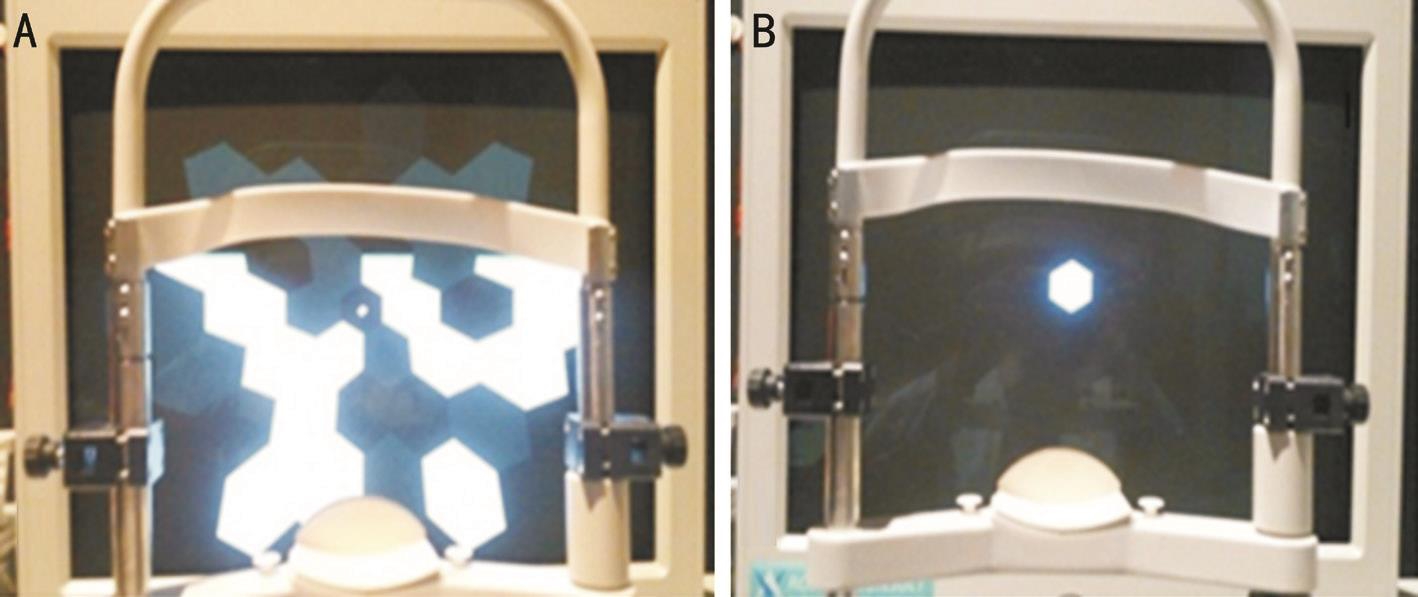
Figure 1 Two protocols used for multifocal ERG recording A: Protocol 1, 37 hexagonal element stimulation; B: Protocol 2, stimulation with one central hexagonal element corresponding to ring 1 and with the other 36 hexagonal elements off-set.
Electrophysiological measurements have been commonly used to provide objective information about retinal function. Full-field electroretinography (ERG) allows recording of electrical responses originating from the entire retina when stimulated with a full- field light source. Macular function can be evaluated electrophysiologically from focal macular ERG (fERG) or multifocal ERG (mfERG)recordings[1-10]. While fERG allows for electrophysiological response to light stimuli presented in a limited area of the central retina (4-9 retinal degrees)[10-11], the mfERG is recording the responses from several different retinal areas as well as the fovea[12–15]. Even though retinal topographic function is measured at almost 50 degrees post pole during mfERG, the central hexagonal element is considered the most valuable segment by clinical ophthalmologists because it represents macular function. Thus, maintaining stable central fixation during mfERG is very important for obtaining accurate topographical ERG information. However, it is sometimes difficult for the patient to concentrate on the fixation target on the ERG monitor during mfERG because multiple segments are continuously changing on the screen, which is a problem that can be exacerbated by impaired macular function. Testing the macular area with a single hexagonal element may increase fixation stability during the test because patients see only the single stimulating hexagon in which the fixation target is embedded. The first-order component is the average response for a particular retinal area separated from other regions according to stimulus hexagons and is calculated as a crosscorrelation between the response cycle and the m-sequence[16].However, we questioned whether the actual mfERG response is spatially in fluenced by stimulation of nearby retinal regions or not, although it is an independently calculated value.
