One-year outcomes of intravitreal conbercept combined rescue therapy for polypoidal choroidal vasculopathy in a Chinese population: a real-life clinical data
Hui-Jun Qi, En-Zhong Jin, Ming-Wei Zhao
Department of Ophthalmology, Ophthalmology & Optometry Center, Peking University People’s Hospital, Beijing Key Laboratory of Diagnosis and Therapy of Retinal and Choroid Diseases, Beijing 100044, China
Abstract● AlM: To evaluate the real-life clinical outcomes of intravitreal injection of conbercept combined rescue therapy for polypoidal choroidal vasculopathy (PCV).● METHODS: This was an open label, single center, and interventional study. All enrolled patients were treated initially with three consecutive monthly intravitreal conbercept injections (0.5 mg). Additional conbercept injections were administered upon substantial polyp regression with improved visual acuity (VA). Eyes with partial or no polyp regression and poor VA were rescue treated with photodynamic therapy (PDT) for subfoveal polyps or thermal laser photocoagulation for extrafoveal polyps. Best-corrected visual acuity (BCVA), central foveal thickness (CFT) and polyp regression were observed as primary outcomes. Side effects were also collected during the follow-up period.● RESULTS: A total of 56 eyes (56 patients) with PCV were included. BCVA increased significantly from the baseline of 43.52±24.21 letters to 55.88±21.94 letters (P<0.001) at 12mo,while CFT decreased significantly from 457.41±207.86 μm to 247.98±127.08 μm (P<0.001). All patients showed polyp regression. Twenty-three eyes achieved complete polyp regression after the three initial injections, which increased to 44 eyes at 12mo. Seventeen eyes underwent rescue therapy, among which 2 eyes treated with PDT and 15 eyes treated with laser photocoagulation. A mean of 4.30±1.43 injections were given per eye. No intraocular in flammation, retinal or vitreous hemorrhage, or systemic complication occurred.● CONCLUSlON: Conbercept is an effective and safe option for the treatment of PCV in Chinese population. The treatment regimen of three initial conbercept injections followed by additional injections or rescue therapies is efficacious for treating PCV.
INTRODUCTION
Idiopathic polypoidal choroidal vasculopathy (PCV) was first described by Yannuzzi et al[1] in 1990 and was later renamed PCV. It is characterized by orange-red polyps with or without branching vascular networks (BVN), and often leads to pigment epithelial detachment. Advances in the understanding and diagnosis of PCV have increased the detection rate of this disease in the Asian populations, particularly the Chinese population, in which PCV now accounts for 25%-50% of agerelated macular degeneration (AMD)[2]. PCV and choroidal neovascularization (CNV) are the two main subtypes of exudative AMD and share similar clinical features and risk factors. Though a recent study has found that the genes associated with PCV are different from that associated with AMD[3], previous studies also showed PCV and CNV shared many risk genes like CFH, ARMS2, HTRA1 and CETP. The current evidence is not sufficient to indicate that PCV and CNV are different diseases. However, the two subtypes differ in epidemiological, clinical characteristics and treatment response, with PCV more commonly seen in Asians and CNV more commonly seen in Caucasians[4-7]. In the present study, patients with PCV were enrolled based on clinical presentation and confirmation on indocyanine green angiography (ICGA).
Despite its variable prognosis, PCV has significantly higher risk of complete vision loss resulting from suprachoroidal or vitreous humoral hemorrhage than AMD. Uyama et al[8] reported that untreated PCV resolved by itself in approximately 50% of patients. However, the other half of the patients experienced severe vision loss resulting from pigment epithelial detachment and retinal detachment[8]. There is no consensus on the optimal treatment methods of PCV. The currently used treatments include thermal laser photocoagulation, photodynamic therapy(PDT), anti-vascular endothelial growth factor (VEGF)therapy, intravitreal or subtenon injection of triamcinolone acetonide, and pneumatic displacement. Most of these treatments can only manage the symptoms and are not very effective[2]. PDT is the most widely used treatment for PCV and is associated with improved or stabilized vision and an 80%-95% polyp regression rate[9-18]. However, PDT has no effect on BVN[13,15,19] and the long-term (3-5y) visual outcomes of PDT monotherapy was poor with a polyp regrowth rate of 59%-79%[20-23]. Moreover, it has been reported that PDT was associated with subretinal and vitreous hemorrhage, retinal pigment epithelium tear, and subsequent deterioration in visual acuity (VA)[24-26].
As we all know, VEGF plays an important role in the pathogenesis of macular edema. In comparison with non-PCV patients, PCV patients have higher VEGF levels in the vascular endothelial cells, retinal pigment epithelium cells, and aqueous humor[27-28]. Ranibizumab and a flibercept are both anti-VEGF agents and have been used to treat PCV. Ranibizumab was superior to PDT in improving VA of PCV patients but not in inducing polyp regression[29-30]. Ranibizumab monotherapy was effective in reducing subretinal fluid accumulation and stabilizing VA of PCV patients, but with low polyp regression rates ranging 23%-40%[29,31-37]. Aflibercept was effective in treating PCV patients refractory to ranibizumab and was associated with a higher rate of polyp regression[38-40]. Thus, a combination therapy of PDT and intravitreal injection of anti-VEGF agents is often recommended for treating PCV.
