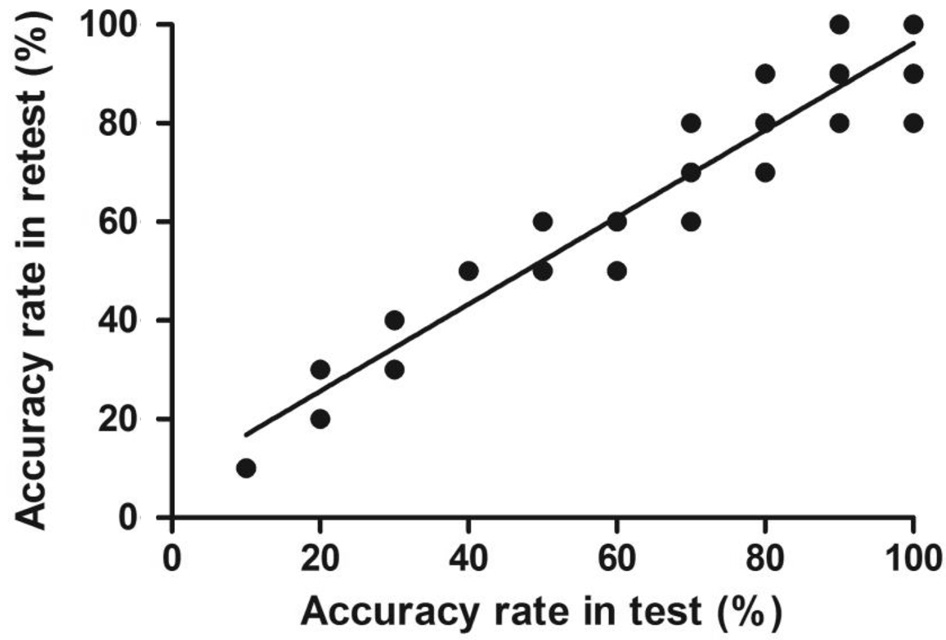
Figure 1 Test-retest correlation of the dynamic stereopsis program(including repetitive marking points for the same correct answer fill rate).
I ntermittent exotropia [X(T)] is one of the most common forms of childhood strabismus[1]. Strabismus prevents the normal development of binocular sensory neurons in the visual cortex, and the eye turn is visible during stressful situations or when the person is tired, ill or anxious, which may have a negative effect on the quality of binocular visual functions[2],such as divergence[3], fusion[4] and stereoacuity[5]. Traditional stereopsis examinations include the Titmus stereo test, the Lang stereo test and the Frisby stereo test[6]. Deterioration of near stereoacuity to below normal thresholds and the loss of near stereoacuity are considered signs of X(T) severity, although such factors are rarely reported[7-8]; however, researchers have suggested that patients with X(T) may retain good near stereoacuity[9]. We suggest that one reason underlying these inconsistent conclusions is the vergence fluctuation,which has been recognized as a characteristic of X(T)[10]. The inconsistency of vergence deviations increases the difficulty of detecting true binocular function via the traditional static stereopsis test in X(T) patients in everyday life[11] because the stimulus’ frontoparallel location and its dot pattern are
constant and fixed while retinal images are always in motion in a dynamic world[6]. Therefore, a comprehensive evaluation of binocular function that utilizes a dynamic stereopsis test is warranted.
Both static and dynamic stereoacuity can be reduced in the central visual field of patients with strabismus and other binocular vision anomalies[12]. Theoretically, at least two possible methods of generating dynamic stereopsis are available: a mechanism that detects binocular disparity at different times (disparity only) and a mechanism that detects interocular velocity differences (motion only)[11]. Interestingly,a poor correlation is observed between these two routes,even in normal adults, which suggests that humans use two distinct mechanisms to extract binocular information of motion-in-depth. Our previous study focused on a computer program that generates dynamic random-dot stereograms,which has been shown to be a useful method for measuring dynamic stereopsis[13-14]. This program has three indexes:motion+disparity, motion only and disparity only, from which the disparity and motion cues can be included or omitted independently based on the stereoacuity of 600 arc seconds.This program demonstrated that binocular depth-from-motion was present in approximately half of the esotropic patients without fine stereopsis, suggesting that separate mechanisms may underlie static stereopsis and motion[13]. However, the condition of dynamic stereopsis in X(T) patients remains unclear.
