Table 1 Ultimate stress and modulus at 10% strain mean±SD
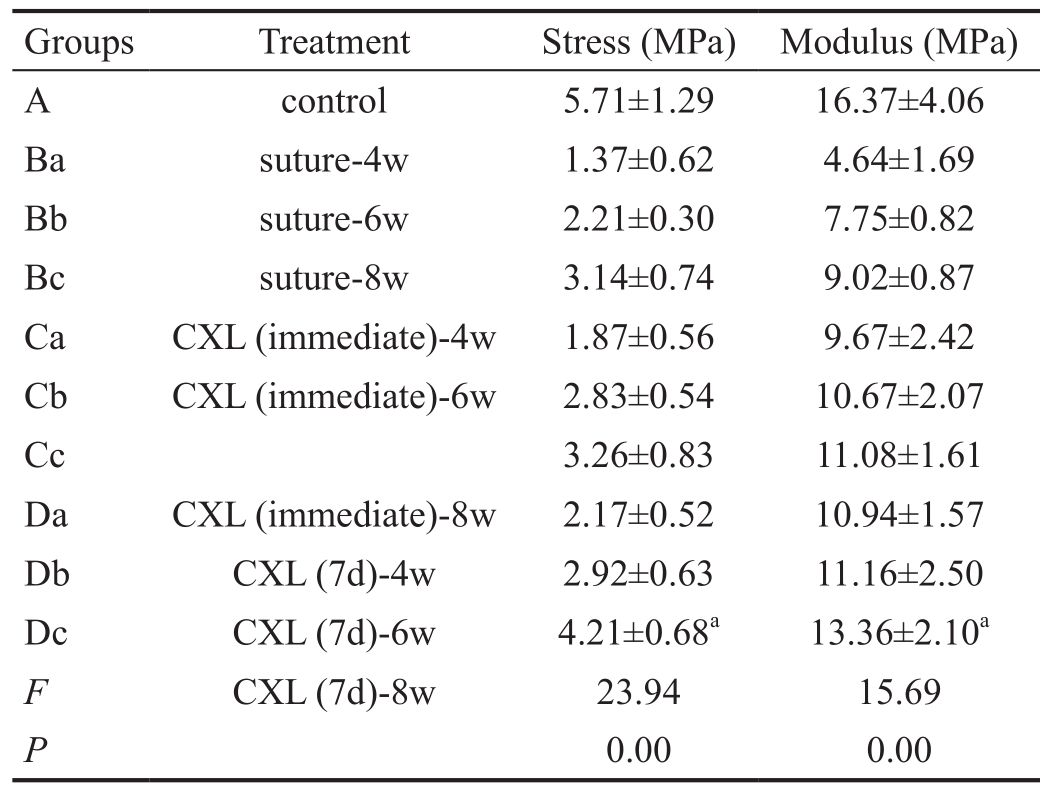
aP<0.05.
?
The cornea is the transparent tissue covering the front of the eye. The penetrating corneal trauma is very common due to directly contacting the external environment[1].Epithelial-stromal injury of the cornea initiates a complex stromal response that can lead to the formation of corneal scar[2-3]. The scar leads to opacity of the cornea and weakened biomechanical properties. When intraocular pressure increases continuously, corneal scar could develop to corneal staphyloma.Corneal scar tissue’s impact on biomechanical properties of the cornea has been documented in an experiment report[4].
The biomechanics of collagen depends on the collagen density and the covalent cross-linking between collagen molecules[5].Therefore, cross-linking plays an important role in the strength of collagen tissue. Over the past decade, a technique to generate corneal collagen cross-linking (CXL) was invented by Seilor[6]. CXL induced by ribo flavin/ultraviolet A (UVA) has a stiffening effect that increases resistance of the collagen against proteolytic enzymes, and increases the collagen diameter which could improve the biomechanics of the cornea[7-12]. CXL was originally designed to improve the corneal biomechanics and prevent further development of keratoconus[13]. CXL was also used for the treatment of corneal ulcers, which healed with scar tissue and no complications[14-15]. CXL has also been reported successfully in preventing the progress of keratectasia after refractive surgery[16]. To the best of our knowledge, there was no report about CXL in corneal scar tissue which induced by penetrating corneal trauma. The purpose of the present study is to evaluate the biomechanics of corneal scar which treated with ribo flavin/UVA.