Conbercept is a new anti-VEGF agent developed by Chengdu Kanghong Pharmaceutical Group and was approved by the China Food and Drug Administration for the treatment of exudative macular degeneration. It is a fusion protein consisting of a human immunoglobulin Fc region, the extracellular domain 2 of VEGF receptor 1, and the extracellular domains 3 and 4 of VEGF receptor 2[41]. Conbercept is similar to a flibercept in structure but has a very high affinity to VEGF with a mean IC50 of 10 pmol/L. In addition, it blocks VEGF-induced cell proliferation[41-42]. The efficacy of conbercept used for PCV treatment had been reported in AURORA Study[43].
Since the combination therapy was recommended, PDT was applied in patients got poor VA improvement and no polyp regression. In the current study, the authors also performed laser photocoagulation for patients with poor VA improvement or stubborn polyps located at extra-macular area. The present study aims to assess the one-year efficacy and safety of intravitreal conbercept injection combined with PDT or laser photocoagulation in treating PCV in a real-world clinical setting.
SUBJECTS AND METHODS
Ethical Approval This study was approved by the Ethical Committee and Institutional Review Board of Peking University People’s Hospital (Beijing, China), which was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from the each patient before receiving the intravitreal injection of anti-VEGF agents.
Study Design and Patient Selection This is an open label,single center, interventional, prospective study. Fifty-six PCV patients (56 eyes) who were treated at the Eye Center of Peking University People’s Hospital from May 2014 to May 2016 were included. All the enrolled patients were consecutive patients. The inclusion criteria were the presence of PCV and treatment-naive status. Patients who had previously received treatment for PCV were excluded from the present study.Patients with other eye diseases such as glaucoma, diabetic retinopathy, rhegmatogenous retinal detachment and macular hole, which may affect VA, were also excluded. All patients received at least three intravitreal injections of conbercept and were followed up for 12mo. The best-corrected visual acuity(BCVA) testing, dilated fundoscopy and optical coherence tomography (OCT) were performed monthly in all enrolled eyes; and repeated fluorescein angiography (FA) and ICGA exam were performed at 3 and 12mo.
Diagnosis of Polypoidal Choroidal Vasculopathy The eyes were examined using ICGA performed with a Heidelberg Retina Angiography 2 (Heidelberg Engineering GmBH,Dossenheim, Germany). The diagnostic criteria of PCV were: 1) single or multiple focal nodular or grape-like lesions of hyperfluorescence from the choroidal circulation within the first six minutes; 2) one or more of the following clinical presentations: nodular appearance on stereoscopic view of ICGA, hypofluorescent halo surrounding the focal hyperfluorescence, BVN, presence of pulsation, presence of orange-red subretinal nodules with corresponding indocyanine green hyper fluorescence, or submacular hemorrhage[44].
Treatments All patients received an initial treatment of three monthly intravitreal injections of 0.5 mg conbercept.No further treatment was administered if the patient showed complete polyp regression after the initial treatment. Patients with partial polyp regression and good VA (BCVA≥70 letters)received additional conbercept injections. Patients with partial polyp regression but poor VA (BCVA<70 letters) and those who were refractory to three consecutive monthly conbercept injection with no polyp regression received rescue therapies.The rescue therapies included thermal laser photocoagulation for extrafoveal polyps and PDT for subfoveal polyps. In the present study, no targeted treatment was performed for BVN.
Outcome Measurement All patients were assessed at baseline and thereafter monthly for BCVA presented as ETDRS letter scores, intraocular pressure, and fundus examinations. Central foveal thickness (CFT) was measured with the software (version 2016.2.0.35) of RTVue XR Avanti(Optovue, Inc., Fremont, CA, USA). Polyp regression was assessed with ICGA at three months and 12mo follow-up visits. Polyp regression was considered complete if no polyp could be observed with ICGA. Partial regression was defined as a decrease in the polyp size of more than 10% of baseline.No regression was defined as a decrease or increase in the polyp size of less than 10% of baseline. Good BCVA was defined as a fifteen-letter increase in the ETDRS letter score compared with baseline or a score of greater than 70 letters after three injections of conbercept.
Statistical Analysis All data are presented as mean±standard deviation. Statistical analyses were performed with SPSS 20.0(IBM, USA). Within group data were compared using non parametric analysis (Wilcoxon matched-pair test) for different time point, and between group data were also compared using t test. A P-value less than 0.05 was considered as statistically significant.
RESULTS
Patients Fifty-six PCV patients (56 eyes) were included in the present study and all finished the 12mo follow-up. There were 31 males and 25 females with a mean age of 68.21±11.20y.BVN was observed in 18/56 (32.14%) eyes (Table 1). For the enrolled patients, 39/56 (69.64%) received intravitreal injection of conbercept (IVC) monotherapy. Two eyes were treated with PDT for subfoveal polyps and 15 eyes were treated with thermal laser photocoagulation for extrafoveal polyps.