The characteristics of X(T) patients were assessed by comparing static and dynamic stereopsis, and we aimed to answer three questions: 1) Do X(T) patients with stereoblindness diagnosed by stereograms have the potential for stereopsis? 2) Is there a difference between static and dynamic stereopsis in X(T) patients? 3) What is the relationship among the age of onset, the duration of the disease and ocular deviations and stereopsis? In this study, we aimed to compare static and dynamic stereopsis, including motion stereopsis and disparity stereopsis, in 57 X(T) patients and 55 normal subjects through traditional methods and Ai-Hou Wang’s method.
Ethical Approval This observational, cross-sectional,nonconsecutive case study was conducted by recruiting volunteers with X(T) as well as healthy subjects who visited the Optometry Clinic. The research was performed according to the tenets of the Declaration of Helsinki. The study protocol was approved by the institutional Ethics Committee. All subjects provided informed consent after receiving both written and verbal explanations of the nature and intent of the study.
Subjects and Inclusion Criteria This study included 57 patients with X(T) [the X(T) group] and 55 normal subjects(the normal group). The inclusion criteria for the X(T) group were as follows: 1) near and distance exodeviation angles greater than 5 prism degrees (PD) and alternate cover tests and X(T) diagnosis by an experienced doctor; 2) between 5 and 30 years old; 3) 0.0 or better best corrected visual acuity (BCVA;logMAR, log of the minimum angle of resolution); and 4)absence of other ocular disorders or other systemic diseases.The inclusion criteria for the normal group were as follows: 1)between 5 and 30 years old; 2) 0.0 or better logMAR BCVA;3) near and distance exodeviation angles less than 5 PD and alternate cover tests; and 4) no history of any ocular pathology or systemic disease. All participants in both groups wore full optical correction devices during testing.
Binocular Vision Function Examination After a routine ophthalmic examination, the normal group was tested to determine the feasibility and accuracy of the dynamic stereopsis test. In the X(T) group, the included indexes were as follows: 1) the dynamic stereopsis test, which included motion+disparity, motion-only and disparity-only parts; 2) the angle of ocular deviation, 3) the Bagolini striated lens test, 4)the disease course, and 5) the Titmus stereopsis test.
Prism cover test: The angles of deviation for distance (6 m)and near (33 cm) vision were measured using the alternate prism cover test with spectacle corrections when required. The deviation after occlusion of one eye for 1h was remeasured to distinguish the simulated X(T) from true divergence excess X(T). Then, we classified the results according to Kushner’s classification scheme for X(T)[15].
Bagolini striated lens test: Each observer viewed a light source(30 cd/m2) held at 33 cm while wearing Bagolini striated lenses under low ambient illumination (5 lx). Under normal viewing conditions, the participants with normal binocular function perceived an “X”, which represents a combination of the “/” seen by one eye and the “\” seen by the other. However,participants with suppression (SUP) perceive only one line (/ or \)within the region affected by the suppression scotoma[16].
Age of onset and disease course: The age of onset was recorded using data from the patients’ files and determined based on the history given by the patients or their parents or confirmed by inspecting old photographs, and the time since onset was calculated and used as the disease course.
Static and Dynamic Stereoacuity Measurement The stereoacuity measurement was divided into static and dynamic stereopsis. The static Titmus stereo test detected stereoacuity of 40, 50, 60, 80, 100, 140, 200, 400 or 800 arc seconds using circular figures, and each measurement was repeated twice.
For the dynamic stereopsis measurement, each patient sat facing a screen at a distance of 55.5 cm with a green filter in front of the right eye and a red filter in front of the left eye.Three tests were performed, with test 1 (motion+disparity)designed to test binocular motion-in-depth elicited by interocular disparity cues and/or binocular depth-from-motion elicited by movement cues, test 2 (disparity only) designed to test only stereopsis, and test 3 (motion only) designed to tested only binocular depth-from-motion without disparity cues (the online website for these programs is https://pan.baidu.com/s/1I0StAl9ynsQeytNybR3IEw). Specifically, four rectangles were included in these three tests, and one of these four randomly provides a depth cue and moves back and forth in the “Z” direction. The patients were required to select the correct answer continuously 10 times in a forced-choice manner, and eight correct answers (80%) were judged as accurate[11]. Normal subjects performed the dynamic stereopsis test first to show that it was feasible; then, one-eyed viewing through Bangerter filters with densities of 0.2 was performed to simulate suppression. In our previous study, stereopsis was demonstrated to be destroyed with this method, and a corrective lens on the other eye was used to test the traditional and dynamic stereopsis function[17-18]. Each measurement was repeated twice to avoid any learning effects.
