Valproic acid’s effects on visual acuity in retinitis pigmentosa: a systemic review and Meta-analysis
Wen-Jun Chen, Li Ma, Ming-Shu Li, Xiang Ma
Department of Ophthalmology, the First Affiliated Hospital of Dalian Medical University, Dalian 116011, Liaoning Province,China
Abstract● AlM: To gain a better understanding of the overall efficacy of valproic acid (VPA) treatment for retinitis pigmentosa (RP).● METHODS: Publications in PubMed, EMBASE, Cochrane Library, Web of Science and Clinicaltrials.gov were searched for clinical trials of patients with RP assigned to treatment with VPA. Patients’ pre- and post-treatment visual field(VF) and best-corrected visual acuity (BCVA) scores were extracted and compared to assess changes.● RESULTS: A total of 78 reports were retrieved and 6 studies involving 116 patients were included in the Meta-analysis.The combined results showed a significant decrease in logarithm of minimal angle of resolution (logMAR) scores,calculated using baseline and post-treatment BCVA(P<0.00001, mean difference=-0.05, 95%Cl: -0.05, -0.04,I2=36%) scores, which means there was considerable improvement in visual acuity. Meanwhile, more BCVA changes were observed in short-term (≤6mo) treatment studies (P<0.00001, mean difference=-0.05, 95%Cl: -0.05,-0.04, I2=38%), studies conducted in Asia (P<0.00001,mean difference=-0.05, 95%Cl: -0.05, -0.04, I2=4%), studies with a sample size of 30 or fewer patients (P<0.00001,mean difference=-0.05, 95%Cl: -0.05, -0.04, I2=38%) and prospective studies (P<0.00001, mean difference=-0.05,95%Cl: -0.05, -0.04, I2=0%). However, VPA’s effect on VF was inconsistent across studies (P=0.75, mean difference=-22.76, 95%Cl: -160.56, 115.05, I2=68%).● CONCLUSlON: This Meta-analysis reveals that most RP patients who were treated with VPA showed improvement in BCVA. However, its effect on VF remains inconsistent.VPA may be a promising treatment for RP.
INTRODUCTION
Retinitis pigmentosa (RP) refers to a disease about progressive degeneration of retina. Usually, RP begins from the mid periphery and spreads to macula and fovea.It presents as blindness at night and then develop to be the narrowing of visual fields (VF), causing tunnel vision and the legal blindness or complete blindness at last[1]. RP can lead to gradual dysfunction as well as the loss of rod photoreceptors in cells. It firstly impacts the night vision in mid-peripheral retina rich in rods and then impacts the night vision in central retina rich with cones. As a result, the cones will be eventually lost due to the disease process or following the loss of rods[2]. The prevalence of RP is about 1:4000[1]. RP itself exhibits a high heterogeneity as over 50 genes have mutations which can lead to non-syndromic RP. As reported, there are about 3100 kinds of mutations in these genes by now[3].
At present, RP cannot be treated as it is impossible to hinder the loss of photoreceptors as well as function. The use of neuroprotectors is a basal treatment mode, which adopts many trials to evaluate their curative effects in the treatment of RP,including neurotrophic factors, vitamin A, DHA, and lutein[4].In recent years, the valproic acid (VPA) has drawn people’s attention as it has the potential to treat RP. Functioning as an emotional and anticonvulsant stabilizer, VPA, to our knowledge, can lead to the inhibitory effects against gammaaminobutyric acid (GABA) in central nervous system[5]. Based on empirical evidence, VPA is likely to effectively treat people suffering retinal dystrophies due to its inhibitory effect on histone deacetylase[6] and in flammatory response pathway through microglial cell apoptosis[7-8]. However, its therapeutic benefits in RP remain inconclusive and controversial.According to Clemson et al[9], an average four-month VPA treatment will contribute to the improvement on bestcorrected visual acuity (BCVA) and VF for 5/7 RP patients.In another study, Bhalla et al[10] retrospectively studied 31 patients suffering various pigmentary retinal dystrophies after treatment with VPA for an average of 9.8mo. In comparison with publication of Clemson et al[9] , Bhalla et al[10] found that the severity of VF kept decreasing in 4/5 patients and averagely, the VF was greatly worsened during the process of being treated with VPA (P=0.002). Besides, the VPA could lead to some undesirable side effects. Recently, Iraha et al[11]found that VF level improved during the 6-month followup period; however, these reversed to baseline values after discontinuing the drug. Therefore, a systemic review together with a Meta-analysis are conducted aiming at evaluating how VPA contributes to RP treatment.