We examined whether results for central hexagonal elements corresponding to the macular area would be identical between conventional mfERG and a single central hexagonal element stimulation, such as fERG, if we applied the same m-sequence and central hexagonal size.
Ethical Approval The study protocol was reviewed and approved by the Institutional Review Board of Korea University Medical Center, Seoul, Korea. All participating subjects submitted their informed consent before the investigation. All research and data collection protocols complied with the Declaration of Helsinki.
This is a prospective study of healthy adults between 23 and 79 years old who visited Korean University Ansan Hospital between December 21, 2016 and June 30, 2017. Thirty healthy subjects with no ocular or systemic diseases were included in the study. Exclusion criteria included best corrected visual acuity (BCVA) <1.0 (decimal), prior ocular surgery, ocular diseases and a medical history of systemic diseases.
All subjects underwent a comprehensive ocular examination,including BCVA, manifest refraction using an auto keratorefractometer (KR-8100; Topcon), slit-lamp examination,intraocular pressure measurement with a pneumatic tonometer(CT-80A; Topcon, Tokyo, Japan), axial length measurement with a biometer (IOL MASTER 500; Carl Zeiss Meditec Inc.,Jena, Germany), ultra-wide-field color fundus photography(Optos California, Optos PLC, Dunfermline, Scotland, UK),and central retinal thickness measurement with spectral domain-optical coherence tomography (Spectralis; Heidelberg Engineering, Dossenheim, Germany). mfERG was recorded after pupil dilation.
mfERG were recorded with a Roland-Consult RetiScan®system (Roland-Consult, Brandenburg an der Havel,Germany). The participants remained in dim light for at least 15min before the recordings were taken, and pre-test light exposure was avoided. Next, their pupils were fully dilated with 1% tropicamide and 2.5% phenylephrine. A Dawson-Trick-Litzkow (DTL) fiber electrode was used as the active electrode. Moderate myopia was fully corrected with glasses for optimal acuity at a viewing distance of 33 cm. Monocular stimulation and monocular recording were performed with the contralateral eye occluded.
The stimulus matrix, which consisted of 37 scaled hexagonal elements, was presented on a high-resolution 21-inch CRT monitor at a frame rate of 60 Hz. The stimulus hexagons were set to modulate between white and black according to a pseudorandom m-sequence (511 short sequences, 16.67ms per frame, 83ms base period =5 CRT frames, one m-stimulus step was followed by 4 dark frames). The Michelson contrast between white and black hexagons was set to 99% (i.e. the luminance was <1 cd/m2 for the black hexagons and 115 cd/m2 for the white hexagons). The surrounding background light was dimmed and a red fixation cross was used. The monitor subtended 50° to 60° at a viewing distance of 26 cm. The central hexagon at ring 1 corresponded to the macular 5° area.mfERG was recorded according to two different protocols:stimulus with 37 hexagonal elements (protocol 1, conventional protocol) and stimulus with a single central hexagonal element corresponding to ring 1 created by deactivating the other 36 hexagonal elements in the conventional protocol (protocol 2; Figure 1). All recordings were monitored in real time throughout the entire testing session and the testing durations for each protocol were measured and compared. The recording was repeated if the recorded signal was not appropriate or if fixation losses were detected.
The first negative component (N1), first positive component(P1), and second negative component (N2) amplitudes and implicit times of ring 1 were measured using the conventional protocol. Additionally, if two consecutive waves appeared serially, the highest point of the second positive component was defined as second positive component (P2) and its amplitude and the implicit time of ring 1 were also measured.In such cases, N2 was defined as the lowest point between two consecutive waves. Statistical analyses were performed with SPSS software, version 20.0 (IBM, Inc., Armonk, NY,USA). Paired and independent t-tests were used to compare differences between ring 1 parameters in each protocol. P values <0.05 were considered statistically significant.
We enrolled 30 eyes of 30 subjects in this study. The clinical characteristics of the study subjects are presented in Table 1.
Two crests appeared after N1 in the plot patterns from protocol 2 and were different from those of protocol 1, which had 37 hexagonal element stimulations (Figure 2).