Ethical Approval The experiments and all animal procedures were approved by the ethics committee of Zunyi Medical College, and followed guidelines of the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Animals Eighty-six healthy rabbits were selected and divided into control group (group A, n=8) and trauma groups[group B (n=27), group C (n=24) and group D (n=27)]. The right corneas of these 78 rabbits in the trauma groups were penetrated using blades. The corneas in group B were treated only with sutures. The corneas in group C were treated with riboflavin/UVA immediately after suturing. The corneas in group D were treated with riboflavin/UVA seven days after suturing. Then groups B, C and D were further divided into subgroups according to the time points of sacrifice, i.e. groups Ba (4wk, n=8), Bb (6wk, n=8) and Bc (8wk, n=11); groups Ca (4wk, n=8), Cb (6wk, n=8) and Cc (8wk, n=8); groups Da (4wk, n=8), Db (6wk, n=8) and Dc (8wk, n=11). Samples from the three rabbits of group Bc and three of group Dc were used to measure the expression of alpha smooth muscle action(α-SMA).
Corneal Penetrating Injury Model Rabbits were anesthetized by intravenous injection of 2% sodium pentobarbital solution (Beijing Pubos Biotechnology Co., Ltd.Beijing, China) and then a full-thickness incision about 6 mm length was cut in the vertical direction around the centre of cornea. The incisions were sutured intermittently with four stitches using 10-0 nylon suture.
Riboflavin/UVA Cross-linking According to Wollensak’s method[17], 30min before the irradiation, 0.1% riboflavin photosensitizer solution (10 mg riboflavin-5-phosphate in 10 mL 20% dextran-T-500; Beijing Solarbio Science &Technology Co., Ltd., Beijing, China) was dropped on the target cornea. UVA irradiation (370 nm) was applied using a single UVA diodes (Zhuhai Tianhui Electronic Co., Zhuhai,China) with an irradiance of 3 mW/cm2 for 30min. During the irradiation, the riboflavin was instilled on the cornea every 3min. Illumination intensity was monitored using a calibrated UV light meter (UV-340A, 290-390 nm, Taiwan, China).
Stress-strain Test At the time points of sacrifice, the rabbits were killed by air embolism. A corneal strip (4.0 mm wide,10.0 mm in length) was cut using a double-blade scalpel.The scar must be placed in the middle of the strip. The corneal strips were preserved less than 24h at 4℃ in a moist chamber. Then the corneal scar thickness was measured using a digimatic calliper [Sanling group (H.K.) Ltd., H.K., China]and a stress-strain test was performed.
The stress-strain tests of the corneal strips were performed using CMT6104 (Meitesi industry system Co. Ltd., Minnesota,USA). The strips were subjected to a stress level of 0.02 MPa and 5 cycles in total of deformation-load testing. Strain was increased linearly at a velocity of 1.5 mm/min, and stress was measured up to scar fracture.
Western Blot Corneal tissues from the three rabbits of group Bc and three of group Dc were excised and homogenized in the immunoprecipitation assay (RIPA) lysis buffer. Proteins in whole lysate (10 μg protein per sample) were electrophoresedby 7.5% SDS-PAGE and then electroblotted onto nitrocellulose membranes which were blocked in 5% skim milk. Subsequently,the membrane was incubated overnight with primary antibodies directed against actin and α-SMA antibody (1:5000,Beijing Boorsen Biotechnology Co., Ltd., Beijing, China)at 4℃. The membranes were washed using tris-buffered saline with Tween 20 (TBST, Cell Signaling Technology) and incubated with a horseradish peroxidase-conjugated secondary antibody for 60min at room temperature (β-actin 1:5000;BoAoSeng, Beijing, China). Chemiluminescence assays were processed using a peroxidase substrate. The immunoblot signal was detected and analyzed using Image J software.
Table 1 Ultimate stress and modulus at 10% strain mean±SD

aP<0.05.
?
Statistical Analysis The ultimate stress and Young’s modulus data at 10% strain were expressed as mean±standard deviation and analysed by the one-way ANOVA (version 17.0, SPSS Inc, Chicago, IL, USA). Normality for continued variables in groups was analyzed using the one-way ANOVA. A value of P<0.05 was considered statistically significant.
The mean thickness of the corneal scar strips was 560±84 μm.There was statistically significant difference among all groups(P<0.05). Group A was 398±7 μm; groups Ba, Bb and Bc were 640±62 μm, 609±63 μm and 579±63 μm, respectively; groups Ca, Cb and Cc were 604±69 μm, 581±76 μm and 537±26 μm,respectively; groups Da, Db and Dc were 603±82 μm, 533±44 μm and 524±19 μm, respectively.