Statistical Analysis The differences between patients with motion-only, disparity-only or motion+disparity stereopsis and those without stereopsis were analyzed statistically using either the Chi-square test or the Mann-Whitney test. The test-retest reliability of dynamic stereopsis in the normal and X(T) groups was appraised using Pearson’s correlation coefficient analysis.P<0.05 was considered statistically significant.
Clinical Demographics This study included 57 patients (37 females and 20 males) in the X(T) group and 55 people (26 females and 29 males) in the normal group. The mean ages of the X(T) group and normal group were 14.8±10.5y and 15.9±2.2y, respectively. The mean deviation angle in the X(T)group was 25.78±15.93 PD, and all the participants in the normal group were nearly orthotropic (less than 5 PD) on the alternate cover test. The mean BCVA was 0.00 logMAR in both groups.
Feasibility and Accuracy of Dynamic Stereopsis All the subjects in the normal group accepted the dynamic stereopsis test, and all the subjects (100%) exhibited good stereoacuity with accuracy rates of more than 80%. To examine the testretest reliability, 85 subjects from two groups repeated the test for a second time and presented a correct answer fill rate from 10% and 20% to 90% and 100%, indicating that the test was highly reliable. The Pearson’s correlation coefficient was 0.901(P<0.001; Figure 1).
Comparison of Static and Dynamic Stereopsis Tests In the normal group, no patients could pass the static as well as the dynamic stereopsis test after using Bangerter filters with densities of 0.2 in one eye, whereas in the X(T) patients diagnosed with stereoblindness via traditional tests, the accuracy rates of the traditional static tests were 31.81% for motion+disparity, 36.36% for motion-only and 45.45% for disparity-only stereopsis (Table 1, Part I).

Figure 1 Test-retest correlation of the dynamic stereopsis program(including repetitive marking points for the same correct answer fill rate).
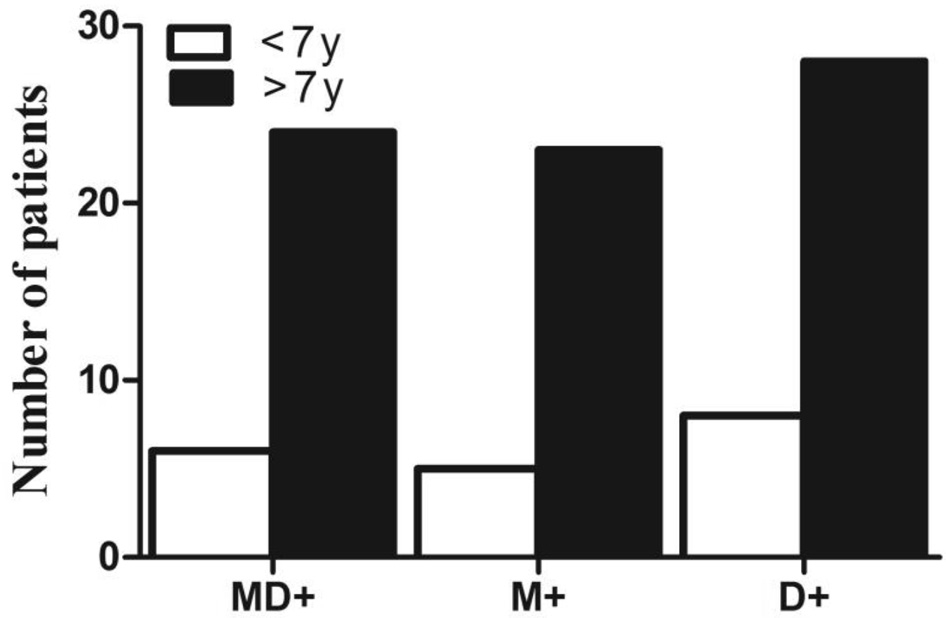
Figure 2 Dynamic stereopsis, including motion+disparity (MD+),motion only (M+) and disparity only (D+), in X(T) patients with an age of onset of less than 7y or more than 7y.
In the motion+disparity test, the Fly (+) X(T) patients (22/35,62.9%) obviously passed the test more easily than the Fly (-)X(T) patients (7/22, 31.8%; P=0.046, Chi-square test), which was also observed for the disparity-only test, with the Fly (+) X(T) patients presenting a greater correct answer fill rate (26/35, 74.3%) than the Fly (-) X(T) patients (10/22,45.5%; P=0.047, Chi-square test); however, no obvious relationship was observed between positive Titmus Fly tests and the motion-only test (P=0.290, Chi-square test).