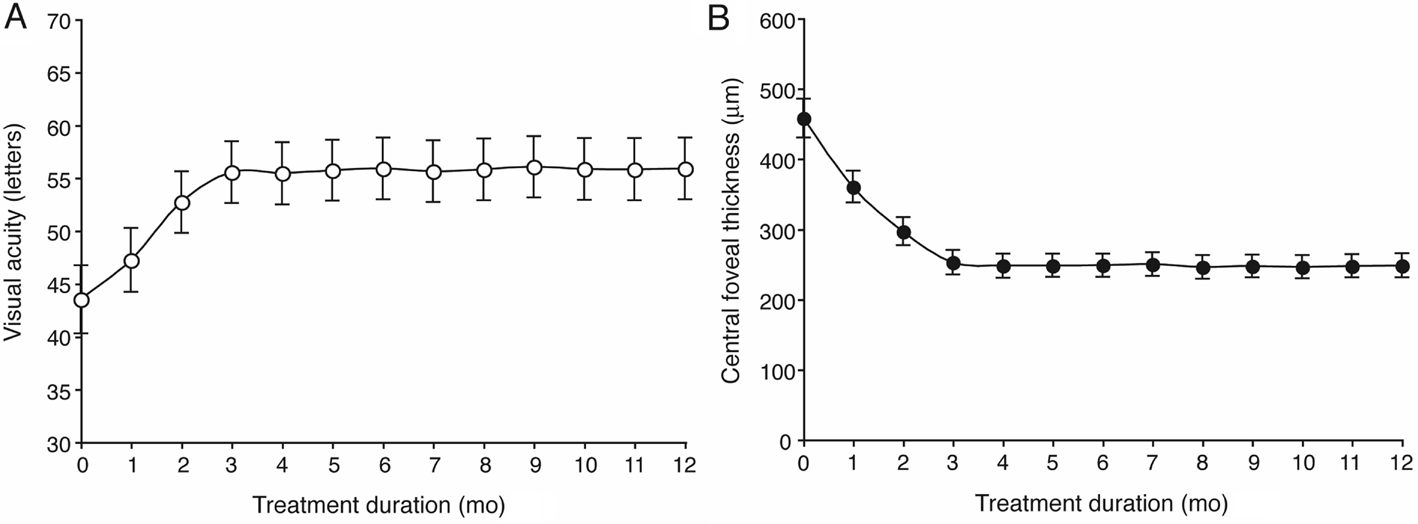
Visual and Anatomical Outcomes Visual and anatomical outcomes improved significantly in comparison with baseline at three months (after initial three monthly injections) and at 12mo (the end of the study). The average BCVA of the 56 eyes increased significantly from the baseline of 43.52±24.21(median 50, from 2 to 80) letters to 52.70±21.97 (median 54, from 5 to 90) letters (P<0.001) at three months and 55.88±21.94 (median 58 , from 5 to 90) letters (P<0.001) at 12mo.Twenty-four eyes showed an increase in BCVA of greater than 15 letters, and the average increase in BCVA was 12.36±15.00(median 10, from -8 to 76) letters at 12mo (Figure 1A).
Table 1 Patients’ demographic information and baseline characteristics
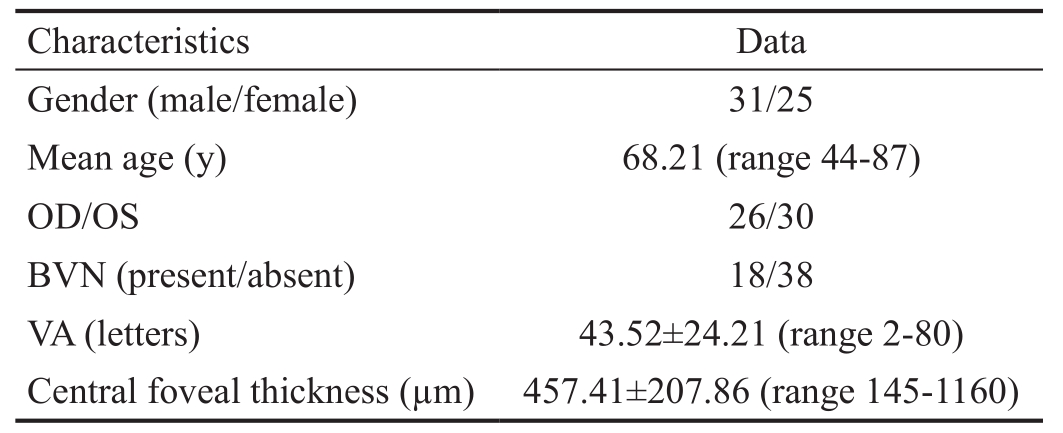
BVN: Branching vascular network; VA: Visual acuity.
?
CFT decreased significantly from the baseline of 457.41±207.86 (median 397, from 145 to 1160) μm to 252.32±130.99(median 210, from 54 to 850) μm (P<0.001) at 3mo and 247.98±127.08 (median 210, from 54 to 850) μm (P<0.001)at 12mo. The average decrease in CFT was 209.43±159.75(median 159, from 1 to 850) μm (Figure 1B).