When compared the value of the ultimate stress and Young’s modulus at 10% strain, corneal strips in CXL groups had higher values than group B, but still lower than the control group (P<0.05). Group Dc had a significant higher value of the stress and modulus than those in other trauma groups (groups B,C, Da and Db; P<0.05; Table 1).
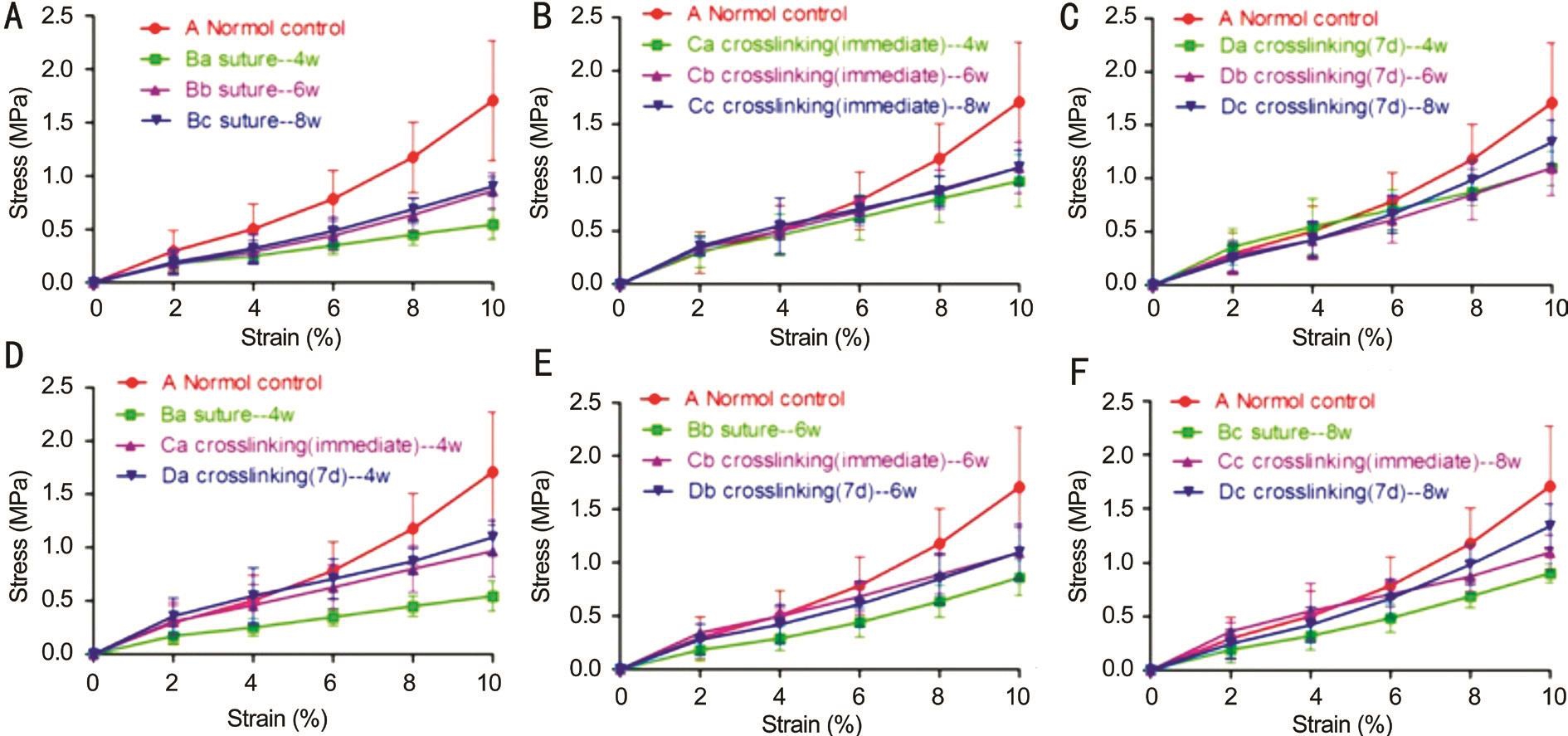
Figure 1 The stress-strain values showed an exponential increase in all groups The ultimate stress and young’s modulus increased with time(A-C). The largest values were observed at 8wk (P<0.05). The values from the CXL groups were lower than that in the control group (P=0.00).The stress of corneal scar in group B was lower than that of group D (P<0.05, D-F).