Dynamic Stereopsis in the X(T) Patients with Different Ages of Onset Patients in the X(T) group were divided into two groups according to the age of onset: younger than 7y and older than 7y. There were 20 patients with an age of onset less than 7y and 37 patients with an age of onset greater than 7y. As shown in Table 1 and Figure 2, 6 of 20 (30%) patients with early onset had positive motion+disparity stereopsis, and 24 of 37 (64.86%) patients with late onset presented positive motion+disparity stereopsis (P=0.015, Chi-square test). In the motion-only test, 5 of the 20 patients (25%) in the early-onsetgroup tested positive, which was a significantly lower rate than the late-onset patients, with 23/37 (62.16%) positive patients in the latter group (P=0.012, Chi-square test). In the disparityonly test, the late-onset patients (28/37, 75.68%) also showed better function than the early-onset patients (8/20, 40%;P=0.011, Chi-square test).
Table 1 Dynamic stereopsis in X(T) patients
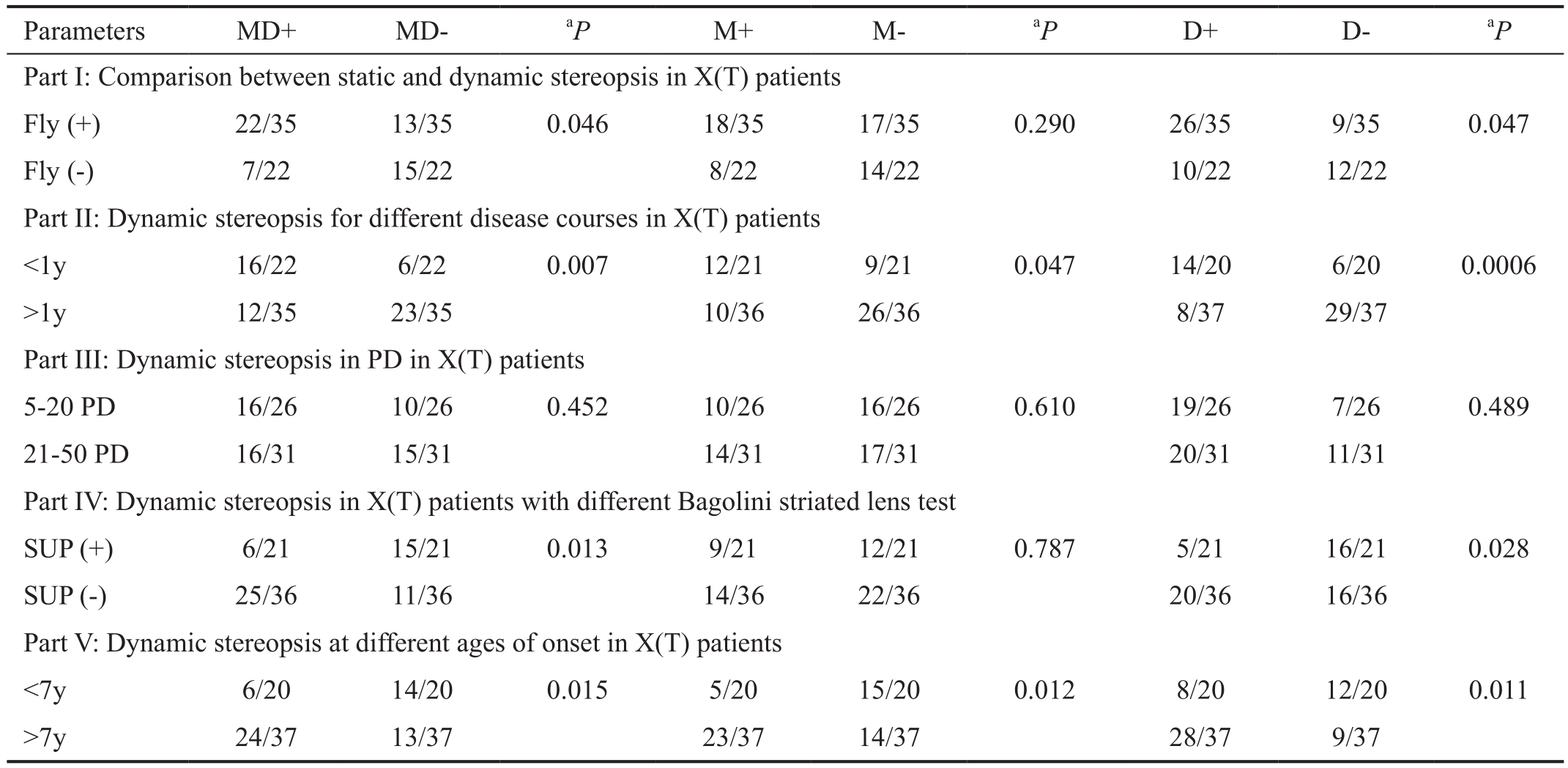
MD: Motion+disparity test; M: Motion-only test; D: Disparity-only test; SUP: Suppression.aChi-square test.