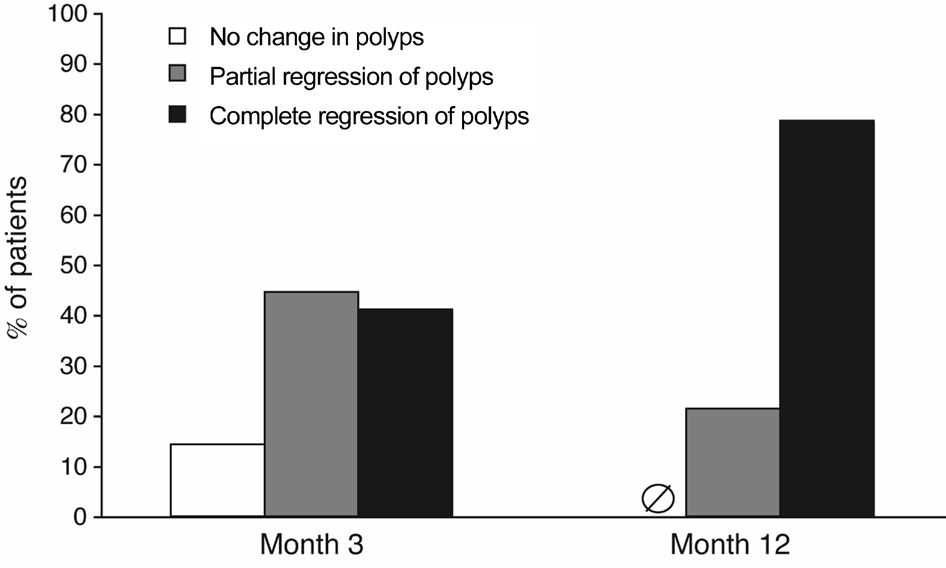
Polyps Regression and Injection Numbers After the initial treatment of three monthly conbercept injections, 23 eyes(41.1%) showed complete polyp regression, 25 eyes (44.6%)showed partial regression, and 8 eyes (14.3%) showed no change in polyps. At 12mo, 44 eyes (78.6%) showed complete polyp regression, and 12 eyes (21.4%) showed partial regression (Figure 2). Of the 44 eyes with complete polyp regression, 27 eyes were treated with conbercept monotherapy of 4.11±1.58 injections per eye, and the other 17 eyes received a mean of 3.82±1.13 conbercept injections (P>0.05) followed by rescue therapies for polyps. Two eyes were treated with PDT for subfoveal polyps and 15 eyes were treated with thermal laser photocoagulation for extrafoveal polyps. The 12 eyes with partial polyp regression at 12mo received a mean of 5.42±1.27 injections. None of these eyes were treated with rescue therapies. Overall, each eye received 4.30±1.43 conbercept injections.
Cases
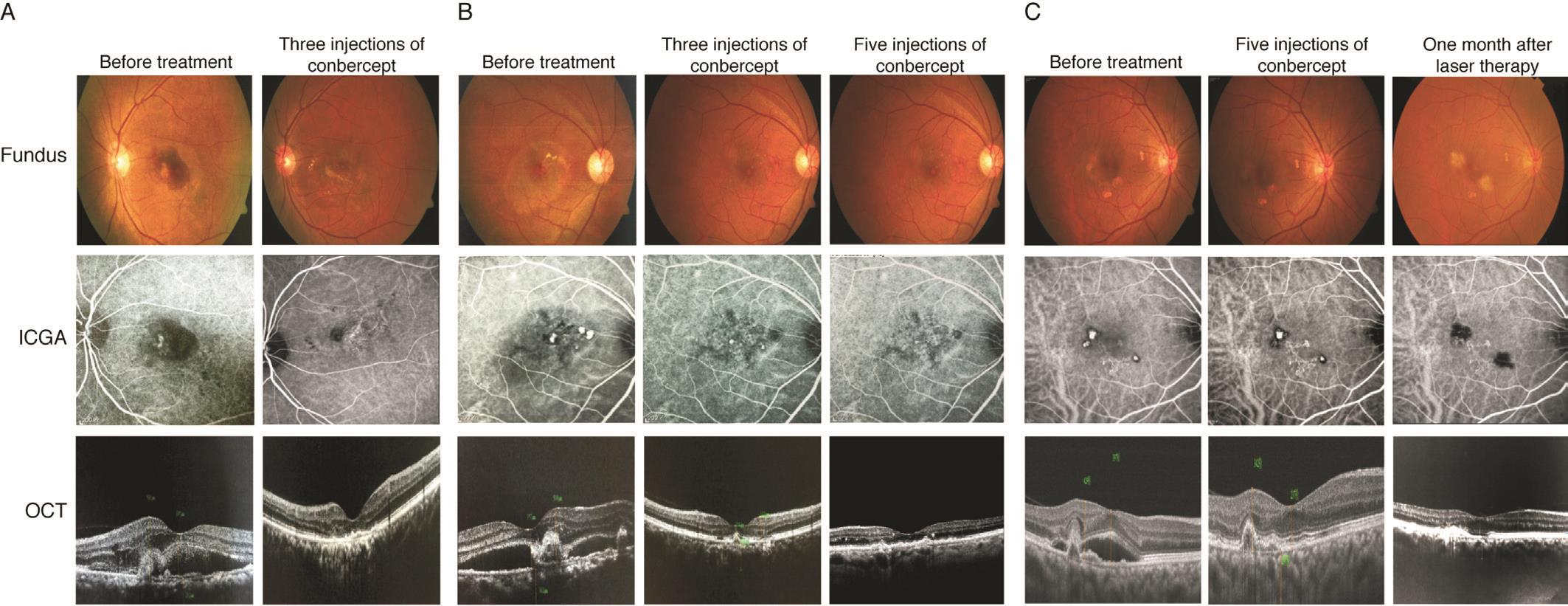
Case A A 62-year-old male experienced visual loss and distortion in the left eye for 20d with a baseline VA of 65 letters and a CFT of 397 μm. After three consecutive conbercept injections, the VA increased to 80 letters and the CFT reduced to 165 μm. The polyps showed complete regression. Figure 3A showed the fundus, ICGA, and OCT before and after the conbercept treatment.