The implicit time for N1 was not significantly different between the two protocols (18.1±1.8ms vs 18.2±1.0ms,P=0.813; Table 2), but the amplitude of N1 in protocol 2 was significantly higher than in protocol 1 (2.5±1.1 μV vs 3.4±1.5 μV, P=0.001). The implicit time for P1 in protocol 1 was shorter than in protocol 2 (37.8±1.8ms vs 40.7±2.4ms,P<0.001). The amplitude of P1 in protocol 2 was significantly higher than in protocol 1 (9.1±3.3 μV vs 6.3±2.7 μV, P=0.001).A prominent P2 wave appeared right next to the P1 wave in protocol 2. The amplitude of P2 was higher than that of P1 in protocol 2 (Figure 2). The implicit time (68.0±4.5ms)and amplitude of P2 (12.3±4.7 µV) in protocol 2 were also significantly longer (P<0.001) and higher (P<0.001) than those of P1 in protocol 1. The mean ratio of the amplitude of P2 in protocol 2 to the amplitude of P1 in protocol 1 was 2.2(range: 1.18-6.95).

Figure 1 Two protocols used for multifocal ERG recording A: Protocol 1, 37 hexagonal element stimulation; B: Protocol 2, stimulation with one central hexagonal element corresponding to ring 1 and with the other 36 hexagonal elements off-set.
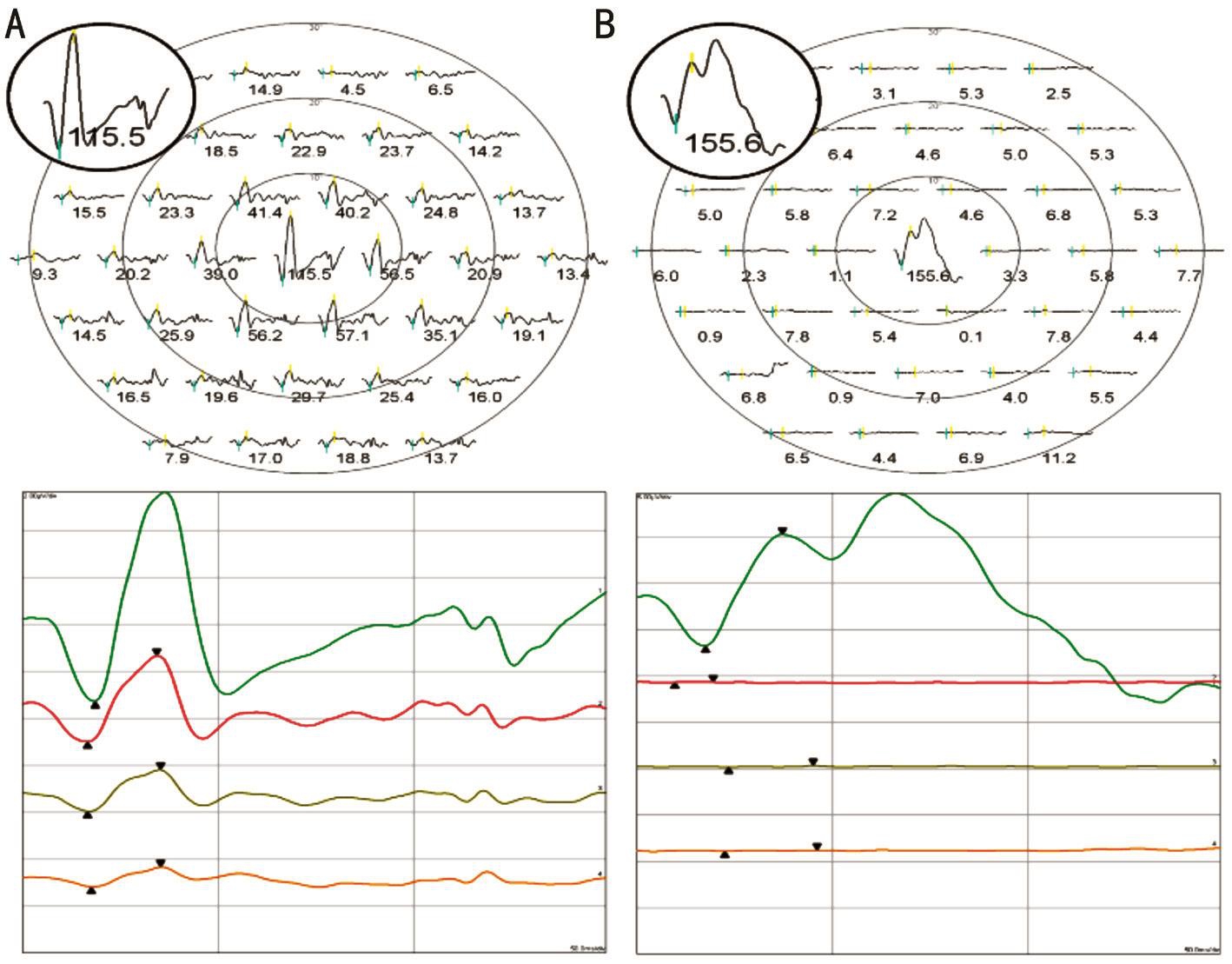
Figure 2 Plot and trace arrays of each recording protocol A: Protocol 1; B: Protocol 2.
