
Figure 1 PCG pedigrees with known CYP1B1 mutation Squares represent male and circles female individuals. The black squares and circles denote the affected individuals. The double line between couples indicate consanguineous marriage.
Glaucoma is the second leading cause of blindness after cataract, affecting more than 65 million people worldwide[1]. Primary congenital glaucoma (PCG; OMIM 231300), the most common type of non-syndromic glaucoma occurring in children in first three years of life, is associated with significant visual loss and blindness if left untreated[2].The clinical presentation of PCG is elevated intraocular pressure (IOP), buphthalmos, corneal edema leading to corneal opacification and optic nerve atrophy with subsequent irreversible blindness[3].
The incidence of PCG ranges between 1:5000 to 3:100 000 in western, Australian and African populations[2,4]. It, however,occurs more frequently in populations having a high rate of consanguineous marriages. Previous studies indicate the highest prevalence in Saudi Arabia and Slovakia patients with 1:2500 and 1:1250 respectively[5]. Whereas PCG in Pakistan is nine times higher than Caucasians[6]. The exact etiology of PCG is still not well understood. It may be due to mutations in genes, causing developmental defects in trabecular meshwork and the anterior chamber angle[7].
Mutations in only two genes, CYP1B1 and LTBP2 have been associated with autosomal recessive PCG so far[8], whereas variants in TEK gene has been associated with dominantly inherited PCG[9]. Mutations in CYP1B1 have been reported as a common cause of PCG in different populations with variable frequency ranging from 15% in American population to as high as 92% in Saudi Arabia patients[10]. In Pakistani population, CYP1B1 mutations account for 50% of PCG cases across different ethnicities.
Pakistani population is genetically heterogeneous with a high rate of endogamous marriages. Various novel mutations and genes have been identified by using the consanguineous Pakistani pedigrees. Previously, we screened 20 families affected with PCG for genetic variants and presented the common and novel CYP1B1 mutations[11]. In the present study,additional eleven families were screened for CYP1B1 to extend the mutation spectrum along with in silico functional analysis of novel variants. This may help to have better insight about the pathophysiology of CYP1B1 associated PCG and effective genetic counseling may be provided to affected families.
Ethical Approval The study was approved by Ethical Review Committee of Liaquat University of Medical and Health Sciences and was strictly adhered to tenets of Declaration of Helsinki. Written informed consent was obtained from all participating individuals.
Patients’ Enrollment and Ophthalmological Examination All affected individuals and their normal family members were interviewed in detail to record the medical history and to confirm the genetic nature of the disorder. Pedigrees were drawn to assess the mode of inheritance and all patients underwent detailed ophthalmological examination, which included determination of anterior chamber angle, visual acuity, measurement of IOP, examination of the fundus, the status of optic nerve head, and measurement of the cup-disc ratio (CDR).
DNA Extraction and Mutation Screening Totally 10 mL blood was collected from all participants in EDTA filled tubes. Genomic DNA was extracted from leucocytes by using inorganic DNA extraction method as described previously[12].The entire coding region of CYP1B1was amplified by using 5 primer pairs as described earlier[11]. The amplified products were purified and exon-intron boundaries, 5’UTR and 3’UTR regions of CYP1B1 were sequenced as described previously[13].The chromatograms were analyzed by Chromas Lite software version 2.6 and Finch TV software (https://digitalworldbiology.com/FinchTV).

Figure 1 PCG pedigrees with known CYP1B1 mutation Squares represent male and circles female individuals. The black squares and circles denote the affected individuals. The double line between couples indicate consanguineous marriage.
In Silico Functional Analyses Various bioinformatics tools were used to assess the pathogenicity of novel substitution mutations. The biochemical properties of wild type and mutant amino acids and effect of amino acid substitution on protein structure and function was assessed by using HOPE (Have Your Protein Explained), online tool (http://www.cmbi.ru.nl/hope/) and Phyre-2 web server was used to model the mutant protein Polyphen2 (http://www.sbg.bio.ic.ac.uk/phyre2) and SIFT (http://www.sift.jcvi.org) software were used to predict the effect of nucleotide and amino acid substitution in CYP1B1 gene and protein. Clustal Omega protein sequence conservation web tool was used to align the CYP1B1 amino acid sequences in different orthologues to study the conservation status of substituted amino acids (https://www.ebi.ac.uk/Tools/msa/clustalo/).