Case B A 58-year-old male experienced visual loss and distortion in the right eye for 10d with a baseline VA of 70 letters and a CFT of 357 μm. After three conbercept injections,the VA increased to 90 letters and the CFT reduced to 189 μm.Subretinal fluid was completely absorbed, and polyps showed partial regression. After two additional conbercept injections,his VA remained at 90 letters and his CFT further reduced to 182 μm with complete polyp regression. Figure 3B showed the fundus, ICGA, and OCT before the treatment, after three conbercept injections, and after five conbercept injections.
Case C A 61-year-old female experienced visual loss and distortion in the right eye for 7d with baseline VA of 64 letters and CFT of 389 μm. After three consecutive conbercept injections, the VA increased to 80 letters and the CFT reduced to 211 μm, with complete absorption of the subretinal fluid and partial polyp regression. After two additional conbercept injections, her VA remained at 80 letters and her CFT further reduced to 190 μm. However, the polyps still showed partial regression by ICGA analysis. Rescue therapy of thermal laser photocoagulation was used to treat the polyps but not BVN.One month later, the polyps showed complete regression and BVN did not change. Her VA remained at 80 letters. Figure 3C showed the fundus, ICGA, and OCT before the treatment, after five conbercept injections, and one month after the thermal laser photocoagulation therapy.
Safety Profile Except injection-related subconjunctival hemorrhage, no other complications were seen such as subretinal or vitreous hemorrhage, intraocular in flammation, or systemic complications.
DISCUSSION
This is an open label, single center, interventional study that evaluated the anatomical and functional outcomes during 12mo follow-up period in patients with PCV treated with either IVC or IVC plus rescue therapy such as PDT or laser photocoagulation. The result of our present study found that intravitreal conbercept injections improved VA and decreased CFT, and induced polyp regression in PCV patients with or without BVN (Figure 3). The rescue therapy for patients with unsatisfactory VA improvement and partial polyps regression got satisfactory outcomes with fewer intravitreal injections by the end of this study. The combined PCV treatment strategy of the current study provided another choice for ophthalmologists.A combined therapy regimen was designed for the present study. PDT and laser photocoagulation was performed as the rescue therapies for patients with stubborn polyps located in extrafoveal or subfoveal. Our results showed that intravitreal conbercept injection is an effective therapy for PCV, which is consistent with previous studies. In addition, the treatment regimen used in our study achieved better outcomes in terms of therapeutic effects and economic burden considerations.The AURORA Study found that conbercept monotherapy was an efficacious and safe treatment for PCV[43], and the LAPTOP Study showed that the efficacy of PDT monotherapy was superior to ranibizumab monotherapy[29]. Similar to the PrONTO study[45], the AURORA Study found that conbercept treatment-related improvements in VA and anatomical outcomes plateaued after three injections, and polyp regression increased only by 10.1% after an average of 6.6 additional injections. Therefore, we designed a treatment regimen of three initial conbercept injections followed by additional injections given to patients who responded well to the treatment. These patients should have good treatment response with substantial polyp regression and BCVA improvement. Otherwise,rescue therapies were considered for patients who showed no significant polyp regression. Our treatment regimen was associated with a greater decrease in CFT and a similar polyp regression rate in comparison with the conbercept treatment regimen used in the AURORA Study. Moreover, our treatment regimen was associated with significantly fewer injections(4.30±1.43 per eye) than the AURORA Study (9.6±3 per eye,P<0.001), which may reduce the economic burden of patients.Previous studies have suggested that anti-VEGF therapy in combination with PDT is effective in PCV treatment[44,46]. The Fujisan study found that initial PDT combined with anti-VEGF therapy could reduce the number of injections required[47].However, PDT is associated with hypoperfusion and subretinal hemorrhage. Furthermore, Nemoto et al[48] reported that PDT in combination with intravitreal ranibizumab improved VA for the first ninth months, but the beneficial effect decreased gradually and disappeared the following year. Our treatment regimen employed differential rescue therapies for polyps at different locations, which include thermal laser photocoagulation for extrafoveal polyps and PDT for subfoveal polyps. In our study,15 eyes were treated for extrafoveal polyps and two eyes for subfoveal polyps, which is consistent with a previous finding that extrafoveal polyps were more prevalent in the Chinese population[49]. Our patients responded well to the thermal laser photocoagulation after three injections of conbercept, and the subretinal fluid and blood were absorbed. Recurrent polyps occurred in one of the two eyes that underwent PDT, and was treated with subsequent thermal laser photocoagulation.
For the rescue of polypoidal lesions of PCV, aflibercept monotherapy over 1y for eyes with PCV in previous studies showed the complete polyp regression rate of 48.0% to 52.9%[50-51]. In the present study, 41.1% and 78.6% PCV patients showed complete polyp regression three months and 12mo after the initial three monthly treatment of conbercept injection.The regression rate of our study was almost the same as that of the a flibercept monothereapy. For the other anti-VEGF agent as ranibizumab, the 1y complete resolution of polypoidal lesions was reported as 19% in a Japanese study[52]. In another study,the regression rate of polyp was performed as 40% by Hikichi et al[53]. Although direct comparisons between studies should not be made, our results suggested the similar effectiveness of conbercept with aflibercept in terms of completely resolving of polyps in PCV. The different regression rates of polyp may be associated with the different pharmacological properties. As we all know, the binding target of ranibizumab was VEGF-A.But for conbercept and aflibercept, VEGF-B and placental growth factor may also play important role[54-55].