Table 1 Clinical characteristics of the study subjects
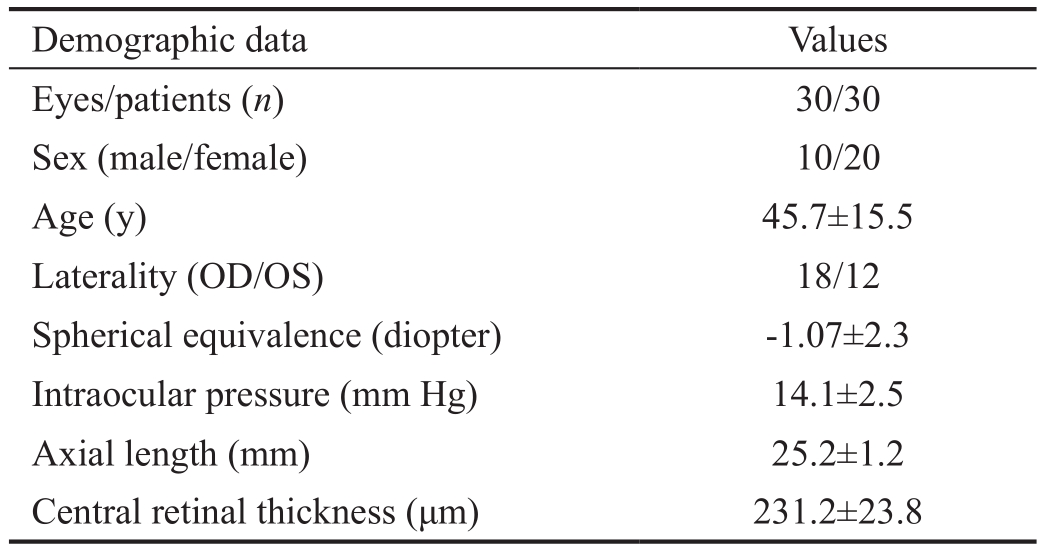
Demographic data Values Eyes/patients (n) 30/30 Sex (male/female) 10/20 Age (y) 45.7±15.5 Laterality (OD/OS) 18/12 Spherical equivalence (diopter) -1.07±2.3 Intraocular pressure (mm Hg) 14.1±2.5 Axial length (mm) 25.2±1.2 Central retinal thickness (μm) 231.2±23.8
The difference in implicit time between the N1 and P1 in protocol 1 was smaller than that in protocol 2 (19.7±1.6ms vs 22.6±2.6ms, P<0.001) and the time between N2 and P2 was significantly shorter than the time between N1 and P1 in protocol 2 (17.70±3.5ms vs 22.6±2.6ms, P<0.001; Table 3).The difference in amplitude between N2 and P2 was smallerthan the difference between N1 and P1 in protocol 2, but not significant so (5.5±2.7 μV vs 5.5±2.0 μV, P=0.594). Therewere no significant differences in duration of recording across protocols (Table 2).
