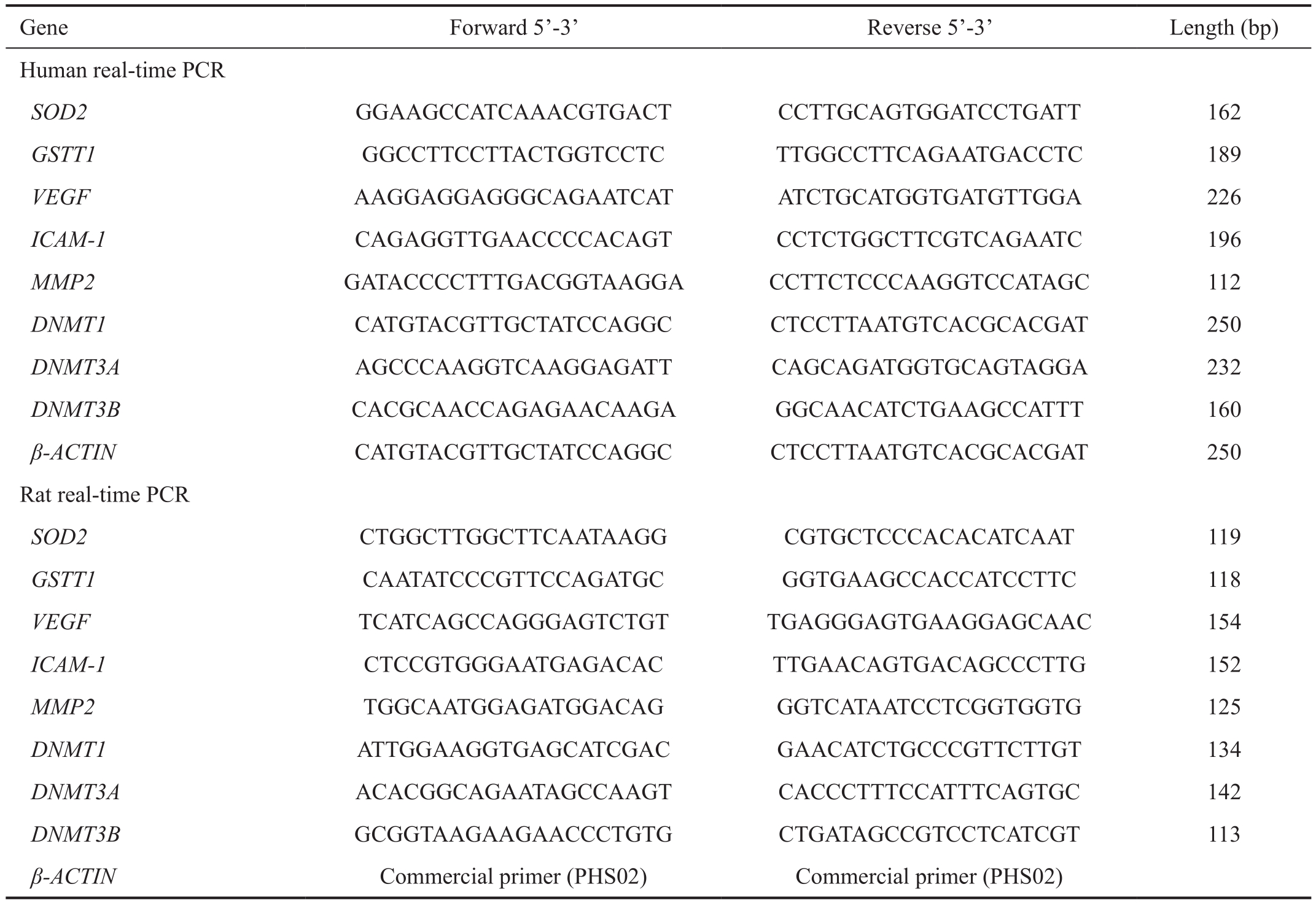
Gene Forward 5’-3’ Reverse 5’-3’ Length (bp)Human real-time PCR SOD2 GGAAGCCATCAAACGTGACT CCTTGCAGTGGATCCTGATT 162 GSTT1 GGCCTTCCTTACTGGTCCTC TTGGCCTTCAGAATGACCTC 189 VEGF AAGGAGGAGGGCAGAATCAT ATCTGCATGGTGATGTTGGA 226 ICAM-1 CAGAGGTTGAACCCCACAGT CCTCTGGCTTCGTCAGAATC 196 MMP2 GATACCCCTTTGACGGTAAGGA CCTTCTCCCAAGGTCCATAGC 112 DNMT1 CATGTACGTTGCTATCCAGGC CTCCTTAATGTCACGCACGAT 250 DNMT3A AGCCCAAGGTCAAGGAGATT CAGCAGATGGTGCAGTAGGA 232 DNMT3B CACGCAACCAGAGAACAAGA GGCAACATCTGAAGCCATTT 160 β-ACTIN CATGTACGTTGCTATCCAGGC CTCCTTAATGTCACGCACGAT 250 Rat real-time PCR SOD2 CTGGCTTGGCTTCAATAAGG CGTGCTCCCACACATCAAT 119 GSTT1 CAATATCCCGTTCCAGATGC GGTGAAGCCACCATCCTTC 118 VEGF TCATCAGCCAGGGAGTCTGT TGAGGGAGTGAAGGAGCAAC 154 ICAM-1 CTCCGTGGGAATGAGACAC TTGAACAGTGACAGCCCTTG 152 MMP2 TGGCAATGGAGATGGACAG GGTCATAATCCTCGGTGGTG 125 DNMT1 ATTGGAAGGTGAGCATCGAC GAACATCTGCCCGTTCTTGT 134 DNMT3A ACACGGCAGAATAGCCAAGT CACCCTTTCCATTTCAGTGC 142 DNMT3B GCGGTAAGAAGAACCCTGTG CTGATAGCCGTCCTCATCGT 113 β-ACTIN Commercial primer (PHS02) Commercial primer (PHS02)
Human Retinal Endothelial Cells Human eyes were obtained from the Eye Bank of the Second Xiangya Hospital(Changsha, China) after corneal transplantation. Human retinal endothelial cells (HRECs) were prepared as previously described[12] and were cultured in human endothelial-serum free medium (HE-SFM; Gibco, Grand Island, NY, USA)which is supplemented with 10% fetal bovine serum, 5 ng/mL recombinant human β-endothelial cell growth factor (β-ECGF;R&D Systems, Minneapolis, MN) and 1% insulin-transferrinselenium. Cells from passages 3 to 5 were incubated in normal glucose (NG, 5 mmol/L) or high glucose (HG, 30 mmol/L)with or without 5-aza-dC (5 μmol/L, 10 μmol/L).