Since either PDT or anti-VEGF therapies was invalid for BVN[13,16,19,43], we did not consider BVN as an efficacy indicators.A previous study showed that BVN was still present and even enlarged after two years of ranibizumab treatment[33],and aflibercept was found to have no effect on BVN[38].Furthermore, in the AURORA Study, BVN was present in 94.4% of patients at 12mo despite the initial reduction in the BVN rate[43]. Therefore, our study did not distinguish BVN but focused on treating the polyps, which avoided hemorrhage resulting from polyp rupture and damage to the retina, with additional benefit of reducing the cost of treatment.
Our results were also consistent with a pharmacology study that found conbercept was well tolerated and was associated with very few adverse events[42]. The most common adverse event in our study that was associated with conbercept injection was subconjunctival hemorrhage caused by intravitreal injection itself, which did not require treatment. No serious adverse events occurred in our study such as retinal detachment, intraocular in flammation, subretinal or subvitreal hemorrhages, and systemic complications.
There are also several limitations in our study that must be mentioned. Firstly, it was conducted with a relatively short period of follow-up. Secondly, the number of the enrolled patients was relatively small and a sham injection group was not used, leading to lower strength of convincing. Since the use of conbercept in clinical practice has been approved for only few years, more cases remain to be collected. Undoubtedly,studies with a larger sample size are required. Thirdly, the availability of conbercept in real world clinical practice was another limitation. By now, conbercept can only be used in China. But in view of the effectiveness for the treatment of PCV, the phrase III clinical trail of conbercept had been approved by FDA in United States since 2016. We have reason to believe that conbercept could be available in other countries in the near future.
The last but not the least, our treatment regimen of three initial conbercept injections followed by subsequent rescue therapies was a safe and effective treatment for PCV. Frequent injections of anti-VEGF agents or receiving PDT at patients’own expense is undoubtedly a heavy burden for PCV patients in China, and a more economical treatment program was required. Since the anti-VEGF agents were still thought to be the best choice for VA improvement, the combined therapy was attempted in our present study. Laser photocoagulation,as a cheap and convenient treatment, was combined with IVC,for the treatment of PCV with poor regression extra-macular polyps. Complete regression of polyps was observed at the end of the present study for the conbercept combined therapy group. Further investigation with a larger patient sample and longer follow-ups and control groups are necessary to confirm the efficacy of conbercept in treating PCV.
ACKNOWLEDGEMENTS
Conflicts of Interest: Qi HJ, None; Jin EZ, None; Zhao MW, None.
REFERENCES
1 Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina 1990;10(1):1-8.
2 Wong RL, Lai TY. Polypoidal choroidal vasculopathy: an update on therapeutic approaches. J Ophthalmic Vis Res 2013;8(4):359-371.
3 Huang L, Zhang H, Cheng CY, et al. A missense variant in FGD6 confers increased risk of polypoidal choroidal vasculopathy. Nat Genet 2016;48(6):640-647.
4 Wong CW, Yanagi Y, Lee WK, Ogura Y, Yeo I, Wong TY, Cheung CMG. Age-related macular degeneration and polypoidal choroidal vasculopathy in Asians. Prog Retin Eye Res 2016;53:107-139.
5 Ciardella AP, Donsoff IM, Huang SJ, Costa DL, Yannuzzi LA.Polypoidal choroidal vasculopathy. Surv Ophthalmol 2004;49(1):25-37.6 Gomi F, Ohji M, Sayanagi K, Sawa M, Sakaguchi H, Oshima Y, Ikuno Y, Tano Y. One-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology 2008;115(1):141-146.
7 Laude A, Cackett PD, Vithana EN, Yeo IY, Wong D, Koh AH, Wong TY, Aung T. Polypoidal choroidal vasculopathy and neovascular agerelated macular degeneration: same or different disease? Prog Retin Eye Res 2010;29(1):19-29.
8 Uyama M, Wada M, Nagai Y, Matsubara T, Matsunaga H, Fukushima I,Takahashi K, Matsumura M. Polypoidal choroidal vasculopathy: natural history. Am J Ophthalmol 2002;133(5):639-648.
9 Akaza E, Yuzawa M, Matsumoto Y, Kashiwakura S, Fujita K, Mori R.Role of photodynamic therapy in polypoidal choroidal vasculopathy. Jpn J Ophthalmol 2007;51(4):270-277.
10 Chan WM, Lam DS, Lai TY, Liu DT, Li KK, Yao Y, Wong TH.Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: one-year results of a prospective case series.Ophthalmology 2004;111(8):1576-1584.
11 Gomi F, Tano Y. Polypoidal choroidal vasculopathy and treatments.Curr Opin Ophthalmol 2008;19(3):208-212.
12 Lai TY, Chan WM. An update in laser and pharmaceutical treatment for polypoidal choroidal vasculopathy. Asia Pac J Ophthalmol (Phila)2012;1(2):97-104.
13 Lee WK, Lee PY, Lee SK. Photodynamic therapy for polypoidal choroidal vasculopathy: vaso-occlusive effect on the branching vascular network and origin of recurrence. Jpn J Ophthalmol 2008;52(2):108-115.
14 Mauget-Faÿsse M, Quaranta-El Maftouhi M, De La Marnièrre E, Leys A. Photodynamic therapy with vertepor fin in the treatment of exudative idiopathic polypoidal choroidal vasculopathy. Eur J Ophthalmol 2006;16(5):695-704.
15 Mori R, Yuzawa M, Lee Z, Haruyama M, Akaza E. Factors influencing visual outcome of polypoidal choroidal vasculopathy one year after photodynamic therapy. Graefes Arch Clin Exp Ophthalmol 2010;248(9):1233-1239.