Eleven consanguineous pedigrees affected with PCG,including 51 affected individuals (27 males and 24 females)were enrolled. Medical history showed PCG with no other anomaly. The pedigree analysis revealed the recessive mode of inheritance with consanguinity in different generations (Figures 1-3). The clinical examination showed bilateral glaucoma in all patients, whereas parents and siblings were normal.Sequencing analysis of CYP1B1 revealed five pathogenic variants in seven families (7/11; 64%), including two novel variants. Five families showed 2 known mutations, p.R390H, a frequent mutation was found in four families, whereas p.P437L was observed once in a family segregating with PCG.
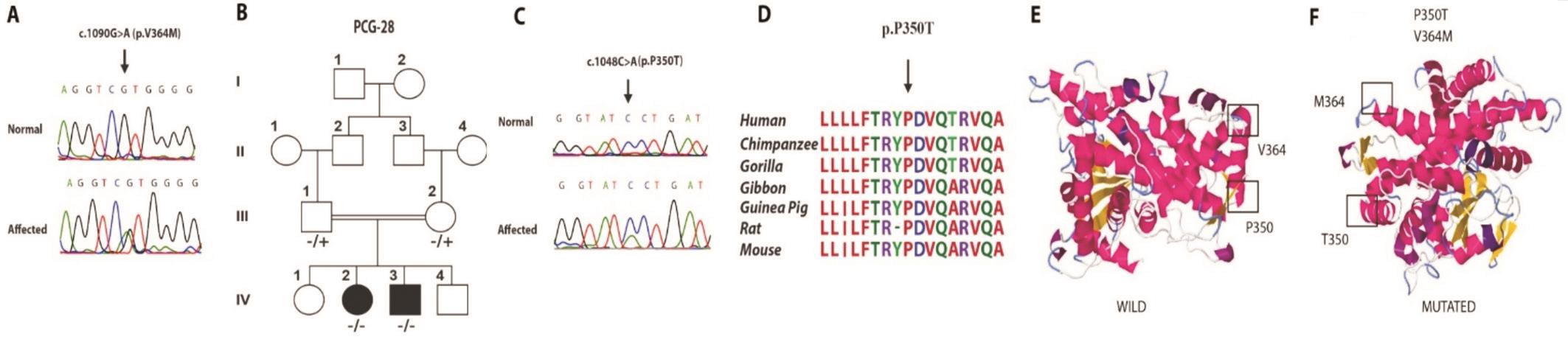
Figure 2 Pedigree with novel compound heterozygous mutation of CYP1B1 A: Mutated and normal chromatogram of p.V364M; B:Pedigree; C: Mutated and normal chromatogram of p.P350T; D: Multiple sequence alignment of CYP1B1 proteins from 7 species. Arrow indicates the mutated conserved amino acid; E: Protein modeling of CYP1B1 gene along with wild type amino acid residues of Proline 350 and Valine 364; F: Mutated protein model showing p.P350T and p.V364M.
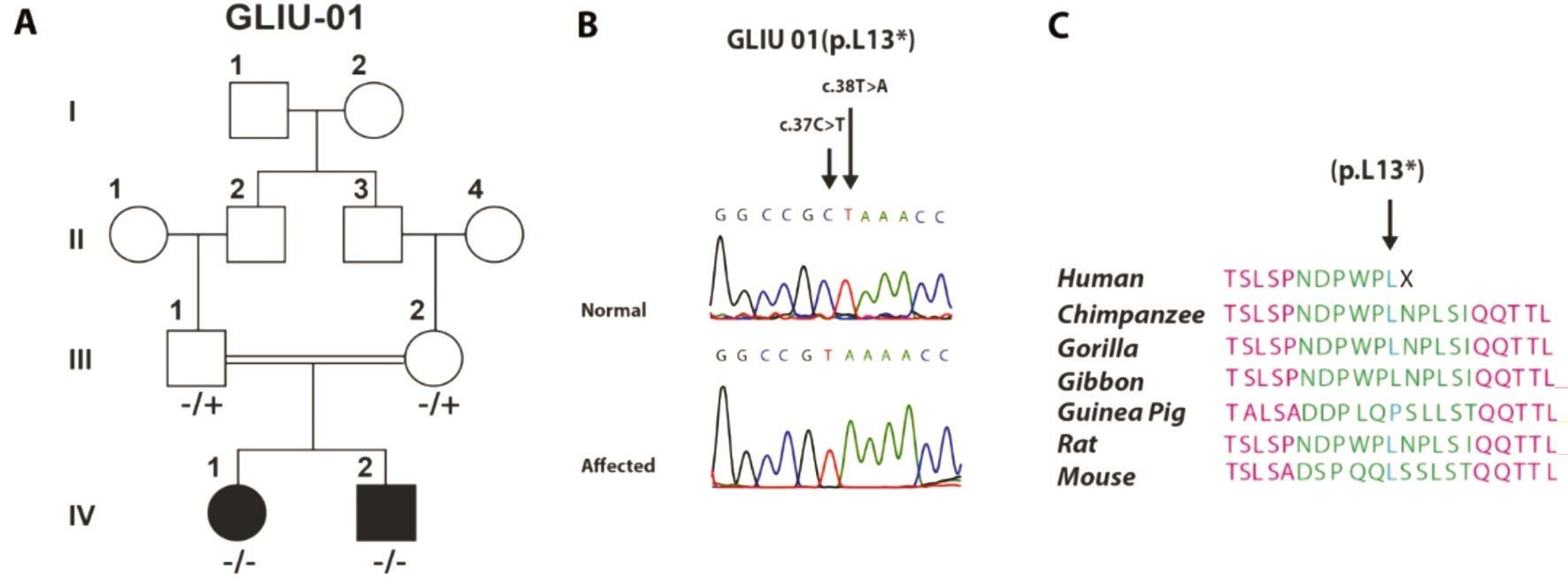
Figure 3 Pedigree with novel nonsense mutations A: Pedigree; B: Mutated and normal chromatograms of p.L13*; C: Sequence alignment showing point of truncation of CYP1B1 protein.
Four families (PCG-22, PCG-23, PCG-26 and PCG-27)harbored a frequent mutation, p.R390H, in a homozygous state (4/7; 57%; Figure 1). All 27 affected individuals (15 males and 12 females) in these four families had the onset of glaucoma up to first three years of life. The patients with early trabeculectomy had low IOP and the preserved vision .Whereas the patients who did not undergo any surgical intervention had elevated IOP, severe corneal opacity and poor visual acuity.The mean IOP in patients carrying p.R390H was 38 mm Hg(Table 1). One patient, PCG-22 IV:9 had phthisical eyes at the age of 42y and was blind. Interfamily phenotypic changes due to early interventions were seen in family PCG-23: patients V:4 to V:7 underwent bilateral trabeculectomy at the early stage and had controlled IOP, near to normal CDR with improved and restored vision. Whereas patients IV:3, IV:4,IV:5 and IV:6 were not surgically treated and had severe corneal opacity and megalocornea. The patients had poor visual acuity up to the perception of light, whereas the patient IV:5 was blind with maximum IOP 42 mm Hg. The detailed clinical findings of all families with p.R390H mutation are described in Table 1.