The stress-strain values increased exponentially in all study groups. The ultimate stress and Young’s modulus of the corneal strips increased when the time after the injury getting longer(Figure 1A-1C). The highest value in the trauma groups was obtained in group Dc (P<0.05). However, the value of group Dc was still lower than that of the control group (P<0.01). The stress values of corneal strips in group D were significantly higher than those in group B (P<0.05; Figure 1D-1F).α-SMA was measured by Western blot in groups Bc and Dc. The relative expression of α-SMA were 0.28±0.11 and 0.65±0.20, respectively. The difference of the above values was statistically significant (t=-2.48, P=0.048; Figure 2).
The cornea is a viscoelastic tissue that is composed of a large number of collagen fibers. The polar region of a collagen molecule cross-links the non-polar region of another collagen molecule via covalent and electrostatic attraction. Thus, the collagen fibers have very high tenacity. Because of its high tenacity and strong tensile resistance, collagen fibers play an important role in maintaining corneal tension[18]. When the collagen structure is affected by external stimuli, its chemical and physical properties would change, such as elasticity and stiffness. The corneal wound healing response usually contributes to a restore of normal stromal structure and function[19-20]. During the repair process after injury, fibroblasts underwent the migration and proliferation, and part of them would transform into myo fibroblasts[21-22]. The biomechanical properties of the corneal scar after the excimer laser in situ keratomileusis surgery was found weak and easily broken in bruises. Clinically, corneal scar is the prominent location of corneal staphyloma, and it also indicates that the resistance of scar tissue to intraocular pressure is decreased. To protect the corneal endothelium, lens and retina, the minimum corneal thickness for performing CXL is 400 μm[23-25]. In the present study, the mean thickness of corneal scars was 560±84 μm.Our experiment was safe to the rabbits.
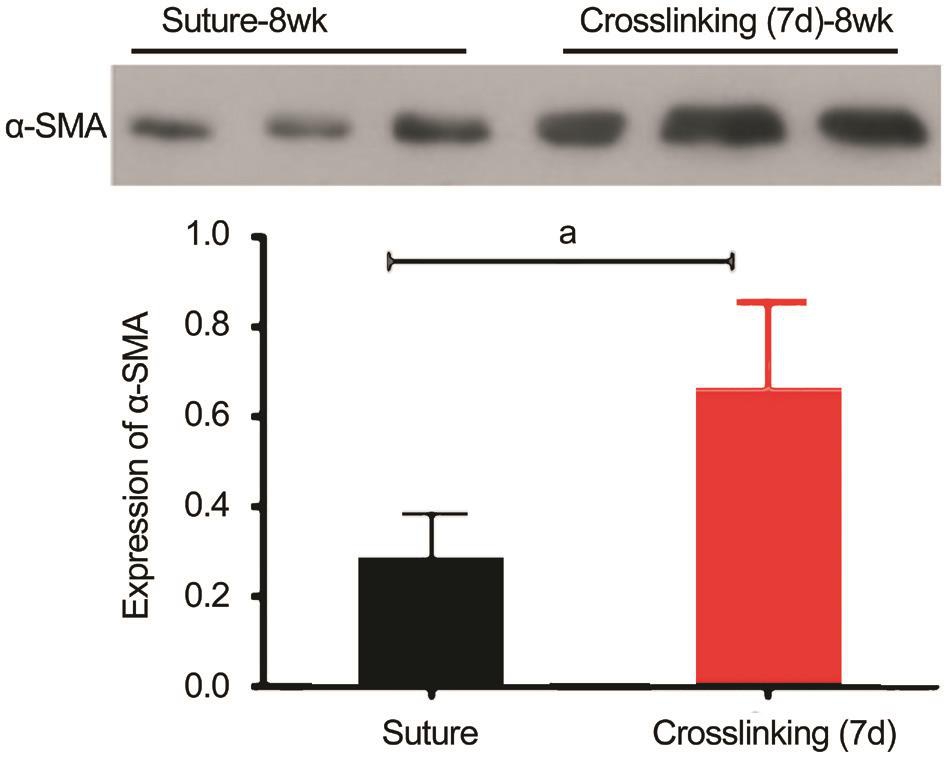
Figure 2 Relative expression of α-SMA evaluated by Western blotting Value in group Dc was significantly higher than that in group Bc.aP<0.05.