16 Otani A, Sasahara M, Yodoi Y, Aikawa H, Tamura H, Tsujikawa A,Yoshimura N. Indocyanine green angiography: guided photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol 2007;144(1):7-14.
17 Silva RM, Figueira J, Cachulo ML, Duarte L, Faria de Abreu JR, Cunha-Vaz JG. Polypoidal choroidal vasculopathy and photodynamic therapy with vertepor fin. Graefes Arch Clin Exp Ophthalmol 2005;243(10):973-979.
18 Spaide RF, Donsoff I, Lam DL, Yannuzzi LA, Jampol LM, Slakter J,Sorenson J, Freund KB. Treatment of polypoidal choroidal vasculopathy with photodynamic therapy. Retina 2002;22(5):529-535.
19 Wakabayashi T, Gomi F, Sawa M, Tsujikawa M, Tano Y. Marked vascular changes of polypoidal choroidal vasculopathy after photodynamic therapy. Br J Ophthalmol 2008;92(7):936-940.
20 Akaza E, Yuzawa M, Mori R. Three-year follow-up results of photodynamic therapy for polypoidal choroidal vasculopathy. Jpn J Ophthalmol 2011;55(1):39-44.
21 Leal S, Silva R, Figueira J,Cachulo ML, Pires I, de Abreu JR, Cunha-Vaz JG. Photodynamic therapy with vertepor fin in polypoidal choroidal vasculopathy: results after 3 years of follow-up. Retina 2010;30(8):1197-1205.
22 Tsuchiya D, Yamamoto T, Kawasaki R, Yamashita H. Two-year visual outcomes after photodynamic therapy in age-related macular degeneration patients with or without polypoidal choroidal vasculopathy lesions. Retina 2009;29(7):960-965.
23 Lee YA, Yang CH, Yang CM, Ho TC, Lin CP, Huang JS, Chen MS. Photodynamic therapy with verteporfin for polypoidal choroidal vasculopathy treatment: 3-year results in Taiwan. Taiwan J Ophthalmol 2012;2(2):64-67.
24 Hirami Y, Tsujikawa A, Otani A, Yodoi Y, Aikawa H, Mandai M,Yoshimura N. Hemorrhagic complications after photodynamic therapy for polypoidal choroidal vasculopathy. Retina 2007;27(3):335-341.
25 Musashi K, Tsujikawa A, Hirami Y, Otani A, Yodoi Y, Tamura H,Yoshimura N. Microrips of the retinal pigment epithelium in polypoidal choroidal vasculopathy. Am J Ophthalmol 2007;143(5):883-885.
26 Ojima Y, Tsujikawa A, Otani A, Hirami Y, Aikawa H, Yoshimura N.Recurrent bleeding after photodynamic therapy in polypoidal choroidal vasculopathy. Am J Ophthalmol 2006;141(5):958-960.
27 Matsuoka M, Ogata N, Otsuji T, Nishimura T, Takahashi K,Matsumura M. Expression of pigment epithelium derived factor and vascular endothelial growth factor in choroidal neovascular membranes and polypoidal choroidal vasculopathy. Br J Ophthalmol 2004;88(6):809-815.
28 Tong JP, Chan WM, Liu DT, Lai TY, Choy KW, Pang CP, Lam DS.Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol 2006;141(3):456-462.
29 Oishi A, Kojima H, Mandai M, et al. Comparison of the effect of ranibizumab and verteporfin for polypoidal choroidal vasculopathy:12-month LAPTOP study results. Am J Ophthalmol 2013;156(4):644-651.30 Inoue M, Arakawa A, Yamane S, Kadonosono K. Long-term outcome of intravitreal ranibizumab treatment, compared with photodynamic therapy, in patients with polypoidal choroidal vasculopathy. Eye (Lond)2013;27(9):1013-1020; quiz 1021.
31 Cho HJ, Kim JW, Lee DW, Cho SW, Kim CG. Intravitreal bevacizumab and ranibizumab injections for patients with polypoidal choroidal vasculopathy. Eye (Lond) 2012;26(3):426-433.
32 Gomi F, Sawa M, Sakaguchi H, Tsujikawa M, Oshima Y, Kamei M,Tano Y. Efficacy of intravitreal bevacizumab for polypoidal choroidal vasculopathy. Br J Ophthalmol 2008;92(1):70-73.
33 Hikichi T, Higuchi M, Matsushita T, Kosaka S, Matsushita R, Takami K,Ohtsuka H, Kitamei H, Shioya S. Results of 2 years of treatment with asneeded ranibizumab reinjection for polypoidal choroidal vasculopathy. Br J Ophthalmol 2013;97(5):617-621.
34 Lai TY, Chan WM, Liu DT, Luk FO, Lam DS. Intravitreal bevacizumab(Avastin) with or without photodynamic therapy for the treatment of polypoidal choroidal vasculopathy. Br J Ophthalmol 2008;92(5):661-666.
35 Yamashiro K, Tomita K, Tsujikawa A, Nakata I, Akagi-Kurashige Y,Miyake M, Ooto S, Tamura H, Yoshimura N. Factors associated with the response of age-related macular degeneration to intravitreal ranibizumab treatment. Am J Ophthalmol 2012;154(1):125-136.
36 Marcus DM, Singh H, Lott MN, Singh J, Marcus MD. Intravitreal ranibizumab for polypoidal choroidal vasculopathy in non-Asian patients.Retina 2013;33(1):35-47.