One novel allele, c.1048 C>A (p.P350T) was segregating with PCG in the compound heterozygous state with another reported allele, c.1090G>A (p.V364M) in two affected individuals of PCG-28. The parents were heterozygous for each allele (Figure 2).c.1090G>A (p.V364M ) was first reported in a compound heterozygous state in a PCG patient of Japanese origin(Figure 2)[14]. Both affected members in our study had the congenital onset of bilateral glaucoma. Patients did not provide any history of ophthalmological surgery for glaucoma and showed bilaterally enlarged cornea and reduced visual acuity(Table 1). IOP was elevated and maximum recorded IOP was 40 mm Hg in the patient IV:2. CDR could not be measured due to bilateral corneal opacities. HOPE online protein prediction tool described this novel variant as damaging as the mutant residue was bigger and less hydrophobic than the wild-type.Polyphen2 described this change as probably damaging with a score of 0.993. SIFT predicted this amino acid substitution as damaging, mutation taster predicted c.1048 C>A transversion as disease causing and PROVEAN web tool predicted this change as deleterious.
The second novel variant was a non-sense variant found in family GLIU-01, resulted due to a homozygous transition and transversion on two adjacent bases, c.37C>T and c.38T>A, causing premature stop codon at the same positionthus truncating the protein, p.(L13*). Both affected were homozygous, whereas parents were carriers and variants segregated with the PCG phenotype (Figure 3). Medical history showed that early preventive bilateral trabeculectomy in both patients led to the preservation of vision, although the cornea was enlarged but without any corneal opacities. IOP was near to normal and fundus examination showed normal CDR in both affected.
Table 1 Clinical features of known and novel CYP1B1 variants associated with PCG in Pakistani patients

NPL: No perception of light; PL: Perception of light; HM: Hand movement; NR: Not recordable; IOP: Intraocular pressure; CDR: Cup-Disc Ratio; Trabe: Trabeculectomy.