Rats Sprague-Dawley (SD) rats (male, 6 weeks old, 200 g)were purchased from the Animal Center of Southern Medical University, Guangzhou, China. The rats were divided to three groups: control group, diabetic rats and diabetic rats treated with 5-aza-dC. Diabetes was induced in the rats (male, 6 weeks old, 200 g) by streptozotocin (STZ, 65 mg/kg body weight). Glucose level of tail vein blood of the rats was analyzed with an automated Accu-Chek glucometer (Roche Diagnostics, Shanghai, China) to confirm diabetic status 72h after intraperitoneal injection and every 2wk. Rats with ≥250 mg/dL blood glucose level were deemed diabetic rats and were raised for 10wk. 5-aza-dC was administered intraperitoneally for the last 4wk every day (1 mg/kg·d). The rats were housed in specific-pathogen-free conditions in the Animal Laboratory Center of the Second Xiangya Hospital, Central South University.Gene Expression Primary cultured HRECs and rat retinas were used to extracted total RNA by Trizol (Invitrogen Life Techologies, Grand Island, NY, USA) according the manufacturer’s instructions. The RNA was reverse transcribed applying the Takara First Strand Synthesis kit (TaKaRa,Dalian, China) to synthesis cDNA. Real-time quantitative PCR was performed using SYBR Green PCR kit (TaKaRa, Dalian,China) on an ABI Prism 7000 system. PCR procedure was set as the following: denaturing at 95℃ for 5min, followed by 40 cycles of denaturation at 95℃, annealing at 60℃, and extension at 72℃ for 10s, respectively. The sequences of the primers used in the experiments are summarized in Table 1.
Protein Expression Rat retinas or harvested cells were treated by SDS sample buffer to extract protein. The 15 g of protein was separated using SDS-PAGE, transferred to PVDF membrane and immunoblotted with antibodies for MnSOD (Novus Biologicals, Colorado, USA; NB100-1992)used at 1:1000 dilutions and GSTT1 (Abcam, Beverly, MA;ab175418) used at 1:500 dilutions. Antibody for β-actin(1:2000) was purchased from Invitrogen Life Techologies(Grand Island, NY, USA). After incubated with goat anti-rabbit HRP-conjugated antibody, the protein bands were revealed using SignalFire™ ECL Reagent (Cell Signaling Technology,Danvers, MA, USA).