MATERIALS AND METHODS
Identification and Selection of Studies Literature search was independently performed by two investigators (Chen WJ and Li MS) in the PubMed, EMBASE, Cochrane Library, Web of Science and Clinicaltrials.gov. databases without restricting the publication language. All related studies available as of December 10, 2017 were retrieved. We used the following key words: (“valproic acid” or “propylisopropylacetic acid”or “2-propylpentanoic acid” or “divalproex” or “depakene”or “depakine” or “convulso fin” or “depakote” or “vupral” or“divalproex sodium” or “semisodium valproate” or “ergenyl” or“magnesium valproate” or “valproate” or “valproate sodium”or “sodium valproate” or “calcium valproate” or “valproate calcium” or “dipropyl acetate”) and (“retinitis pigmentosa”or “rod cone dystrophy” or “pigmentary retinopathy” or“retinopathy, pigmentary” or “tapetoretinal degeneration”). In addition, the reference lists of all retrieved studies are involved to determine other appropriate studies.
Integration and Elimination Standard Eligible studies had to satisfy specific standards: 1) research design: randomized controlled trials (RCTs), Non-randomized comparative researches including single-arm researches, cross-over researches and cohort researches; 2) experiment objects: RP patients; 3) intervention:topical and oral VPA; 4) results variables: baseline and posttreatment BCVA and VF was included. Duplications, reviews,case reports, meeting abstract, animal/in vitro studies, unrelated topic, and author’s response, were not included.
Data Collection Two reviewers (Chen WJ and Li MS)collected the information as follows from the suitable studies:the first author’s name, the publication year, the research design, the number of patients/eyes, sex, intervention, mean age, BCVA, VF, the length of treatment, and quality scores.The analysis on only data for the last treatment were conducted on the condition that the researchers reported original data with all stages of follow-up included. Disagreements were resolved by discussing all items with another reviewer (Ma L).
Quality Assessment Through applying a Jadad scale[12],RCTs were evaluated. Single-arm, cross-over, and cohort studies were assessed applying the Newcastle-Ottawa Scale(NOS)[13] at the same time. With the application of the NOS scale, comprehensive research quality was determined as bad(score=0-3), fairly good (score=4-6), or excellent (score=7-9).
Analysis on Data Analysis on data involved is carried out applying Review Manager [RevMan (Computer program),Version 5.3, Copenhagen: The Nordic Cochrane Centre,The Cochrane Collaboration, 2014]. The result variables were BCVA and VF. In an authentic publication, if visual acuity (VA) was presented in the form of Snellen VA, the data was changed to logMAR values to make data analysis easier. According to report before, “counting fingers” (CF),“hand motion” (HM), “light perception” (LP), and “no light perception”(NLP) were assigned a logMAR value of 1.85,2.3, 2.7, and 3.0, respectively[14-15]. The average variation in VA or VF ranging from baseline to last treatment points were combined and counted applying inverse variance ways.The combined average differences and 95% confidence intervals (CI) were figured out applying the fixed-effect or random-effect model. The data test of Cochran Q for heterogeneity over researches and the I2 data which quantifies the percentage of total variation attributable to between-study heterogeneity were figured out. The Q data was regarded as with statistical significance if P<0.1, and I2 values higher than 50% demonstrated elevated heterogeneity. When testing the significant heterogeneity outcomes from the random-effect model were employed; otherwise, the fixed-effect model was applied. Analysis on heterogeneity was conducted with the I2 data and determined as follows: low (25% to 50%), moderate(50% to 75%), or high (>75%)[16]. Analysis on subgroup were carried out according to the length of treatment, location,and sample size. An I2 value > 50% was determined as heterogeneity, and a random-effects model was subsequently employed to the statistics. If not, a fixed-effects model was used to pool the statistics. A P value less than 0.05 was regarded as statistical significance.