?
Family PCG-24 with two affected individuals had a reported p.P437L mutation in CYP1B1 gene (Figure 1). The patients manifested PCG symptoms soon after their birth during the first year and underwent bilateral trabeculectomy thereafter(Table 1). Individuals IV:1 had elevated IOP with bilateral megaolocornea with opacities and decreased visual acuity withthe perception of light only. Whereas patient IV:2 had also megalocornea with increased lacrimation and photophobia.
Table 2 CYP1B1 haplotypes associated with CYP1B1 mutations in Pakistani PCG patients
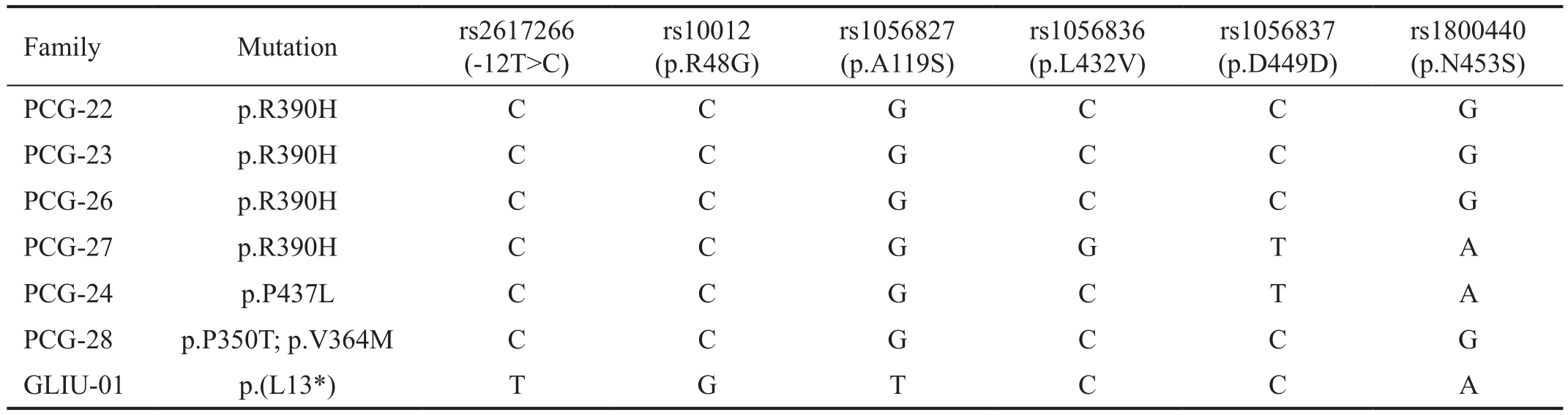
Family Mutation rs2617266(-12T>C)rs10012(p.R48G)rs1056827(p.A119S)rs1056836(p.L432V)rs1056837(p.D449D)rs1800440(p.N453S)PCG-22 p.R390H C C G C C G PCG-23 p.R390H C C G C C G PCG-26 p.R390H C C G C C G PCG-27 p.R390H C C G G T A PCG-24 p.P437L C C G C T A PCG-28 p.P350T; p.V364M C C G C C G GLIU-01 p.(L13*) T G T C C A
Six intragenic SNPs, rs2617266, rs10012, rs1056827,rs1056836, rs1056837 and rs1800440 were used to construct the haplotype associated with different CYP1B1 mutations.Three different haplotypes were found in the study; C-C-G-CC-G was found in 4 families, containing p.R390H in 3 families and 1 family having a compound heterozygous variant,p.V364M and p.P350T. C-C-G-G-T-A was found associated with p.R390H in one family and p.P437L in two families,whereas, T-G-T-C-C-A was found in one family having novel non sense variant L13* (Table 2).
PCG is a rare type of glaucoma, mostly affecting children up to 3 years of age and may cause irreversible blindness in neonatal or early infantile period[15]. PCG is recessively inherited and a cause of irreversible vision impairment in consanguineous pedigrees[16]. Previous studies confirm the CYP1B1 mutations as the major cause of PCG in consanguineous Pakistani families and non-penetrance of known CYP1B1 variants has also been described. This study was aimed to sequence CYP1B1 gene in 11 consanguineous families affected with PCG to find out disease causing variant and study its phenotypic presentations.