Nuclear Fraction EpiQuik™ Nuclear Extraction Kit(Epigentek, Farmingdale, NY, USA) was used to prepare nuclear extract according to manufacturer’s protocol. Rat retinas or harvested cells were lysed by lysis buffer 1 supplied in the nuclear extraction kit for 10min and then centrifuged for 1min at 12 000 rpm. The supernatant was discarded and the nuclear pellet was re-suspended in lysis buffer 2 supplied in the nuclear extraction kit, incubated on ice for 10min with intermittent vortexing and then centrifuged for 1min at 12 000 rpm.The supernatant was used as the ‘nuclear’ fraction.
DNA Methyltransferase Activity DNA methyltransferase(DNMT) activity in the nuclear fraction was analyzed by EpiQuik DNMT activity/inhibitor assay kit (Epigentek,Farmingdale, NY, USA) following the manufacturer’s instructions. Nuclear extract prepared from the retinas or HRECs was incubated with S-adenosymethionine (AdoMet)and a universal proprietary DNMT substrate in the DNMT assay buffer at 37℃ for 2h. The blank contained only AdoMet and the substrate without nuclear extract, while the positive control contained AdoMet/substrate with the purified DNMT enzyme.
Statistical Analysis All data were expressed as mean±SD and analyzed using SPSS 16.0 software (SPSS Inc., Chicago,IL, USA). Differences among groups were analyzed by Oneway ANOVA. A value of P<0.05 was considered statistically significant.
RESULTS
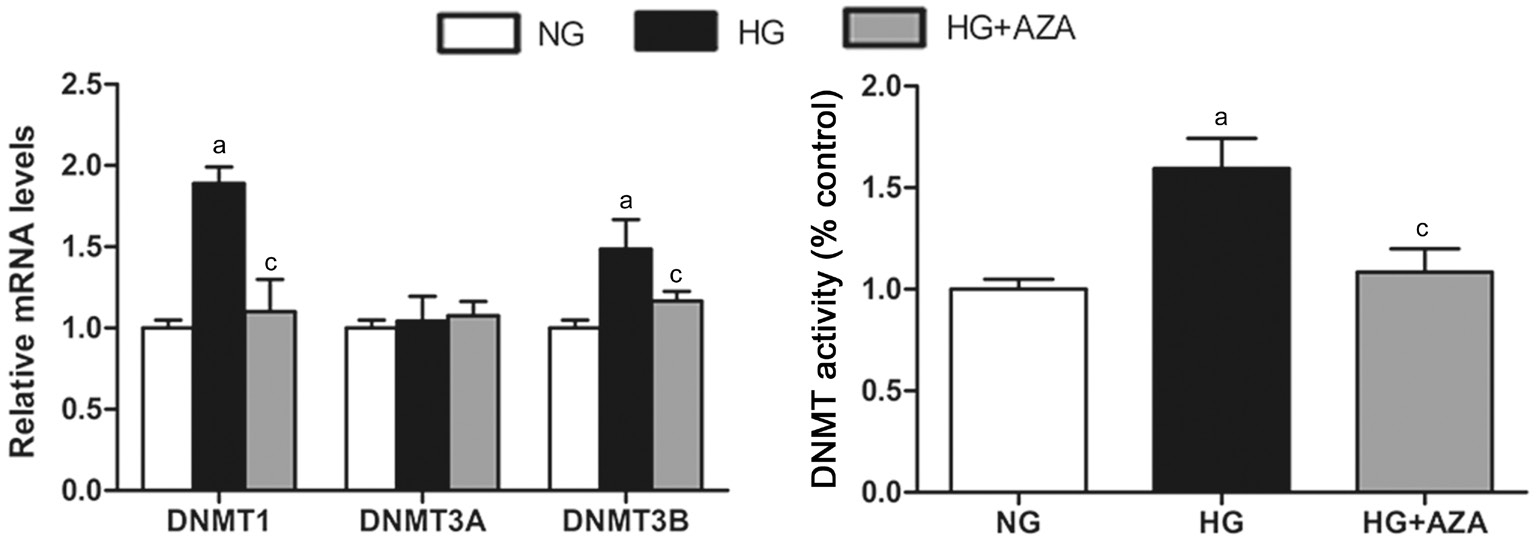
5-aza-dC Reversed DNMT Expression and Activity Induced by HG in HRECs Aberrant expressions of different DNMTs have been observed in various abnormalities. We investigated the changes of DNMTs in HRECs under HG condition and found that hyperglycemia resulted in statistically significant increases in DNMT1 and DNMT3B, but not DNMT3A. 5-aza-dC effectively suppressed the HG-induced DNMTs expressions to different degrees. DNMT activity increased significantly by HG treatment in the retina, and was blocked by 5-aza-dC (Figure 1).