Table 2 Ring 1 parameters and recording duration for each protocol
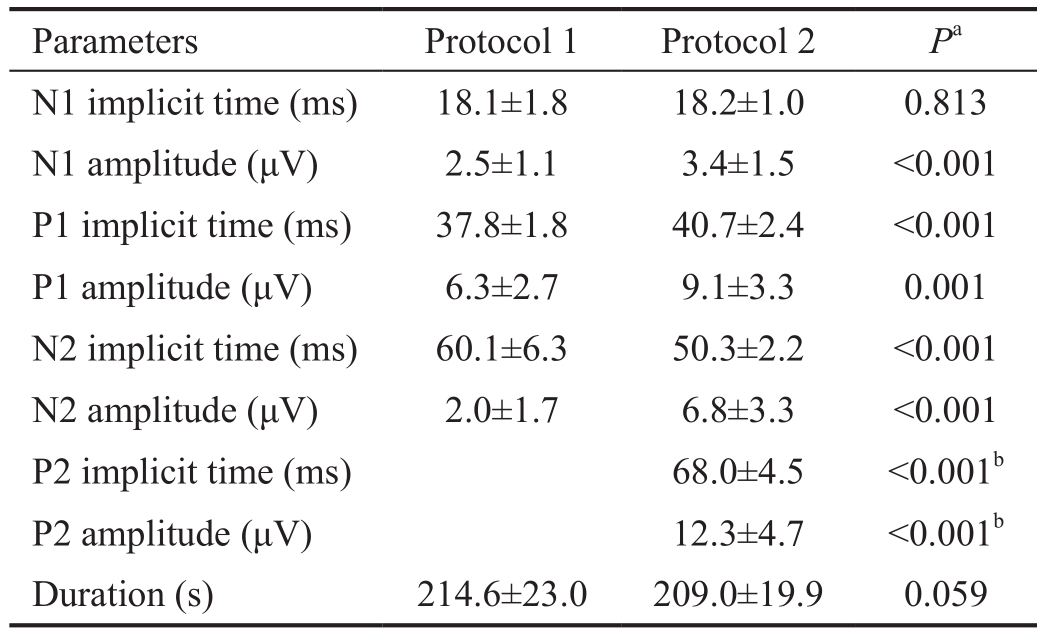
N1: First negative component; N2: Second negative component; P1:First positive component; P2: Second positive component.aPaired t-test;bComparison with P1 parameters in protocol 1.
Parameters Protocol 1 Protocol 2 Pa N1 implicit time (ms) 18.1±1.8 18.2±1.0 0.813 N1 amplitude (μV) 2.5±1.1 3.4±1.5 <0.001 P1 implicit time (ms) 37.8±1.8 40.7±2.4 <0.001 P1 amplitude (μV) 6.3±2.7 9.1±3.3 0.001 N2 implicit time (ms) 60.1±6.3 50.3±2.2 <0.001 N2 amplitude (μV) 2.0±1.7 6.8±3.3 <0.001 P2 implicit time (ms) 68.0±4.5 <0.001b P2 amplitude (μV) 12.3±4.7 <0.001b Duration (s) 214.6±23.0 209.0±19.9 0.059
Table 3 Differences in implicit time and amplitude between Nx-Px components in each protocol
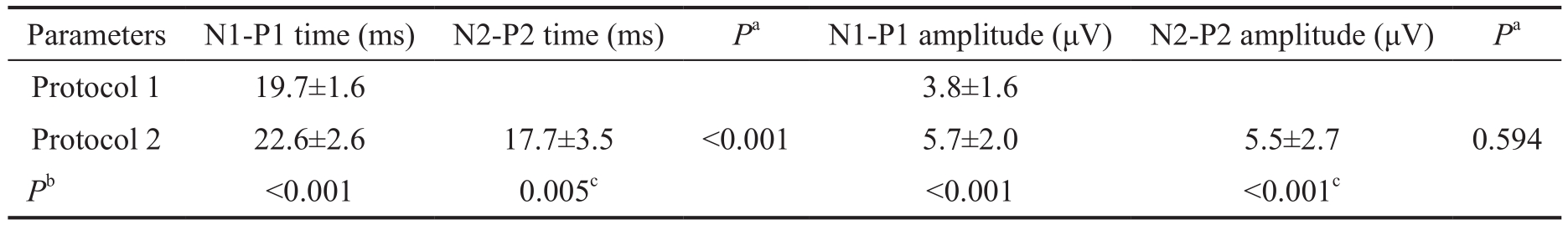
N1: First negative component; N2: Second negative component; P1: First positive component; P2: Second positive component.aIndependent t-test;bPaired t-test;cComparison with N1-P1 parameters in protocol 1.