Five pathogenic CYP1B1 variants were segregating with PCG in 7 families (7/11), including two novel mutations. p.R390H was found in four families (4/7; 57%), thus remained the most common variant. It supported the previous finding of p.R390H (50%) in the cohort of 20 PCG affected families[11].p.R390H has remained the frequent CYP1B1 variant causing PCG among patients of different ethnic backgrounds of Pakistan. All our patients carrying p.R390H belonged to Sindhi Ethnic group, whereas a recent study based on PCG patients of Punjabi ethnicity also reported the highest frequency of p.R390H (13/23; 57%)[17]. The overall frequency of p.R390H is 46% (45/102) when combined all the Pakistani patients having CYP1B1 associated PCG (Table 3) and is higher than neighboring world populations[18-20]. p.R390H also show phenotypic variability among Pakistani patients; It has been associated with Juvenile-onset open angle glaucoma and with primary open angle glaucoma in a single Pakistani family[21-22]. In this study, 27 affected individuals from four p.R390H mutated consanguineous families have age ranging from 2.5y to 42y.All the patient had onset of bilateral glaucoma in first three years of life. The patients without any surgical intervention had vision loss along with corneal opacities, elevated IOP and increased CDR thus indicating the importance of timely surgical intervention for prevention of glaucoma symptoms and restoration of vision. Furthermore, all the individuals carrying single allele of p.R390H were phenotypically normal.p.P437L is the second known mutation found in two affected members of the family PCG24 (Figure 1). This CYP1B1 variant was first reported in the homozygous state in a Turkish PCG affected family[23], and as compound heterozygous in Brazilian patients[24]. p.P437L has been first time reported in PCG patients with Pakistani origin. The different clinical presentation was observed in both affected: the patient IV:1 had bilateral corneal opacity, whereas patient IV:2 had clear cornea in both eyes (Table 2). Although trabeculectomy was done in both affected but the patient IV:1 had elevated IOP.This is contrary to the patients homozygous for p.R390H,where the IOP was controllable after trabeculectomy (Table 1).This indicates that there may be other factors, responsible for clinical variations in the PCG patients. Moreover, functional characterization of p.P437L shows that it affects only the enzyme activity and there is no impact on intracellular localization and folding of the protein[23,25]. Whereas, p.R390H,substitution may lead to in disruption of salt bridge formation and normal functioning of the CYP1B1 protein[26]. This difference in position of both residues may also be responsible for variable clinical presentation.
Compound heterozygous CYP1B1 variants, p.V364M and p.P350T were found segregating with the disease phenotype in PCG 28 family (Figure 2). p.V364M was first reported in compound heterozygous state in a Japanese patient along with p.324Infs104*[14], whereas p.P350T is a novel variant and it has not been reported so far, nor it was found in ExAC 1000 genome browser and human gene mutation database. The twoaffected individuals having age ranging between 8-10y with the congenital onset of glaucoma did not respond positively to surgical interventions to control the glaucomatous changes and restore the vision in both patients, which is contrary to the clinical outcome of p.R390H (Table 1). Valine at 364 and proline at 350 are in the highly conserved position in J-helix of cytochrome P450 protein (Figure 2). It may have a role for the proper folding and heme-binding properties of the CYP1B1 protein[14]. In silico functional analysis of the novel substitution of p.P350T variant revealed the change as pathogenic and damaging to normal structure and function of the CYP1B1 protein (Figure 2). Furthermore, proline is known for providing rigidity to protein structure, its substitution with threonine at this position may lead to loss of rigidity in CYP1B1 protein which might be important for its functioning. In addition,comparative analysis of multiple sequences of 7 species revealed that proline at 350 is highly evolutionary conserved in different species (Figure 2).
Table 3 CYP1B1 mutations found in PCG patients of Pakistani origin

NA: Not available.aReported first in Pakistani PCG patients.