Parameters MD+ MD-aP M+ M-aP D+ D-aP Part I: Comparison between static and dynamic stereopsis in X(T) patients Fly (+) 22/35 13/35 0.046 18/35 17/35 0.290 26/35 9/35 0.047 Fly (-) 7/22 15/22 8/22 14/22 10/22 12/22 Part II: Dynamic stereopsis for different disease courses in X(T) patients<1y 16/22 6/22 0.007 12/21 9/21 0.047 14/20 6/20 0.0006>1y 12/35 23/35 10/36 26/36 8/37 29/37 Part III: Dynamic stereopsis in PD in X(T) patients 5-20 PD 16/26 10/26 0.452 10/26 16/26 0.610 19/26 7/26 0.489 21-50 PD 16/31 15/31 14/31 17/31 20/31 11/31 Part IV: Dynamic stereopsis in X(T) patients with different Bagolini striated lens test SUP (+) 6/21 15/21 0.013 9/21 12/21 0.787 5/21 16/21 0.028 SUP (-) 25/36 11/36 14/36 22/36 20/36 16/36 Part V: Dynamic stereopsis at different ages of onset in X(T) patients<7y 6/20 14/20 0.015 5/20 15/20 0.012 8/20 12/20 0.011>7y 24/37 13/37 23/37 14/37 28/37 9/37
Dynamic Stereopsis in the X(T) Patients with Different Disease Courses We assigned the patients to one of two groups based on disease course: a short-course group for patients with a disease course of less than 1y (22, 38.6%) and a long-course group for patients with a disease course of more than 1y (35, 61.4%). In Table 1 Part II and Figure 3, regardless of whether the motion+disparity, motion-only or disparityonly test was used, the patients in the short-course group displayed a significantly greater correct answer fill rate than the patients in the long-course group, and the correct answer fill rates for the motion+disparity, motion-only and disparityonly tests were 72.7%, 57.1% and 70%, respectively (P=0.007 for motion+disparity, P=0.047 for motion-only and P=0.002 for disparity-only, Chi-square test). In other words, those with a disease course of more than 1y had worse dynamic stereopsis than those with a disease course of less than 1y.
Dynamic Stereopsis in X(T) Patients with Different Angles of Ocular Deviation Based on the angles of ocular deviations,the X(T) patients were divided into 2 groups: a 5-20 PD group (26 patients) and a 21-50 PD group (31 patients). The motion+disparity, motion-only and disparity-only stereopsis cases did not show significant differences between these two groups (P=0.452, 0.610 and 0.489, respectively, Chi-square test; Table 1 Part III).
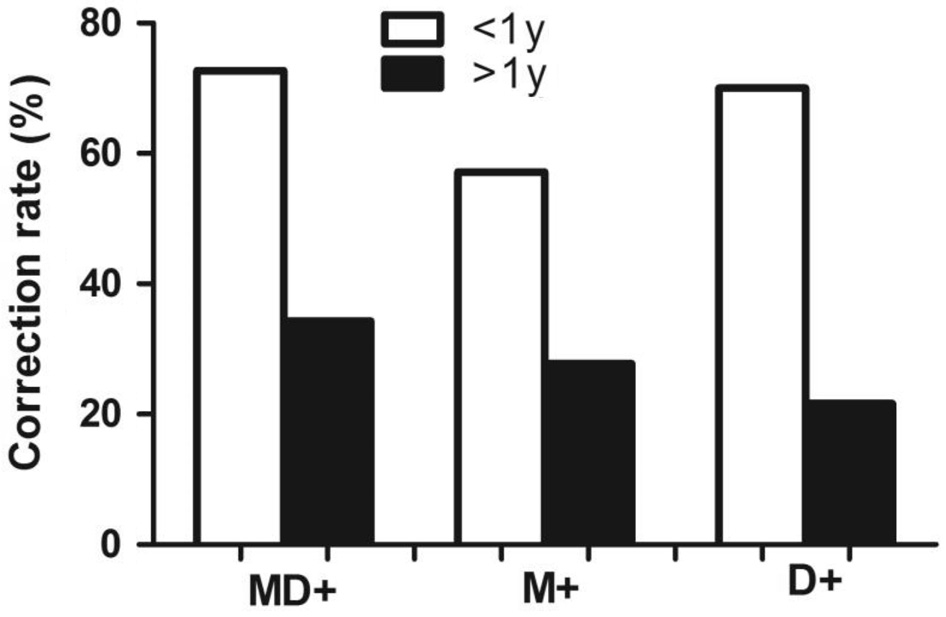
Figure 3 Dynamic stereopsis, including motion+disparity (MD+),motion only (M+) and disparity only (D+), in X(T) patients with a disease course of less than 1y or more than 1y.