RESULTS
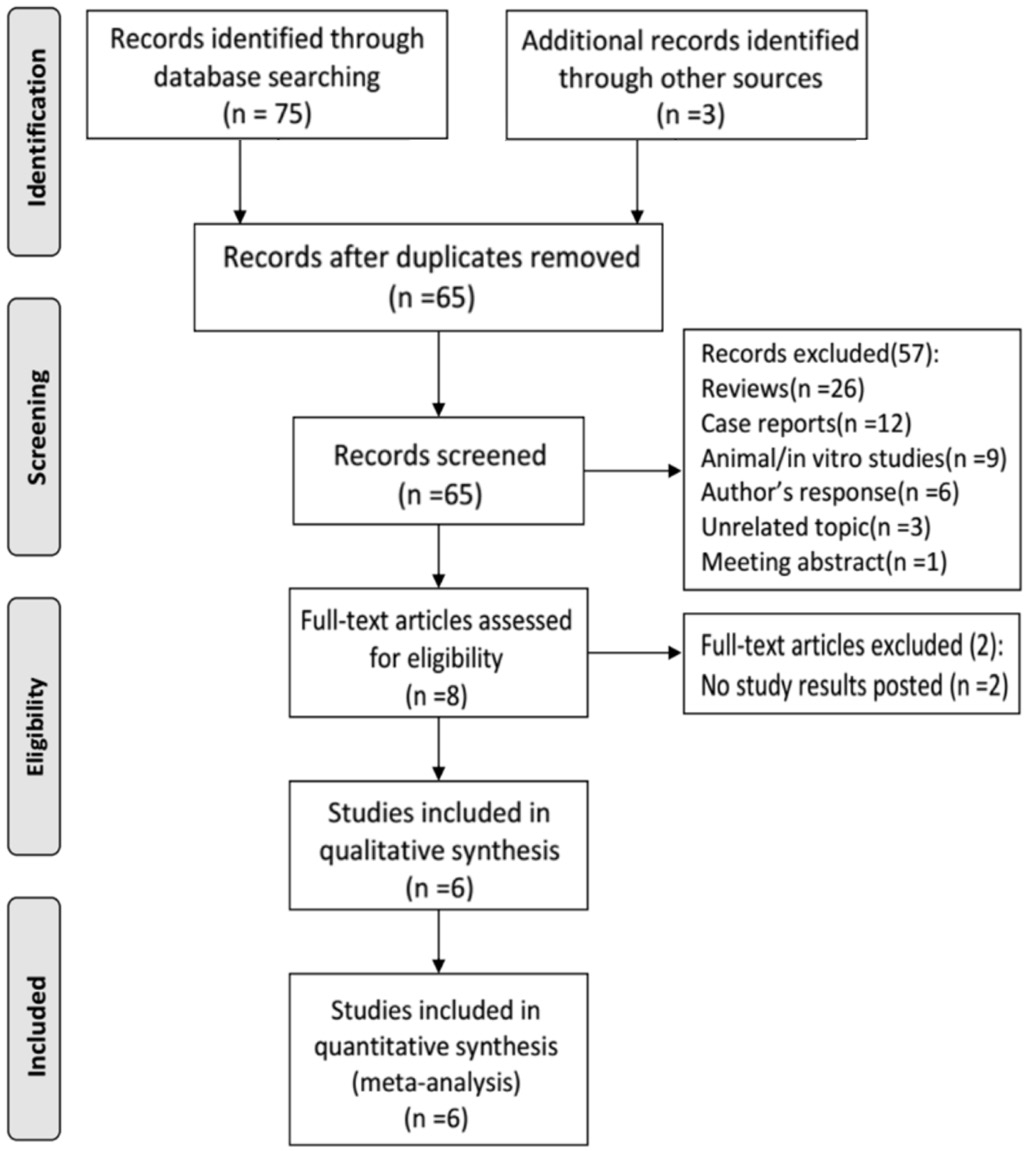
Overall Characteristics of Eligible Studies Figure 1 shows the study inclusion flow in this Meta-analysis. A total of 78 reports were initially identified. Of these, 13 were excluded based on the exclusion criteria listed above, including 13 duplications, 26 reviews, 12 case reports, 9 animal or in vitro studies, 6 authors’ responses, 3 unrelated topics, 1 meeting abstract, and 2 studies without posted results. The 6 remaining clinical reports (1 clinical trial and 5 full-text) that met the inclusion criteria were analyzed[9-11,17-19]. These 6 reports included 1 randomized controlled trial (RCT), 3 retrospective studies, and 2 prospective studies. A total of 203 eyes in 116 patients were included in this Meta-analysis. Additionally, the overall quality scores of the included studies are presented in Table 1.