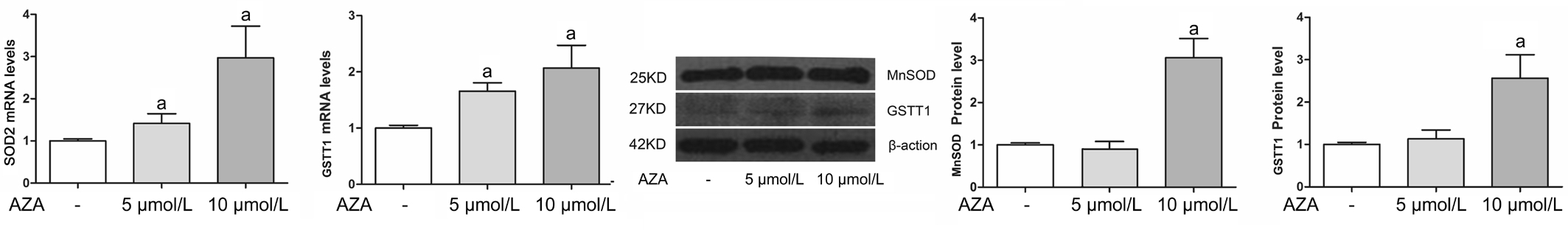
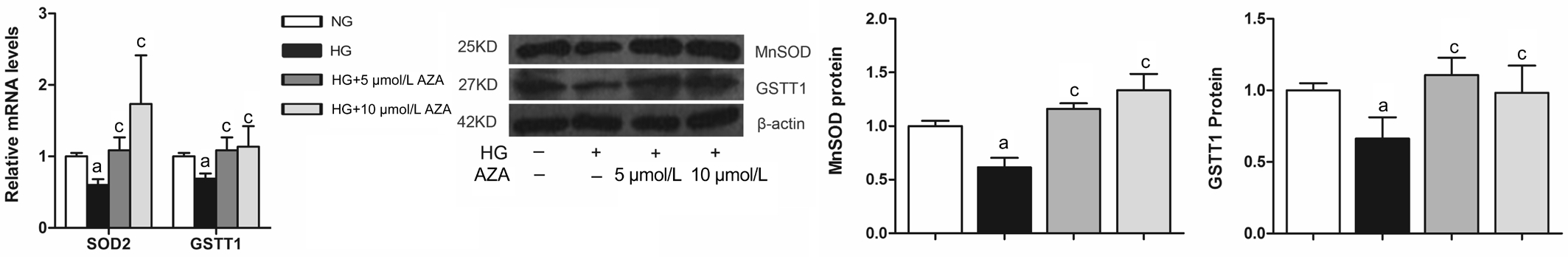
Changes of MnSOD and GSTT1 in HRECs Under NG and HG Condition Changes of methylation state of antioxidant enzymes have been implicated in several pathological processes. We here tested whether the induction of DNA hypomethylation with 5-aza-dC could affect MnSOD and GSTT1 levels both in NG and HG condition. As shown in Figure 2, in NG condition for 2d, 5-aza-dC up-regulated MnSOD and GSTT1 levels. HG treatment (30 mmol/L) for 4d decreased the gene expressions and proteins of MnSOD and GSTT1. 5-aza-dC treatment effectively reversed HG-induced MnSOD and GSTT1 suppression (Figure 3).
5-aza-dC Caused Reversal of HG-induced Changes of Exacerbating Factors in HRECs Antioxidant therapies inhibited key exacerbating factors in DR progression. We thus tested the effects of 5-aza-dC on the expressions of vascular endothelial growth factor (VEGF), intercellular adhesion molecule 1 (ICAM-1) and matrix metalloproteinase 2(MMP2) levels, which are related to the vascular permeability and inflammation in HRECs in HG condition. Compared with control groups, treatment of HG resulted in significant increases in the levels of the target cytokines. 5-aza-dC mitigated the up-regulations of all the three exacerbating factors (Figure 4).