The commonly used index to evaluate the biomechanical properties of a viscoelastic tissue is Young’s modulus, which is the ratio of stress to strain[26-27]. There was a report that after corneal penetrating injury, the tension of the rabbit cornea increased with the prolongation of time and reaches maximum at 6wk[28]. However, in the present study, the corneal tensile strength gradually increased and reached a highest value at 8wk which was the final time point of the study. The reason of a longer restore time in our study than that in the literature may due to a larger lesion we made than the lesion size reported in the other study. Because when the incision was larger, the stability of the cornea was worse, and the recovery time of the tissue repair was increased. The results of the present study demonstrated that CXL increased the biomechanical characteristics of the scar formed in the penetrating corneal injury. CXL could be used to remove sutures earlier when a corneal penetrating injury occurs. Our data showed that the best effect of improving biomechanics of a corneal scar could be induced by CXL at seven days after trauma. We speculated that it may due to the activation and transdifferentiation of fibroblasts to myo fibroblasts, and large amount of collagen was synthesized.
Myofibroblasts express α-SMA which is a cytoskeletal protein[29] and it can make myofibroblasts contractile[30]. The corneal tissue does not express α-SMA. The expression of α-SMA can be used to determine whether the myo fibroblasts appear after the corneal trauma[31-32]. There was study report that the epithelial cells proliferated significantly and the number of fibroblasts increased after photorefractive keratectomy, and the expression of α-SMA was obvious[33].α-SMA connects with extracellular collagen and fibronectin via integrin a2bl and a5b1, enhances the ability of wound tissue to resist external tension[34]. α-SMA has been reported that has impact on biomechanics of the sclera[35]. The expression of α-SMA was observed at one week after corneal trauma occurred. CXL may increase the expression of α-SMA by affecting myofibroblasts. Our Western blotting tests showed that expression of α-SMA was significantly increased in the group performing CXL at seven days after corneal lesion suturing. It documented that CXL induced by ribo flavin/UVA increased α-SMA and enhanced the biomechanical properties of corneal scar tissue. Further studies are needed to confirm the long term effect of CXL with ribo flavin/UVA, which could be a potential therapy to prevent the progression of corneal staphyloma.
Foundations: Supported by the National Natural Science Foundation of China (No.81660169); the Science and Technology Foundation of Zunyi [No.(2014)94].
Confiicts of Interest: Cai YH, None; Liu TX, None; Li HX,None.
1 Bhupally AK, Chigiri SS, Swathi M, Rohini M, Shruthi T. Ocular trauma. Int J Res Med Sci 2015;3(12):3714-3719.
2 Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol 2007;127(3):526-537.
3 Torricelli AA, Santhanam A, Wu JH, Singh V, Wilson SE. The corneal fibrosis response to epithelial-stromal injury. Exp Eye Res 2016;142: 110-118.
4 Corr DT, Hart DA. Biomechanics of scar tissue and uninjured skin. Adv Wound Care 2013;2(2):37-43.
5 Usha R, Ramasami T. Effect of pH on dimensional stability of rat tail tendon collagen fiber. Journal of Applied Polymer Science 2000;75(13):1577-1584.
6 Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue.Exp Eye Res 1998;66(1):97-103.
7 Tomkins O, Garzozi HJ. Collagen cross-linking: strengthening the unstable cornea. Clin Ophthalmol 2008;2(4):863-867.
8 Wollensak G. Corneal collagen crosslinking: new horizons. Expert Review of Ophthalmology 2010;5(2):201-215.
9 Beshtawi IM, O’donnell C, Radhakrishnan H. Biomechanical properties of corneal tissue after ultraviolet-A-riboflavin crosslinking. J Cataract Refract Surg 2013;39(3):451-462.
10 Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after ribo flavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg 2003;29(9):1780-1785.
11 Mazzotta C, Traversi C, Baiocchi S, Caporossi O, Bovone C, Sparano MC, Balestrazzi A, Caporossi A. Corneal healing after riboflavin ultraviolet-A collagen cross-linking determined by confocal laser scanning microscopy in vivo: early and late modifications. Am J Ophthalmol 2008;146(4):527-533.
12 Wollensak G, Wilsch M, Spoerl E, Seiler T. Collagen fiber diameter in the rabbit cornea after collagen crosslinking by ribo flavin/UVA. Cornea 2004;23(5):503-507.
13 De Bernardo M, Capasso L, Lanza M, Tortori A, Iaccarino S, Cennamo M, Borrelli M, Rosa N. Long-term results of corneal collagen crosslinking for progressive keratoconus. J Optom 2015;8(3):180-186.
14 Krader CG. Corneal ulcers respond to CXL therapy. Ophthalmology Times 2012;37(7):53.
15 Ehlers N, Hjortdal J, Nielsen K, Søndergaard A. Riboflavin-UVA treatment in the management of edema and nonhealing ulcers of the cornea. J Refract Surg 2009;25(9):S803-S806.