Parameters N1-P1 time (ms) N2-P2 time (ms) Pa N1-P1 amplitude (μV) N2-P2 amplitude (μV) Pa Protocol 1 19.7±1.6 3.8±1.6 Protocol 2 22.6±2.6 17.7±3.5 <0.001 5.7±2.0 5.5±2.7 0.594 Pb <0.001 0.005c <0.001 <0.001c
In this study, the mfERG response to the central hexagonal element in the single-element stimulation protocol was different from that of conventional multiple element stimulation. In single element stimulation, double-positive waves appeared and the implicit time and the amplitude of P1 and P2 were longer and higher than those of P1 in multipleelement stimulation. The amplitude of N1 in single element stimulation (protocol 2) was also significantly higher than in multiple-element stimulation (protocol 1). Seiple et al[17]reported the similar result with the VERIS system (Electro-Diagnostic Imaging, San Mateo, CA). They found that as the luminance of the surrounding hexagons was increased, the amplitude and implicit time of the center-modulated hexagonal response decreased, which is similar with our result that P1 amplitude and implicit time increase in single-element stimulation protocol hexagon. They also examined the effect of the proximity of the surrounding luminance on the amplitude and implicit time of the central hexagon by separating the central hexagon from the illuminated surround with a dark annulus. The amplitude and implicit time of P1 increased as the width of the dark annulus increased from a 0-deg to a 10-deg.The implicit time of P1 increased about 3ms (from 26.3ms to 29.2ms) over the whole range of annulus widths. In our experiment, the implicit time for P1 also increased about 3ms(37.8ms to 40.7ms). They concluded that the observed effects in the experiment represented a lateral spread of adaptation effects. In the present study, our result showed the different mfERG wave form which was of double-positive wave form.Those difference was might be caused by different type of mfERG machine (Roland vs Veris), electrode type (DTL vs contact lens), ground electrode location (forehead vs ear),bandpass width (10-100 Hz vs 10-300 Hz), base period (83ms vs 26ms), stimulus frequency (60 Hz/16.666ms vs 75 Hz/13.333ms), and the center hexagon luminance (115 cd/m2 vs 340 cd/m2)[18].
Our result of double-positive wave form as well as the increased amplitude and implicit time might be due to the stray light effect inside the eye and loss of lateral inhibition of the inner retina. First, because all the other stimulus elements except the central hexagonal element were blacked out, the light adaptation level of surrounding retina could be lowered, which resulted in the local response alteration and the sensitization of the broad retinal area correspond to the blacked out elements. The blacked out area of the retina became more sensitive to stray lights inside the eye and this increased the electrical response to the stray light component, which might have contributed to changes in waveform shape. Each response in the mfERG is the result of a serial correlation between the stimulation sequence of the particular hexagon and the single continuous ERG record. The peripheral retina will receive equal stray light from the central hexagon whether the sequence has the peripheral hexagon on or off. Thus any stray light response from the peripheral hexagon will be cancelled out. The stray light responses from the peripheral hexagons will correlate, however, with the stimulation sequence of the central hexagon and add positively. In other words, if the stimulus light emerged on a stimulus element illuminates nottargeted retina area, the ERG response from the not-targeted area appears in the response on the targeted retinal area. Thus the central response will be larger and slower, possibly with a substantial rod component, under these conditions relative to the standard mfERG central response. Numerous studies have shown the change of ERG response with light adaptation[19-21].Second, for the loss of lateral inhibition of the inner retina,in the present study, P1 amplitude increase about 50%comparing in single-element stimulation comparing with in multi-element stimulation. Furthermore, much more huge P2 amplitude was noticed after P1 wave in single-element stimulation. It was almost two time bigger than the P1 wave in multi-element stimulation. According to the report by Seiple et al[17], as well as Hood et al[22], the small luminance change in mean level around an already high mean level would not be expected to substantially alter the amplitude or implicit time of the response. Therefore it seemed less like for scatter light alone to raise such a relatively big change of amplitude or implicit time of the P1 or P2 wave. Shen et al[23] demonstrated facilitatory interactions with the mfERG by comparing the amplitude of the mfERG response elicited by single-hexagon stimulus with the sum of the mfERG responses elicited by 7, 19, 37, and 61 hexagon stimuli, and found that the amplitude of the mfERG response to a single large hexagon was significantly smaller than the sum of mfERG responses evoked by 7-61 hexagons covering the same area. Generally lateral inhibition of the inner retina is a known modulation process at the cellular level, such as horizontal cell or amacrine cell, more similar to a receptive field than a gross topographic area, and is often used to explain contrast sensitivity or edge enhancement in image perception[24-27]. Lankheet et al[28]also reported that there is a spread of adaptation over long distances that is mediated through lateral interactions in the horizontal cell network. Since these cells are electrically coupled and have large receptive fields, the cellular processes of lateral inhibition could be expanded into the processes of relatively gross image perception[28-29]. It has been shown that the nature of this feedback depends upon the level of retinal adaptation[30-32]. Given our experimental conditions, lateral inhibition might be lost, and the response of the macular area could be recorded as enhanced and elongated and could appear as overlapping waveforms. The implicit time (50ms) of N2 in single hexagonal element stimulation was significantly shorter than that (60ms) of multiple element stimulation in mfERG.Therefore, the N2 notching observed in the range of 48-52ms between the P1 wave and the P2 wave was elicited by an exaggerated inner retinal response.