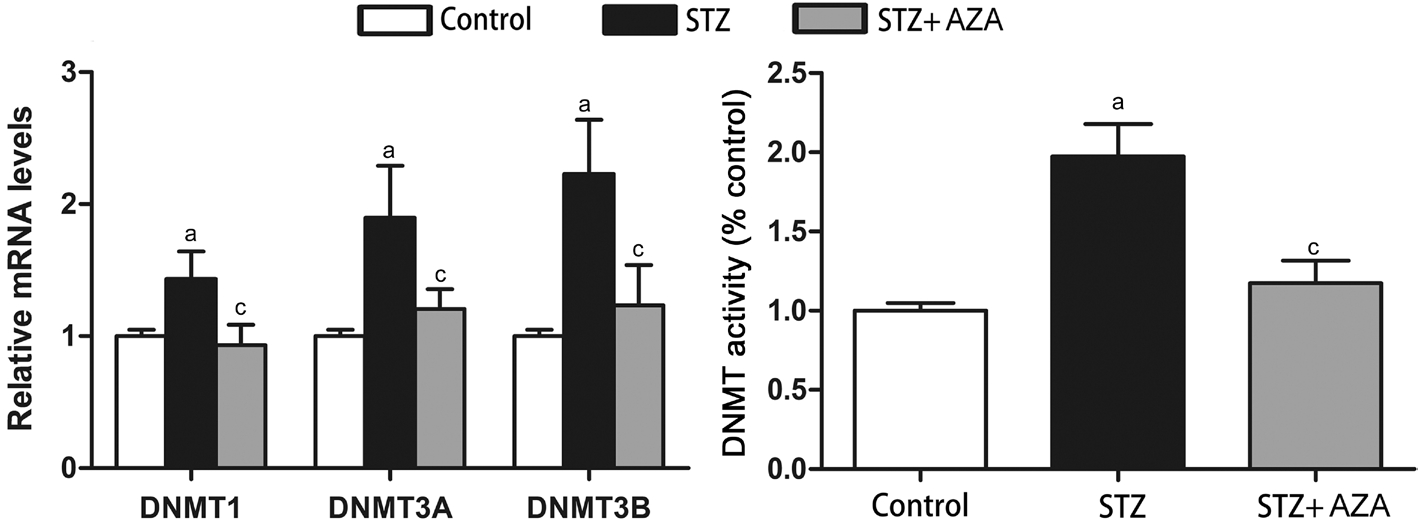
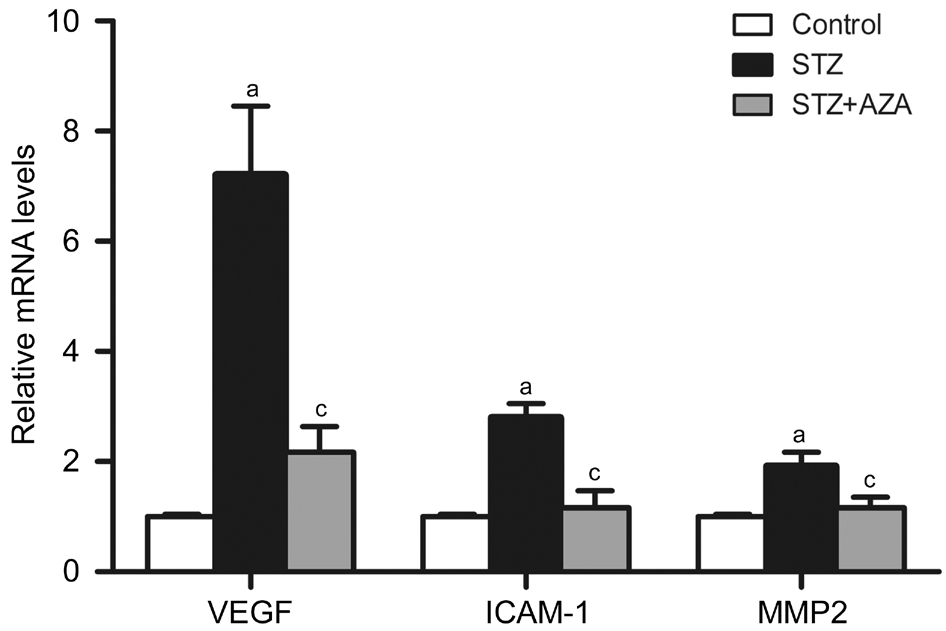
Rats The effects of 5-aza-dC on antioxidant enzymes and the downstream exacerbating factors found in HRECs in vitro were also observed in the retina of diabetic rats in vivo. We found that hyperglycemia resulted in statistically significant increases in all the three DNMTs and total DNMT activity,which were effectively suppressed by 5-aza-dC (Figure 5).MnSOD and GSTT1 expressions in the retina dramatically decreased at mRNA level and protein level in diabetes group.5-aza-dC treatment caused reversal of diabetes-induced MnSOD and GSTT1 suppression to different extent (Figure 6).Compared with control groups, exposure to hyperglycemia resulted in significant increases in the levels of VEGF, ICAM-1 and MMP2, which were reversed by 5-aza-dC (Figure 7).
DISCUSSION
In our present study, we sought to test whether DNMT inhibition by 5-aza-dC affects the critical antioxidant enzymes which are critical in DR, to evaluate its potential values to prevent further progression of DR. We showed that increased DNMT activity and weaken anti-oxidative capacity were blocked by this DNA-demethylating drug, suggesting its potential therapeutic roles in the treatment of DR.