No. Mutation No. of alleles CYP1B1 alleles percentage (%) Associated haplotype of six SNPs References Nucleotide change Amino acid change 1 c.1169G>A p.R390H 46 45 C-C-G-C-C-G C-C-G-G-T-A 11,17,21 2 c.685G>A p.E229K 6 6 T-G-T-C-C-A 11,17,21 3 c.1103G>A p.R368H 4 4 NA 17 4 c.1405C>T p.R469W 2 2 NA 17 5 c.1300T>C p.W434R 2 2 NA 17 6 c.1331G>A p.R444Q 2 2 NA 17 7 c.241T>A p.Y81N 2 2 NA 17 8 c.107G>Aa p.G36D 2 2 C-C-G-C-C-G 11 9 c.198_209del 12a p.G67-70V 2 2 T-G-T-C-C-A 11 10 c.868-869 InCa p.R290Pfs37* 4 4 C-C-G-G-T-A 21 11 c.746G>C p.A115P 2 2 C-C-G-G-T-A 11 12 c.1200_1209dup p.T404Sfs30* 2 2 NA 17 13 c.736_737insTa p.W246Lfs81* 2 2 NA 17 14 c.1325delCa p.P442Qfs15* 2 2 NA 17 15 c.109C>T p.Q37* 2 2 NA 17 16 c.1063C>T p.R355* 2 2 NA 21 17 c.862G>Ca p.A288P 2 2 NA 21 18 c.725A>Ca p.D242A 2 2 NA 21 19 c.1460T>Ga p.L487P 2 2 T-G-T-C-C-A 22 20 c.530T>Ga p.L177R 2 2 C-C-G-G-T-A 22 21 c.1122C>Ga p.D374E 2 2 T-G-T-C-C-A 22 22 c.1311G>A p. P437L 2 2 C-C-G-G-T-A This study 23 c.1090G>A p.V364M 2 2 C-C-G-C-C-G This study 24 c.1048C>Aa p.(P350T) 2 2 C-C-G-C-C-G This study 25 c.37C>T; c.38T>Aa p.(L13*) 2 2 T-G-T-C-C-A This study Total 102 100
The second novel variant, a non-sense mutation p.L13* was segregating with PCG in two affected of family GLIU-01(Figure 3). The simultaneous transition and transversion of two nucleotides, c.37C>T and c.38T>A at same codon, resulted into a premature stop codon in CYP1B1 protein. This type of variant has not been described from Pakistan previously.Moreover, the variant resulted into the smallest CYP1B1 peptide, just 13 amino acids long, lacking all the functional units of CYP1B1 protein (Figure 3). Surprisingly, the phenotype of this null mutation is not as severe as compared to other substitution mutations. The surgical interventions were more successful and ophthalmological examination of both patients showed only reduced visual acuity and controlled IOP (Table 1).This supports the hypothesis that amino acid substitution variants may result in to gain of function or dominant negative effect of the protein as compared to null mutations[27]. This needs further investigation to explore and understand the role of the CYP1B1 enzyme in the development of anomalies causing PCG.
Allelic heterogeneity of CYP1B1 gene has been observed in Pakistani PCG patients and to date, 25 different CYP1B1 variants have been found segregating with PCG in Pakistani patients (Table 3). Twelve variants (48%, 12/25) have been reported first time from Pakistan and have not been found in any other world population (Table 3). Whereas, p.R390H a frequently found variant in Pakistani patients has also been found in other ethnically diverse populations. Moreover, 68%(17/25) of the CYP1B1 variants, found in Pakistani patients are missense substitutions followed by frameshifts and nonsense variants (Table 3). Different position and type of CYP1B1 mutations may affect the protein structure and function in different ways. The compound heterozygous patients may have complex biochemical properties of CYP1B1 protein as compared to patients with homozygous or heterozygous variants[16].In addition, the association of p.R390H heterozygous with primary open-angle glaucoma or juvenile open angle glaucoma in certain populations and normal carriers in families with homozygous affected, support the hypothesis that besides the mutation position and type, unknown genetic or epigenetic factors may in fluence the phenotypic presentations.
Haplotypes of six intragenic SNPs, rs2617266, rs10012,rs1056827, rs1056836, rs1056837 have been widely studied along with CYP1B1 mutations in different populations[28-29].Previously, a distinct haplotype, C-C-G-C-C-G, has been associated with common mutation, p.R390H, in 18 PCG families of Pakistani origin and designated p.R390H as a founder mutation, originated on common haplotype in Indian and Pakistani patients[11,17]. However, in the study, another haplotype, C-C-G-G-T-A has been found associated with p.R390H in one family, whereas 3 families harbored the same previously reported haplotype (Table 3). Similarly,p.R390H has originated on three different haplotypes in Iranian patients including C-C-G-G-T-A, which is a common haplotype in Iranian PCG patient. In addition, C-C-G-GT-A has been reported in association with 50% of CYP1B1 variants[11]. This may suggest the different origin of p.R390H in the Pakistani population and may indicate ancestral lineages between Pakistani and Iranian populations. Moreover, p.P437L and novel compound heterozygous variants (p.P350T and p.V364M) were also associated with C-C-G-C-C-G and C-CG-G-T-A respectively. The novel nonsense variant p.L13* was found associated with T-G-T-C-C-A, haplotype, previously,this haplotype has been associated with an in-frame deletion,p.G67-V70 and p.E229K in Pakistani patients (Table 3)[11].