Table 1 Characteristics of included trails and patients
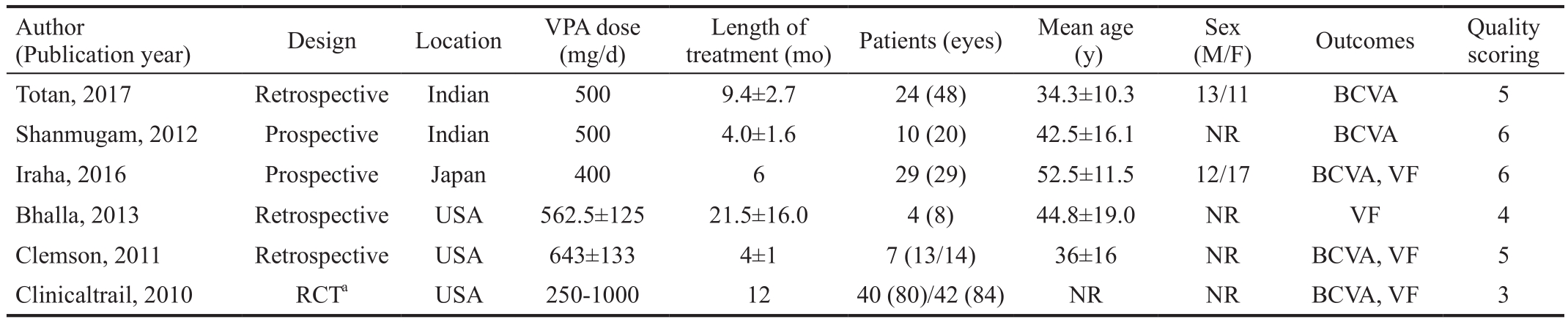
aThe control group is Placebo. RCT: Randomized controlled trail; NR: Not reported; BCVA: Best-corrected visual acuity; VF: Visual field.
(M/F) Outcomes Quality scoring Totan, 2017 Retrospective Indian 500 9.4±2.7 24 (48) 34.3±10.3 13/11 BCVA 5 Shanmugam, 2012 Prospective Indian 500 4.0±1.6 10 (20) 42.5±16.1 NR BCVA 6 Iraha, 2016 Prospective Japan 400 6 