Relationship Between the Bagolini Striated Lens Test and Dynamic Stereopsis in the X(T) Patients For the Bagolini striated lens test, the 36 X(T) patients who passed the test were considered SUP (-), and the 21 patients who did not pass the tests were considered SUP (+). In the motion+disparity and disparity-only tests, more patients in the SUP (-) group passed than in the SUP (+) group (P=0.013 for the motion+disparity test and P=0.028 for the disparity-only test, Chi-square test),and no obvious relationship was observed between the Bagolini striated lens test and the motion-only test (P=0.787; Figure 4 and Table 1 Part IV). In conclusion, the Bagolini striated lens test results were correlated with the motion+disparity and disparity-only tests (P<0.05) but not with the motion-only test.
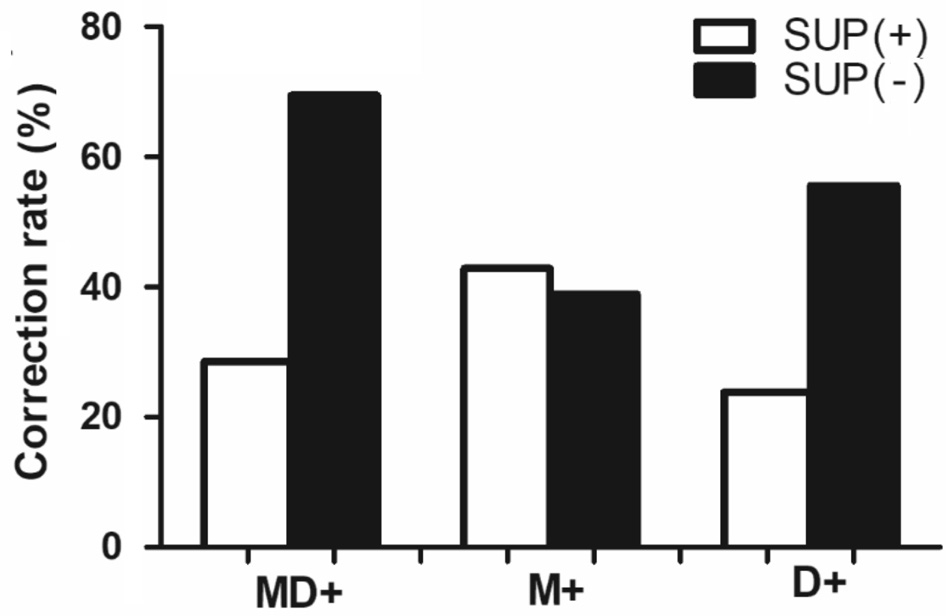
Figure 4 Dynamic stereopsis, including motion+disparity (MD+),motion only (M+) and disparity only (D+), for X(T) patients with different Bagolini striated lens test results as suppression [SUP (+)]or normal binocular function perceived [SUP (-)].
In this study, we revealed the following: 1) dynamic stereopsis showed high test-retest reliability, and individuals with stereoblindness diagnosed by traditional stereograms have the potential for dynamic stereopsis; 2) motion+disparity and disparity-only tests but not the motion-only test of dynamic stereopsis were consistent with the traditional Titmus stereo tests; and 3) long disease courses are associated with a negative effect on dynamic stereopsis, including motion+disparity,motion-only and disparity-only, in X(T) patients, for whom a disease course greater than 1y resulted in comparatively worse performance in the dynamic stereopsis tests.
Although certain X(T) patients were diagnosed as stereoblind with the Titmus stereo test or considered to present suppression with the Bagolini striated lens test in our study[17], they exhibited different positive rates of motion+disparity, motion only or disparity only in the dynamic stereopsis evaluation.This phenomenon indicates that traditional methods cannot fully reflect the visual functions or residual stereopsis[19],and compared with the traditional stereo test, the dynamic stereo test may facilitate the extraction of depth relative to static disparity signals, which is consistent with the results of Hess et al[20] and Tidbury et al[21]. Moreover, the Titmus stereo test and the Bagolini striated lens test were related to the motion+disparity and disparity-only tests of dynamic stereopsis, which did not conform to Huang et al’s[14] results for esotropia patients. We hypothesize that the differences between Huang et al’s[14] study and ours may be due to different types of strabismus; therefore, the damaging mechanism underlying stereopsis is distinctive[22].