37 Chhablani JK, Narula R, Narayanan R. Intravitreal bevacizumab monotherapy for treatment-naive polypoidal choroidal vasculopathy.Indian J Ophthalmol 2014;62(1):97.
38 Ijiri S, Sugiyama K. Short-term efficacy of intravitreal a flibercept for patients with treatment-naive polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol 2015;253(3):351-357.
39 Miura M, Iwasaki T, Goto H. Intravitreal aflibercept for polypoidal choroidal vasculopathy after developing ranibizumab tachyphylaxis. Clin Ophthalmol 2013;7:1591-1595.
40 Yamashita M, Nishi T, Hasegawa T, Ogata N. Response of serous retinal pigment epithelial detachments to intravitreal aflibercept in polypoidal choroidal vasculopathy refractory to ranibizumab. Clin Ophthalmol 2014;8:343-346.
41 Zhang M, Zhang J, Yan M, Li H, Yang C, Yu D. Recombinant antivascular endothelial growth factor fusion protein efficiently suppresses choridal neovasularization in monkeys. Mol Vis 2008;14:37-49.
42 Zhang M, Yu D, Yang C, Xia Q, Li W, Liu B, Li H. The pharmacology study of a new recombinant human VEGF receptor-fc fusion protein on experimental choroidal neovascularization. Pharm Res 2009;26(1):204-210.
43 Qu J, Cheng Y, Li X, Yu L, Ke X; AURORA Study Group. Efficacy of intravitreal injection of conbercept in polypoidal choroidal vasculopathy:subgroup analysis of the AURORA study. Retina 2016;36(5):926-937.
44 Koh AH, Chen LJ, Chen SJ, et al. Polypoidal choroidal vasculopathy:evidence-based guidelines for clinical diagnosis and treatment. Retina 2013;33(4):686-716.
45 Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W, Davis JL, Flynn HW Jr, Esquiabro M. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration:year 2 of the PrONTO Study. Am J Ophthalmol 2009;148(1):43-58.e1.
46 Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y, Kim H, Lai TY, Pilz S,Ruamviboonsuk P, Tokaji E, Weisberger A, Lim TH. EVEREST study:efficacy and safety of vertepor fin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina 2012;32(8):1453-1464.
47 Gomi F, Oshima Y, Mori R, Kano M, Saito M, Yamashita A, Iwata E,Maruko R; Fujisan Study Group. Initial versus delayed photodynamic therapy in combination with ranibizumab for treatment of polypoidal choroidal vasculopathy: the Fujisan Study. Retina 2015;35(8):1569-1576.
48 Nemoto R, Miura M, Iwasaki T, Goto H. Two-year follow-up of ranibizumab combined with photodynamic therapy for polypoidal choroidal vasculopathy. Clin Ophthalmol 2012;6:1633-1638.
49 Wen F. Discussion on the diagnosis and treatment for polypoidal choroidal vasculopathy. Ophthalmology in China 2007;16(6):373-375.
50 Yamamoto A, Okada AA, Kano M, Koizumi H, Saito M, Maruko I,Sekiryu T, Iida T. One-year results of intravitreal a flibercept for polypoidal choroidal vasculopathy. Ophthalmology 2015;122:1866-1872.
51 Inoue M, Yamane S, Taoka R, Arakawa A, Kadonosono K. A flibercept for polypoidal choroidal vasculopathy: as needed versus fixed interval dosing. Retina 2016;36(8):1527-1534.
52 Mori R, Yuzawa M, Akaza E, Haruyama M. Treatment results at 1 year of ranibizumab therapy for polypoidal choroidal vasculopathy in eyes with good visual acuity. Jpn J Ophthalmol 2013;57(4):365-371.
53 Hikichi T, Higuchi M, Matsushita T, Kosaka S, Matsushita R, Takami K, Ohtsuka H, Ariga H. One year results of three monthly ranibizumab injections and as needed reinjections for polypoidal choroidal vasculopathy in Japanese patients. Am J Ophthalmol 2012;154(1):117-124.
54 Papadopoulos N, Martin J, Ruan Q, Ra fique A, Rosconi MP, Shi E, Pyles EA, Yancopoulos GD, Stahl N, Wiegand SJ. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 2012;15(2):171-185.
55 Lu X, Sun X. Profile of conbercept in the treatment of neovascular agerelated macular degeneration. Drug Des Devel Ther 2015;9:2311-2320.
Citation: Qi HJ, Jin EZ, Zhao MW. One-year outcomes of intravitreal conbercept combined rescue therapy for polypoidal choroidal vasculopathy in a Chinese population: a real-life clinical data. Int J Ophthalmol 2019;12(1):51-57
DOl:10.18240/ijo.2019.01.08
● KEYWORDS: conbercept; anti-vascular endothelial growth factor; polypoidal choroidal vasculopathy; intravitreal injection; laser photocoagulation; photodynamic therapy
Received: 2017-06-10 Accepted: 2018-09-06
Correspondence to: Ming-Wei Zhao. Department of Ophthalmology, Ophthalmology & Optometry Center, Peking University People’s Hospital, Beijing Key Laboratory of Diagnosis and Therapy of Retinal and Choroidal Disease,Xizhimen South Street 11, Xi Cheng District, Beijing 100044,China. zhaomingwei64@163.com
Co- first authors: Hui-Jun Qi and En-Zhong Jin