Impair of various anti-oxidative substances in the cells and retina tissues results in the imbalance of redox state,contributing to elevation of oxidative stress, which is considered to play central and critical roles in activations of other metabolic pathways in DR progression[1]. Pharmacologic augmentation of the cell’s antioxidant defenses is reported to be protective for the cells from oxidative stress[2]. MnSOD is responsible for scavenging mitochondrial superoxide. Reverse of MnSOD inhibition by antioxidants or MnSOD over-expression retards the development and progression of DR[13]. Similar to previous studies, our present study also showed that MnSOD was suppressed by hyperglycemia both in the rat retina and HRECs.
As one of the endogenous antioxidants, Glutathione S-transferases (GSTs) are multifunctional and associated with the regulation of oxidative stress and the detoxification of the harmful substances in the body. Human GST gene family includes several variants coded by eight main distinct loci:α (GSTA), μ (GSTM), θ (GSTT), σ (GSTS), κ(GSTK), o(GSTO), and τ (GSTZ)[14]. Retinal GSTs are likely to protect the retina from oxidative insult. Researchers also demonstrated that the GSTT1 null genotype was related to the progression of both nephropathy and retinopathy in diabetes, consistent with its roles in reducing oxidative stress in the pathophysiology of DR[15-16]. In our previous study on resistance gene of DR using cDNA microarray, we have identified that GSTT1 was differentially expressed in peripheral blood between proliferating DR and non-DR patient with diabetes mellitus for more than 15y and speculated that GSTT1 was a protective factor for DR[17]. GSTT1 changes in the retina and its capillary cells in pathological condition have not been reported. We here investigated GSTT1 changes in the retina and retinal cells under diabetic condition and found that GSTT1 was suppressed both in the rat retina in vivo and in HRECs in vitro under diabetic condition. These results expand our previous findings and indicate that GSTT1 decrease subjects the retina more susceptible to oxidative stress related to diabetic damage.Dysregulation of DNA methylation has been reported to regulate MnSOD and GSTs expression in previous studies.In pancreatic carcinoma and multiple myeloma, silencing of SOD2 was observed to result from hypermethylation of CpG islands within the promoter of SOD2[18-19]. In hepatocellular carcinoma, hyperrmethylation of the promoter CpG islands of GSTP1 resulted in epigenetic silencing of GSTP1, indicating the roles of methylation regulations of GST family in disease progression[20]. GSTM1 and GSTM5 were significantly down-regulated in AMD, corresponding to the hypermethylation repression of GSTM1 and GSTM5 in retinal pigment epithelium (RPE)/choroid[21]. During the pathophysiological processes initiated by hyperglycemia in diabetes and its complications,a series of metabolic abnormalities may lead to or result from aberrant DNA methylation patterns[22]. In DR patients, DNA methylation levels are associated with the genesis and the severity of retinopathy[23]. The CpG sites at the regulatory region of the DNA polymerase gamma (POLG1)in the retina are hyper-methylated in diabetic condition, which possibly compromises the transcriptional activity[24]. These findings suggest that extensive gene methylation might exist in retinal abnormalities in diabetic patients.
DNA methylation is established and maintained by three main DNMTs. DNMT1 expressed mainly in proliferating tissues and the activity of DNMT1 is related to DNA replication,mediating the process of methylation pattern copying from the parental to the daughter strand and thus acts primarily as a maintenance methyltransferase. In contrast, DNMT3A and DNMT3B are considered “de novo” methyltransferases which establish new methylation patterns in the cell[25]. In both HRECs and the retina, we detected the expressions of DNMTs and the levels were up-regulated by diabetic stimuli, indicating that gene expression patterns in DR might due to DNMTs dysregulation.
5-aza-dC is widely used as one of the DNA methylation inhibitors and can result in de-methylation of the tumor suppressor genes and thus cause their re-expression[7-8]. As a non-selective DNMT inhibitor, 5-aza-dC has been indicated to be protective in a series of pathological conditions[9-11]. 5-azadC was reported to regulate the angiogenic and anti-angiogenic VEGF variants in human lung microvascular endothelial cells[9]. 5-aza-dC was shown to increase the production of Treg cells in vivo to modulate the airway inflammation in the pathogenesis of asthma and thus imply a novel therapy strategy of asthma[10]. Xiao et al[11] demonstrated that increased DNA methylation induced by norepinephrine played a causal role in programming of heart hypertrophy and reducing cardiac contractility, which could be reversed by treatment of 5-aza-dC. The effects of 5-aza-dC on the retina and retinal cells in diabetic condition remain unknown. We here tested the effects of 5-aza-dC on the diabetic retina and HRECs in diabetic condition and found that under diabetic condition in vivo and in vitro, 5-aza-dC reversed DNMT expression and activity, and readjusted SOD2 and GSTT1 levels disturbed by hyperglycemia. These results indicated that DNA methylation mediating by DNMTs might regulate SOD2 and GSTT1 levels in vitro and in vivo and 5-aza-dC might protect the retina and its cells from oxidative stress injuries.