X(T) can develop in early childhood and is considered to be progressive[23], although the natural history of X(T) remains obscure due to the lack of longitudinal prospective studies.We determined that the disease course of X(T) may affect stereoacuity, as patients with a short disease course of less than 1y presented better motion+disparity, motion-only, and disparity-only test results than patients with a long disease course of more than 1y. Our results were similar to but not in complete agreement with the results of Abroms et al[24], which suggests that subjects had a significantly greater chance of having postoperative fine stereoacuity if they were surgically aligned within 5y of the onset of strabismus. However, in a study by Fawcett et al[25], 12mo or less of constant strabismus led to significantly better random-dot stereoacuity than longer periods, which is consistent with the results of our study.Interestingly, a single statistical multivariate model including disease course, onset times, along with motion and disparity or motion-only or disparity-only results has been developed,which supported the significant correlation observed between disease course and dynamic stereopsis. However, additional subjects should be included to further prove this correlation.Therefore, we deduced that the destruction of dynamic stereopsis is dynamic and cumulative, and a longer disease course is associated with worse progression[26].
Moreover, we also found that patients with an onset before the age of 7y were more easily damaged in the motion-only,disparity-only or motion+disparity tests than patients with onset after 7 years of age. Six years old is an important time point for stereoacuity that enables children to discriminate confusing information[27]. Moreover, our study was consistent with Dekker et al’s[28] report showing that older children(>10.5y) demonstrated better sensory fusion and integration of depth cues in the brain than younger children (<10.5y).However, the age of onset in our study was not perfectly matched with the critical period for stereopsis, thus suggesting that the strabismic period can intersect with the critical period for stereopsis development and maturation[29].
PD value is a common index in X(T) patients for measuring the angle of the strabismus; however, PD value was not correlated with stereopsis for the motion-only, disparity-only or motion+disparity tests, which was consistent with the opinion of Seki et al[30] that deviation angles do not differ between near and distance stereopsis. Therefore, the deviation angle should not be listed as a complete operating indicator, as it does not in fluence stereopsis.
In conclusion, dynamic stereopsis is preserved in certain X(T)cases defined as stereoblindness via static stereo tests. A long disease course was shown to be a negative factor for dynamic stereopsis in X(T) patients which might be associated with worse progression, and provide good references clinically.
Foundations: Supported by National Natural Science Foundation of China (No.81600761); the Natural Science Foundation Team Project of Guangdong Province(No.2015A030312016).
Conflicts of Interest: Zhong J, None; Deng DM, None;Chen ZD, None; Li JR, None; Yuan JP, None; Feng L,None; Wang AH, None; Yu MB, None.
1 Leske DA, Holmes JM, Melia BM; Pediatric Eye Disease Investigator Group. Evaluation of the Intermittent Exotropia Questionnaire using Rasch analysis. JAMA Ophthalmology 2015;133(4):461-465.
2 Firth AY, Davis H, Horwood AM. Binocular visual acuity in intermittent exotropia: role of accommodative convergence. Am J Ophthalmol 2013;155(4):776-777.
3 Liebermann L, Hatt SR, Leske DA, Yamada T, Mohney BG, Brodsky MC, Holmes JM. Assessing divergence in children with intermittent exotropia. Strabismus 2012;20(1):11-16.
4 Feng XL, Zhang XX, Jia YD. Improvement in fusion and stereopsis following surgery for intermittent exotropia. J Pediatr Ophthalmol Strabismus 2015;52(1):52-57.
5 Hatt SR, Leske DA, Mohney BG, Brodsky MC, Holmes JM. Fusional convergence in childhood intermittent exotropia. Am J Ophthalmol 2011;152(2):314-319.
6 Matsuo T, Negayama R, Sakata H, Hasebe K. Correlation between depth perception by three-rods test and stereoacuity by distance Randot Stereotest. Strabismus 2014;22(3):133-137.
7 Saxena R, Kakkar A, Menon V, Sharma P, Phuljhele S. Evaluation of factors influencing distance stereoacuity on Frisby-Davis Distance Test(FD2) in intermittent exotropia. Br J Ophthalmol 2011;95(8):1098-1101.
8 Holmes JM, Hatt SR, Leske DA. Is intermittent exotropia a curable condition? Eye (Lond) 2015;29(2):171-176.
9 Kim DW, Han S, Kim US, Baek SH. Results of conservative management for consecutive esotropia after intermittent exotropia surgery.Eye (Lond) 2015;29(6):776-782.