In the present study, the mfERG response of the central hexagonal element in single-element stimulation was different from the traditional focal ERG waveforms observed in other studies[1-3]. This discrepancy might be due to differences in stimulus parameters between the two procedures. Because there is no established standard for focal ERG, waveforms vary in stimulus diameter, repetition rate, and retinal illuminance.While low stimulus rates (5 Hz) produced a transient ERG with a waveform similar to that of the full- field ERG, including a-waves, b-waves, and oscillatory potentials[15], high repetition rates (≥10 Hz) elicited sinusoidal responses localized with photoreceptors and bipolar contributions[1,33].
Although we argue that the different mfERG responses of the central hexagonal element in single-element stimulation in this study might be associated with not only the stray light, but also by the loss of lateral inhibition of the inner retina, further study, such as central hexagon surrounded by hexagons set the mean luminance (half-illumination of the surrounding stimulus elements) instead of blacking out, is needed to rule out the possibility of a stray light effect.
In conclusion, mfERG responses from a central hexagonal element in single-element stimulation were different from those of conventional multiple-element stimulation. The positive wave was more enhanced and it elongated into two wavelets. More studies are warranted to identify the exact underlying mechanisms of these observations.
Authors’ contributions: All authors have made substantial contributions to the work reported in this manuscript (e.g. data collection, analysis, and writing or editing assistance).
Foundations: Supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2016R1D1A1A02937018); the Bio & Medical Technology Development Program of the NRF, which is funded in part by the Korean government, the Ministry of Science, and ICT(MSIP) (NRF-2017M3A9E2056458).
Conflicts of Interest: Yoo JH, None; Yun C, None; Oh J,None; Kim SW, None.
1 Seiple WH, Siegel IM, Carr RE, Mayron C. Evaluating macular function using the focal ERG. Invest Ophthalmol Vis Sci 1986;27(7):1123-1130.
2 Schönbach EM, Chaikitmongkol V, Annam R, McDonnell EC, Wolfson Y, Fletcher E, Scholl HPN. 7-hexagon multifocal electroretinography for an objective functional assessment of the macula in 14 seconds.Ophthalmic Res 2017;58(2):117-124.
3 Badhani A, Padhi TR, Panda GK, Mukherjee S, Das T, Jalali S.Correlation of macular structure and function in a boy with primary foveomacular retinitis and sequence of changes over 5 years. Doc Ophthalmol 2017;135(1):43-52.
4 Oiwa K, Kataoka K, Maruko R, Ueno S, Ito Y, Terasaki H. Half-dose photodynamic therapy for chronic central serous chorioretinopathy evaluated by focal macular electroretinograms. Jpn J Ophthalmol 2017;61(3):260-266.
5 MacHida S, Toba Y, Nishimura T, Ohzeki T, Murai KI, Kurosaka D. Comparisons of cone electroretinograms after indocyanine green-,brilliant blue G-, or triamcinolone acetonide-assisted macular hole surgery. Graefes Arch Clin Exp Ophthalmol 2014; 252(9):1423-1433.
6 Kominami A, Ueno S, Kominami T, Nakanishi A, Piao CH, Ra E,Yasuda S, Asami T, Terasaki H. Restoration of cone interdigitation zone associated with improvement of focal macular ERG after foveaoff rhegmatogenous retinal reattachment. Invest Ophthalmol Vis Sci 2016;57(4):1604-1611.
7 Andréasson S, Ghosh F. Cone implicit time as a predictor of visual outcome in macular hole surgery. Graefes Arch Clin Exp Ophthalmol 2014;252(12):1903-1909.
8 Koh V, Tan C, Nah G, Zhao P, Yang A, Lin ST, Wong TY, Saw SM, Chia A. Correlation of structural and electrophysiological changes in the retina of young high myopes. Ophthalmic Physiol Opt 2014;34(6):658-666.
9 Takahashi H, Hayashi T, Tsuneoka H, Nakano T, Yamada H, Katagiri S, Fujino Y, Noda Y, Yoshimoto M, Kawashima H. Occult macular dystrophy with bilateral chronic subfoveal serous retinal detachment associated with a novel RP1L1 mutation (P.S1199P). Doc Ophthalmol 2014;129(1):49-56.
10 Varano M, Parisi V, Tedeschi M, Sciamanna M, Gallinaro G, Capaldo N, Catalano S, Pascarella A. Macular function after PDT in myopic maculopathy: psychophysical and electrophysiological evaluation. Invest Ophthalmol Vis Sci 2005;46(4):1453.