The study suggests that though the CYP1B1 mutations are the major cause of PCG in genetically heterogeneous populations,but there may be unknown genetic and environmental factors,responsible for phenotypic variability and success of surgical interventions. Moreover, the type and position of CYP1B1 mutations may affect the PCG development and it needs further investigation to better understand the pathophysiology of the disease. Identification of multiple novel and known variants reaffirm the genetic heterogeneity of the population and suggest the importance of genetic testing and genetic counseling for better management of the disease among consanguineous affected families.
Authors are thankful to all individuals who participated in this study. The study was supported by Pakistan Sciences Foundation Grant (No: Biotech-101) and LUMHS Intramural Funds to Ali M Waryah.
Conflicts of Interest: Waryah YM, None; Iqbal M, None;Sheikh SA, None; Baig MA, None; Narsani AK, None; Atif M, None; Bhinder MA, None; Rahman A, None; Memon AI, None; Pirzado MS, None; Waryah AM, None.
1 Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010.Br J Ophthalmol 2012;96(5):614-618.
2 Ho CL, Walton DS. Primary congenital glaucoma: 2004 update. J Pediatr Ophthalmol Strabismus 2004;41(5):271-288; quiz 300-1.
3 Berraho A, Serrou A, Fritez N, El Annas A, Bencherifa F, Gaboun F, Hilal L. Genotype-phenotype correlation in Moroccan patients with primary congenital glaucoma. J Glaucoma 2015;24(4):297-305.
4 Morales-Fernandez L, Martinez-De-la-casa JM, Garcia-Bella J, Mendez C, Saenz-Frances F, Garcia M, Escribano J, Garcia-Feijoo J. Clinical variability of primary congenital glaucoma in a Spanish family with Cyp1b1 gene mutations. J Glaucoma 2015;24(8):630-634.
5 Moore DB, Tomkins O, Ben-Zion I. A review of primary congenital glaucoma in the developing world. Surv Ophthalmol 2013;58(3):278-285.
6 Bashir R, Sanai M, Azeem A, Altaf I, Saleem F, Naz S. Contribution of GLC3A locus to primary congenital glaucoma in Pakistani population.Pak J Med Sci 2014;30(6):1341-1345.
7 Bejjani BA, Stockton DW, Lewis RA, Tomey KF, Dueker DK, Jabak M, Astle WF, Lupski JR. Multiple CYP1B1 mutations and incomplete penetrance in an inbred population segregating primary congenital glaucoma suggest frequent de novo events and a dominant modifier locus.Hum Mol Genet 2000;9(3):367-374.
8 Reis LM, Tyler RC, Weh E, Hendee KE, Schilter KF, Phillips JA 3rd,Sequeira S, Schinzel A, Semina EV. Whole exome sequencing identifies multiple diagnoses in congenital glaucoma with systemic anomalies. Clin Genet 2016;90(4):378-382.
9 Souma T, Tompson SW, Thomson BR, et al. Angiopoietin receptor TEK mutations underlie primary congenital glaucoma with variable expressivity.J Clin Invest 2016;126(7):2575-2587.
10 Souzeau E, Hayes M, Ruddle JB, Elder JE, Staffieri SE, Kearns LS,Mackey DA, Zhou T, Ridge B, Burdon KP, Dubowsky A, Craig JE.CYP1B1 copy number variation is not a major contributor to primary congenital glaucoma. Mol Vis 2015;21:160-164.
11 Sheikh SA, Waryah AM, Narsani AK, Shaikh H, Gilal IA, Shah K,Qasim M, Memon AI, Kewalramani P, Shaikh N. Mutational spectrum of the CYP1B1 gene in Pakistani patients with primary congenital glaucoma: novel variants and genotype-phenotype correlations. Mol Vis 2014;20:991-1001.
12 Grimberg J, Nawoschik S, Belluscio L, McKee R, Turck A, Eisenberg A. A simple and efficient non-organic procedure for the isolation of genomic DNA from blood. Nucleic Acids Res 1989;17(20):8390.
13 Waryah AM, Narsani AK, Sheikh SA, Shaikh H, Shahani MY. The novel heterozygous Thr377Arg MYOC mutation causes severe Juvenile Open Angle Glaucoma in a large Pakistani family. Gene 2013;528(2):356-359.