10 Adams DL, Economides JR, Horton JC. Incomitance and eye dominance in intermittent exotropia. Invest Ophthalmol Vis Sci 2017;58(10):4049-4055.
11 Serrano-Pedraza I, Clarke MP, Read JC. Single vision during ocular deviation in intermittent exotropia. Ophthalmic Physiol Opt 2011;31(1):45-55.
12 Schor C, Bridgeman B, Tyler CW. Spatial characteristics of static and dynamic stereoacuity in strabismus. Invest Ophthalmol Vis Sci 1983;24(12):1572-1579.
13 Maeda M, Sato M, Ohmura T, Miyazaki Y, Wang AH, Awaya S.Binocular depth-from-motion in infantile and late-onset esotropia patients with poor stereopsis. Invest Ophthalmol Vis Sci 1999;40(12):3031-3036.
14 Huang CH, Wang AH, Hu FR, Tsai TH. Exploring the divergence range for stereopsis maintenance with a computer-simulated troposcope in patients with intermittent exotropia. Invest Ophthalmol Vis Sci 2016;57(10):4493-4497.
15 Kushner BJ, Morton GV. Distance/near differences in intermittent exotropia. Arch Ophthalmol 1998;116(4):478-486.
16 Li JR, Lam CS, Yu MB, Hess RF, Chan LY, Maehara G, Woo GC,Thompson B. Quantifying sensory eye dominance in the normal visual system: a new technique and insights into variation across traditional tests.Invest Ophthalmol Vis Sci 2010;51(12):6875-6881.
17 Odell NV, Hatt SR, Leske DA, Adams WE, Holmes JM. The effect of induced monocular blur on measures of stereoacuity. J AAPOS 2009;13(2):136-141.
18 Li JR, Thompson B, Ding ZF, Chan LYL, Chen X, Yu MB, Deng DM,Hess RF. Does partial occlusion promote normal binocular function?Invest Ophthalmol Vis Sci 2012;53(11):6818-6827.
19 Guo DD, Wu JF, Hu YY, Sun W, Lv TL, Jiang WJ, Wu H, Wang XR,Jonas JB, Bi HS. Stereoacuity and related factors: the Shandong children eye study. PLoS One 2016;11(7):e0157829.
20 Hess RF, Mansouri B, Thompson B, Gheorghiu E. Latent stereopsis for motion in depth in strabismic amblyopia. Invest Ophthalmol Vis Sci 2009;50(10):5006-5016.
21 Tidbury LP, Brooks KR, O’Connor AR, Wuerger SM. A systematic comparison of static and dynamic cues for depth perception. Invest Ophthalmol Vis Sci 2016;57(8):3545-3553.
22 Merriam SW, Kushner BJ. An investigation into the mechanisms causing antipodean strabismus. J AAPOS 2007;11(3):249-253.
23 Hatt SR, Leske DA, Liebermann L, Mohney BG, Brodsky MC,Yamada T, Holmes JM. Associations between health-related quality of life and the decision to perform surgery for childhood intermittent exotropia.Ophthalmology 2014;121(4):883-888.
24 Abroms AD, Mohney BG, Rush DP, Parks MM, Tong PY. Timely surgery in intermittent and constant exotropia for superior sensory outcome. Am J Ophthalmol 2001;131(1):111-116.
25 Fawcett SL, Stager DR Sr, Felius J. Factors in fluencing stereoacuity outcomes in adults with acquired strabismus. Am J Ophthalmol 2004;138(6):931-935.
26 Fawcett SL, Wang YZ, Birch EE. The critical period for susceptibility of human stereopsis. Invest Ophthalmol Vis Sci 2005;46(2):521-525.
27 Nardini M, Bedford R, Mareschal D. Fusion of visual cues is not mandatory in children. Proc Natl Acad Sci U S A. 2010;107(39):17041-17046.
28 Dekker TM, Ban H, van der Velde B, Sereno MI, Welchman AE,Nardini M. Late development of cue integration is linked to sensory fusion in cortex. Curr Biol 2015;25(21):2856-2861.
29 Koc F, Sefi-Yurdakul N. Predictors of stereoacuity outcome in visually mature subjects with exotropia. Eye (Lond) 2016;30(2):264-269.
30 Seki Y, Wakayama A, Takahashi R, Umebara I, Tanabe F, Abe K,Shimomura Y. Influence of test distance on stereoacuity in intermittent exotropia. Strabismus 2017;25(1):12-16.