ROS is considered as a strong stimulus for the release of exacerbating cytokines, including VEGF, ICAM-1 and MMP2,which can damage endothelial cells and increase microvascular permeability. These exacerbating factors can interact with oxidative stress and contributing to the progression of DR.Data from previous studies have confirmed the inhibition roles on these exacerbating factors of antioxidant therapies[26-27]. We here found that 5-aza-dC administration effectively suppressed the exacerbating factors in rat retina and in HRECs under diabetic condition. In general, DNA demethylation leads to chromatin opening and gene expression. Therefore, downregulation of gene expression by 5-aza-dC would indicate an indirect influence, which may due to its ability of restoring anti-oxidative capacity.
In summary, the present study demonstrates that increased DNMTs and the aberrant antioxidative gene expressions,as well as their downstream exacerbating factors can be reversed by a DNA demethylating agent, indicating that DNA-demethylating agent may rescue the anti-oxidative potency in the retina and provides novel therapeutic targets for novel treatments in DR. Considering 5-aza-dC is a nonselective inhibitor, it will be important in the future to make clear via which DNMT 5-aza-dC regulates the antioxidative genes and determine the exact hypermethylation CpG site of the genes in DR. Besides, the side effects including myelosuppression and neurological toxicity of 5-aza-dC while being used in cancer therapy[7-8] may also be taking into consideration. Whether administration of 5-aza-dC would produce any significant change in retinal structure and function, and whether acute inhibition of retinal DNMTs by 5-aza-dC can be tolerated in diabetic condition might also be further research directions.
ACKNOWLEDGEMENTS
Foundations: Supported by the National Natural Science Foundation of China (No.81600757); Department of Science and Technology, Hunan (No.2015TP2007).
Confiicts of Interest: Xie MY, None; Yang Y, None; Liu P,None; Luo Y, None; Tang SB, None.
REFERENCES
1 Calderon GD, Juarez OH, Hernandez GE, Punzo SM, de la Cruz ZD.Oxidative stress and diabetic retinopathy: development and treatment. Eye(Lond) 2017;31(8):1122-1130.
2 Kowluru RA, Abbas SN. Diabetes-induced mitochondrial dysfunction in the retina. Invest Ophthalmol Vis Sci 2003;44(12):5327-5334.
3 Cui Y, Xu X, Bi HS, Zhu Q, Wu JF, Xia X, Qiushi Ren, Ho PC.Expression modification of uncoupling proteins and MnSOD in retinal endothelial cells and pericytes induced by high glucose: the role of reactive oxygen species in diabetic retinopathy. Exp Eye Res 2006;83(4):807-816.
4 Kowluru RA, Kowluru A, Mishra M, Kumar B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res 2015;48:40-61.
5 Mishra M, Kowluru RA. The role of DNA methylation in the metabolic memory phenomenon associated with the continued progression of diabetic retinopathy. Invest Ophthalmol Vis Sci 2016;57(13):5748-5757.
6 Mishra M, Kowluru RA. DNA Methylation-a potential source of mitochondria dna base mismatch in the development of diabetic retinopathy. Mol Neurobiol 2018.
7 Nie J, Liu L, Li X, Han WD. Decitabine, a new star in epigenetic therapy: the clinical application and biological mechanism in solid tumors.Cancer Lett 2014;354(1):12-20.
8 Hackanson B, Daskalakis M. Decitabine. Recent Results Cancer Res 2014;201:269-297.
9 Miller-Kasprzak E, Jagodziński PP. 5-Aza-2’-deoxycytidine increases the expression of anti-angiogenic vascular endothelial growth factor 189b variant in human lung microvascular endothelial cells. Biomed Pharmacother 2008;62(3):158-163.
10 Wu CJ, Yang CY, Chen YH, Chen CM, Chen LC, Kuo ML. The DNA methylation inhibitor 5-azacytidine increases regulatory t cells and alleviates airway inflammation in ovalbumin-sensitized mice. Int Arch Allergy Immunol 2013;160(4):356-364.
11 Xiao DL, Dasgupta C, Chen M, Zhang KL, Buchholz J, Xu ZC, Zhang LB. Inhibition of DNA methylation reverses norepinephrine-induced cardiac hypertrophy in rats. Cardiovasc Res 2014;101(3):373-382.
12 Xie M, Tian J, Luo Y, Wei L, Lin S, Tang S. Effects of 5-aza-2’-deoxycytidine and trichostatin A on high glucose- and interleukin-1βinduced secretory mediators from human retinal endothelial cells and retinal pigment epithelial cells. Mol Vis 2014;20,1411-1421.