11 Paris V, Falsini B. Electrophysiological evaluation of the macular cone system: focal electroretinography and visual evoked potentials after photostress. Semin Ophthalmol 1998;13(4):178-188.
12 Gerth C. Cone-mediated multifocal electroretinogram in age-related macular degeneration. Arch Ophthalmol 2006;124(3):345-352.
13 Parisi V, Ziccardi L, Stifano G, Montrone L, Gallinaro G, Falsini B.Impact of regional retinal responses on cortical visually evoked responses:Multifocal ERGs and VEPs in the retinitis pigmentosa model. Clin Neurophysiol 2010;121(3):380-385.
14 Parisi V, Perillo L, Tedeschi M, Scassa C, Gallinaro G, Capaldo N,Varano M. Macular function in eyes with early age-related macular degeneration with or without contralateral late age-related macular degeneration. Retina 2007;27(7):879-890.
15 Nagata M, Honda Y. The macular and paramacular local electroretinograms of the human retina and their clinical application. The Visual System:Neurophysiology, Biophysics, and Their Clinical Applications. New York,Plenum Press;1972.
16 Sutter E. The interpretation of multifocal binary kernels. Doc Ophthalmol 2000;100(2-3):49-75.
17 Seiple W, Vajaranant TS, Pepperberg DR, Szlyk JP. Lateral spread of adaptation as measured with the multifocal electroretinogram. Vis Neurosci 2001;18(5):687-694.
18 Bock M, Andrassi M, Belitsky L, Lorenz B. A comparison of two multifocal ERG systems. Doc Ophthalmol 1998-1999;97(2):157-178.
19 Li DK, Fang Q, Yu HB. The shift of ERG B-wave induced by hours’dark exposure in rodents. PLoS One 2016;11(8):e0161010.
20 Hamilton R, Graham K. Effect of shorter dark adaptation on ISCEV standard DA 0.01 and DA 3 skin ERGs in healthy adults. Doc Ophthalmol 2016;133(1):11-19.
21 Kondo M, Miyake Y, Piao CH, Tanikawa A, Horiguchi M, Terasaki H. Amplitude increase of the multifocal electroretinogram during light adaptation. Invest Ophthalmol Vis Sci 1999;40(11):2633-2637.
22 Hood DC, Seiple W, Holopigian K, Greenstein V. A comparison of the components of the multifocal and full-field ERGs. Vis Neurosci 1997;14(3):533-544.
23 Shen Y, Racine J, Little JM, Lachapelle P. Facilitatory interactions evidenced with the multifocal ERG [abstract]. Invest Ophthalmol Vis Sci 2010;51:1482.
24 Baylor DA, Fuortes MG, O’Bryan PM. Receptive fields of cones in the retina of the turtle. J Physiol 1971;214(2):265-294.
25 Barnes S, Werblin F. Direct excitatory and lateral inhibitory synaptic inputs to amacrine cells in the tiger salamander retina. Brain Res 1987;406(1/2):233-237.
26 Cook PB, McReynolds JS. Lateral inhibition in the inner retina is important for spatial tuning of ganglion cells. Nat Neurosci 1998;1(8):714-719.
27 Jackman SL, Babai N, Chambers JJ, Thoreson WB, Kramer RH.A positive feedback synapse from retinal horizontal cells to cone photoreceptors. PLoS Biol 2011;9(5):e1001057.
28 Lankheet MJ, Przybyszewski AW, van de Grind WA. The lateral spread of light adaptation in cat horizontal cell responses. Vision Res 1993;33(9):1173-1184.
29 Lamb TD, Simon EJ. The relationship between intercellular coupling and electrical noise in turtle photoreceptors. J Physiol 1976;263(2):257-286.
30 Vrolijk PC, van der Wildt GJ. Foveal inhibition measured with suprathreshold stimuli. Vision Res 1985;25(10):1413-1421.
31 Lankheet MJ, van Wezel RJ, van de Grind WA. Effects of background illumination on cat horizontal cell responses. Vision Res 1991;31(6):919-932.
32 Ichinose T, Lukasiewicz PD. Inner and outer retinal pathways both contribute to surround inhibition of salamander ganglion cells. J Physiol 2005;565(Pt2):517-535.
33 Bush RA, Sieving PA. Inner retinal contributions to the primate photopic fast flicker electroretinogram. J Opt Soc Am A Opt Image Sci Vis 1996;13(3):557-565.