16 Vinciguerra P, Camesasca FI, Albè E, Trazza S. Corneal collagen crosslinking for ectasia after excimer laser refractive surgery: 1-year results. J Refract Surg 2010;26(7):486-497.
17 Wollensak G, Iomdina E. Long-term biomechanical properties of rabbit cornea after photodynamic collagen crosslinking. Acta Ophthalmol 2009;87(1):48-51.
18 Lewis PN, White TL, Young RD, Bell JS, Winlove CP, Meek KM.Three-dimensional arrangement of elastic fibers in the human corneal stroma. Exp Eye Res 2016;146:43-53.
19 Couture C, Zaniolo K, Carrier P, Lake J, Patenaude J, Germain L,Guérin SL. The tissue-engineered human cornea as a model to study expression of matrix metalloproteinases during corneal wound healing.Biomaterials 2016;78:86-101.
20 Li Y, Chen HJ, Zhang H, Wu J, Hu YT, Ma ZZ. Effects of different sutures on fibrosis and wound healing in a rabbit model of corneal wounds. Exp Ther Med 2016;12(5):2827-2834.
21 Torricelli AA, Santhanam A, Wu JH, Singh V, Wilson SE. The corneal fibrosis response to epithelial-stromal injury. Exp Eye Res 2016;142:110-118.
22 West-Mays JA, Dwivedi DJ. The keratocyte: corneal stromal cell with variable repair phenotypes. Int J Biochem Cell Biol 2006;38(10):1625-1631.
23 Caporossi A, Baiocchi S, Mazzotta C, Traversi C, Caporossi T.Parasurgical therapy for keratoconus by riboflavin-ultraviolet type a rays induced cross-linking of corneal collagen. J Cataract Refract Surg 2006;32(5):837-845.
24 Kohlhaas M, Spoerl E, Schilde T, Unger G, Wittig C, Pillunat LE.Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet a light. J Cataract Refract Surg 2006;32(2):279-283.
25 Hafezi F, Mrochen M, Iseli HP, Seiler T. Collagen crosslinking with ultraviolet-A and hypoosmolar riboflavin solution in thin corneas. J Cataract Refract Surg 2009;35(4):621-624.
26 Battaglioli JL, Kamm RD. Measurements of the compressive properties of scleral tissue. Invest Ophthalmol Vis Sci 1984;25(1):59-65.
27 Hollman KW, Emelianov SY, Neiss JH, Jotyan G, Spooner GJ, Juhasz T, Kurtz RM, O’Donnell M. Strain imaging of corneal tissue with an ultrasound elasticity microscope. Cornea 2002;21(1):68-73.
28 Liu JL, Lian LY, Liu H, Zhang XR. Corneal strength change and keratocan expression of corneas during the healing of penetrating injury in rabbits. Chin J Exp Ophthalmol 2015;33(7):611-615.
29 Ishizaki M, Zhu G, Haseba T, Shafer SS, Kao WW. Expression of collagen I, smooth muscle alpha-actin, and vimentin during the healing of alkali-burned and lacerated corneas. Invest Ophthalmol Vis Sci 1993;34(12):3320-3328.
30 Jester JV, Ho-Chang J. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res 2003;77(5):581-592.
31 Nakamura K, Kurosaka D, Yoshino M, Oshima T, Kurosaka H. Injured corneal epithelial cells promote myodifferentiation of corneal fibroblasts.Invest Ophthalmol Vis Sci 2002;43(8):2603-2608.
32 Mohan RR, Gupta R, Mehan MK, Cowden JW, Sinha S. Decorin transfection suppresses profibrogenic genes and myo fibroblast formation in human corneal fibroblasts. Exp Eye Res 2010;91(2):238-245.
33 Zhang J, Zheng L, Chen Q, Ju Y. Expression of α-smooth muscle actin in corneal fibroblasts after PRK and LASIK. Chin Ophtal Res 2005;23(2):121-124.
34 Jester JV, Petroll WM, Barry PA, Cavanagh HD. Expression of alphasmooth muscle (alpha-SM) actin during corneal stromal wound healing.Invest Ophthalmol Vis Sci 1995;36(5):809-819.
35 Phillips JR, McBrien NA. Pressure-induced changes in axial eye length of chick and tree shrew: significance of myofibroblasts in the sclera.Invest Ophthalmol Vis Sci 2004;45(3):758-763.