13 Kowluru RA, Kowluru V, Xiong Y, Ho YS. Overexpression of mitochondrial superoxide dismutase in mice protects the retina from diabetes-induced oxidative stress. Free Radical Biology and Medicine 2006;41(8):1191-1196.
14 Pemble S, Schroeder KR, Spencer SR, Meyer DJ, Hallier E, Bolt HM,Ketterer B, Taylor JB. Human glutathione S-transferase theta (GSTT1):cDNA cloning and the characterization of a genetic polymorphism.Biochem J 1994;300(Pt 1):271-276.
15 Cilenšek I, Mankoč S, Petrovič MG, Petrovič D. GSTT1 null genotype is a risk factor for diabetic retinopathy in caucasians with type 2 diabetes, whereas GSTM1 null genotype might confer protection against retinopathy. Dis Markers 2012;32(2):93-99.
16 Doney AS, Lee S, Leese GP, Morris AD, Palmer CN. Increased cardiovascular morbidity and mortality in type 2 diabetes is associated with the glutathione Stransferase theta-null genotype: a Go-DARTS study.Circulation. 2005;111(22): 2927-2934.
17 Andina Hu. Study of the associated factors of the presence of proliferative diabetic retinopathy in type 2 diabetic patients. Doctor thesis,Zhongshan Ophthalmic Center, Sun Yat-sen University, 2011.
18 Hodge DR, Peng B, Pompeia C, Thomas SB, Cho E, Clausen PA,Marquez VE, Farrar WL. Epigenetic silencing of manganese superoxide dismutase (SOD-2) in KAS 6/1 human multiple myeloma cells increases cell proliferation. Cancer Biol Ther 2005;4(5):585-592.
19 Hurt EM, Thomas SB, Peng B, Farrar WL. Molecular consequences of SOD2 expression in epigenetically silenced pancreatic carcinoma cell lines. Br J Cancer 2007;97(8):1116-1123.
20 Li QF, Li QY, Gao AR, Shi QF. Correlation between promoter methylation in the GSTP1 gene and hepatocellular carcinoma development: a meta-analysis. Genet Mol Res 2015;14(2):6762-6772.
21 Hunter A, Spechler PA, Cwanger A, Song Y, Zhang Z, Ying GS,Hunter AK, Dezoeten E, Dunaief JL. DNA methylation is associated with altered gene expression in AMD. Invest Ophthalmol Vis Sci 2012;53(4):2089-2105.
22 Wegner M, Neddermann D, Piorunska-Stolzmann M, Jagodzinski PP. Role of epigenetic mechanisms in the development of chronic complications of diabetes. Diabetes Res Clin Pract 2014;105(2):164-175.
23 Maghbooli Z, Hossein-nezhad A, Larijani B, Amini M, Keshtkar A.Global DNA methylation as a possible biomarker for diabetic retinopathy.Diabetes Metab Res Rev 2015;31(2):183-189.
24 Tewari S, Zhong Q, Santos JM, Kowluru RA. Mitochondria DNA replication and DNA methylation in the metabolic memory associated with continued progression of diabetic retinopathy. Invest Ophthalmol Vis Sci 2012;53(8):4881-4888.
25 Dupont C, Armant DR, Brenner CA. Epigenetics: definition,mechanisms and clinical perspective. Semin Reprod Med 2009;27(5):351-357.
26 Goto H, Nishikawa T, Sonoda K, Kondo T, Kukidome D, Fujisawa K, Yamashiro T, Motoshima H, Matsumura T, Tsuruzoe K, Araki E.Endothelial MnSOD overexpression prevents retinal VEGF expression in diabetic mice. Biochem Biophys Res Commun 2008;366(3):814-820.
27 Kowluru RA, Kanwar M. Oxidative stress and the development of diabetic retinopathy: contributory role of matrix metalloproteinase-2. Free Radic Biol Med 2009;46(12):1677-1685.
Citation: Xie MY, Yang Y, Liu P, Luo Y, Tang SB. 5-aza-2’-deoxycytidine in the regulation of antioxidant enzymes in retinal endothelial cells and rat diabetic retina. Int J Ophthalmol 2018;12(1):1-7
DOl:10.18240/ijo.2019.01.01
● KEYWORDS: DNA methylation; diabetic model; 5-aza-dC;oxidative stress; in flammation; human retinal endothelial cells; rat
Received: 2018-06-25 Accepted: 2018-10-15
Correspondence to: Shi-Bo Tang. Aier Research Institute of Ophthalmology, Aier School of Ophthalmology, Central South University, No.198 Furong Middle Road, Changsha 410015,Hunan Province, China. tangshibo@vip.163.com