14 Ohtake Y, Kubota R, Tanino T, Miyata H, Mashima Y. Novel compound heterozygous mutations in the cytochrome P4501B1 gene(CYP1B1) in a Japanese patient with primary congenital glaucoma.Ophthalmic Genet 2000;21(3):191-193.
15 Chen L, Huang L, Zeng A, He J. CYP1B1 gene mutations with incomplete penetrance in a Chinese pedigree with primary congenital glaucoma: a case report and review of literatures. Int J Clin Exp Med 2015;8(8):14538-14541.
16 Stoilov IR, Costa VP, Vasconcellos JP, Melo MB, Betinjane AJ, Carani JC, Oltrogge EV, Sarfarazi M. Molecular genetics of primary congenital glaucoma in Brazil. Invest Ophthalmol Vis Sci 2002;43(6):1820-1827.
17 Rauf B, Irum B, Kabir F, Firasat S, Naeem MA, Khan SN, Husnain T,Riazuddin S, Akram J, Riazuddin SA. A spectrum of CYP1B1 mutations associated with primary congenital glaucoma in families of Pakistani descent. Hum Genome Var 2016;3:16021.
18 Chitsazian F, Tusi BK, Elahi E, et al. CYP1B1 mutation profile of Iranian primary congenital glaucoma patients and associated haplotypes. J Mol Diagn 2007;9(3):382-393.
19 Tanwar M, Dada T, Sihota R, Das TK, Yadav U, Dada R. Mutation spectrum of CYP1B1 in North Indian congenital glaucoma patients. Mol Vis 2009;15:1200-1209.
20 Li N, Zhou Y, Du L, Wei M, Chen X. Overview of Cytochrome P450 1B1 gene mutations in patients with primary congenital glaucoma. Exp Eye Res 2011;93(5):572-579.
21 Micheal S, Ayub H, Zafar SN, Bakker B, Ali M, Akhtar F, Islam F,Khan MI, Qamar R, den Hollander AI. Identification of novel CYP1B1 gene mutations in patients with primary congenital and primary openangle glaucoma. Clin Exp Ophthalmol 2015;43(1):31-39.
22 Firasat S, Riazuddin SA, Khan SN, Riazuddin S. Novel CYP1B1 mutations in consanguineous Pakistani families with primary congenital glaucoma. Mol Vis 2008;14:2002-2009.
23 Stoilov I, Akarsu AN, Alozie I, Child A, Barsoum-Homsy M, Turacli ME, Or M, Lewis RA, Ozdemir N, Brice G, Aktan SG, Chevrette L,Coca-Prados M, Sarfarazi M. Sequence analysis and homology modeling suggest that primary congenital glaucoma on 2p21 results from mutations disrupting either the hinge region or the conserved core structures of cytochrome P4501B1. Am J Hum Genet 1998;62(3):573-584.
24 Della Paolera M, de Vasconcellos JP, Umbelino CC, Kasahara N,Rocha MN, Richeti F, Costa VP, Tavares A, de Melo MB. CYP1B1 gene analysis in primary congenital glaucoma Brazilian patients: novel mutations and association with poor prognosis. J Glaucoma 2010;19(3):176-182.
25 Medina-Trillo C, Ferre-Fernandez JJ, Aroca-Aguilar JD, Bonet-Fernandez JM, Escribano J. Functional characterization of eight rare missense CYP1B1 variants involved in congenital glaucoma and their association with null genotypes. Acta Ophthalmol 2016;94(7):e555-e560.
26 Su CC, Liu YF, Li SY, Yang JJ, Yen YC. Mutations in the CYP1B1 gene may contribute to juvenile-onset open-angle glaucoma. Eye (Lond)2012;26(10):1369-1377.
27 Sena DF, Finzi S, Rodgers K, Del Bono E, Haines JL, Wiggs JL.Founder mutations of CYP1B1 gene in patients with congenital glaucoma from the United States and Brazil. J Med Genet 2004;41(1):e6.
28 Bagiyeva S, Marfany G, Gonzalez-Angulo O, Gonzalez-Duarte R.Mutational screening of CYP1B1 in Turkish PCG families and functional analyses of newly detected mutations. Mol Vis 2007;13:1458-1468.
29 Chakrabarti S, Kaur K, Kaur I, Mandal AK, Parikh RS, Thomas R, Majumder PP. Globally, CYP1B1 mutations in primary congenital glaucoma are strongly structured by geographic and haplotype backgrounds. Invest Ophthalmol Vis Sci 2006;47(1):43-47.