29 (29) 52.5±11.5 12/17 BCVA, VF 6 Bhalla, 2013 Retrospective USA 562.5±125 21.5±16.0 4 (8) 44.8±19.0 NR VF 4 Clemson, 2011 Retrospective USA 643±133 4±1 7 (13/14) 36±16 NR BCVA, VF 5 Clinicaltrail, 2010 RCTa USA 250-1000 12 40 (80)/42 (84) NR NR BCVA, VF 3 Author(Publication year) Design Location VPA dose (mg/d)treatment (mo) Patients (eyes) Mean age (y)Length of Sex
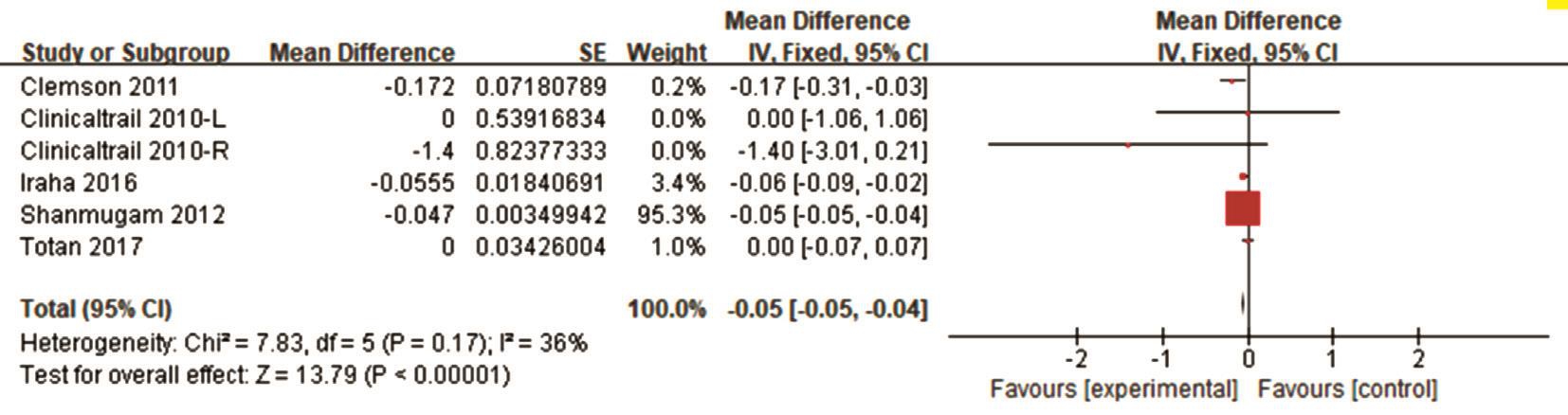
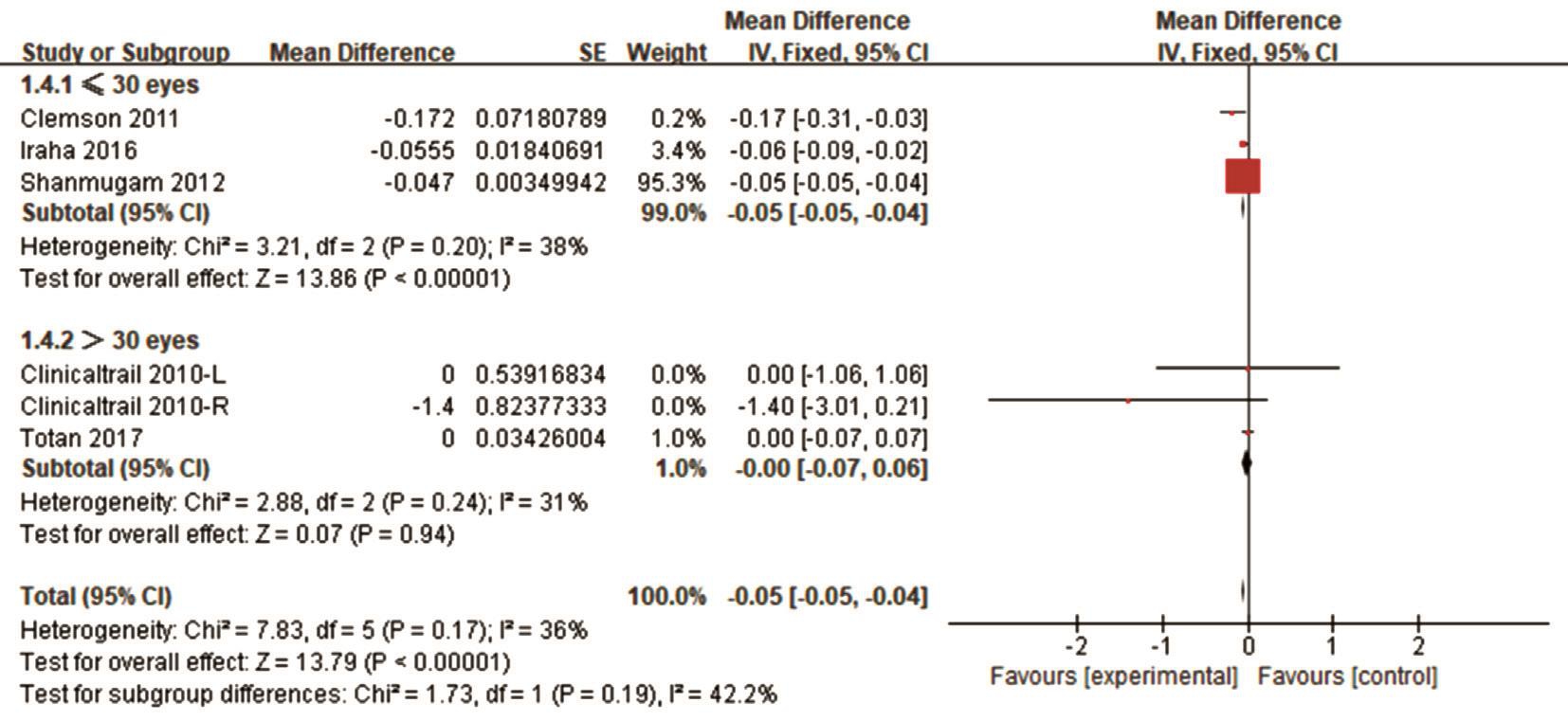
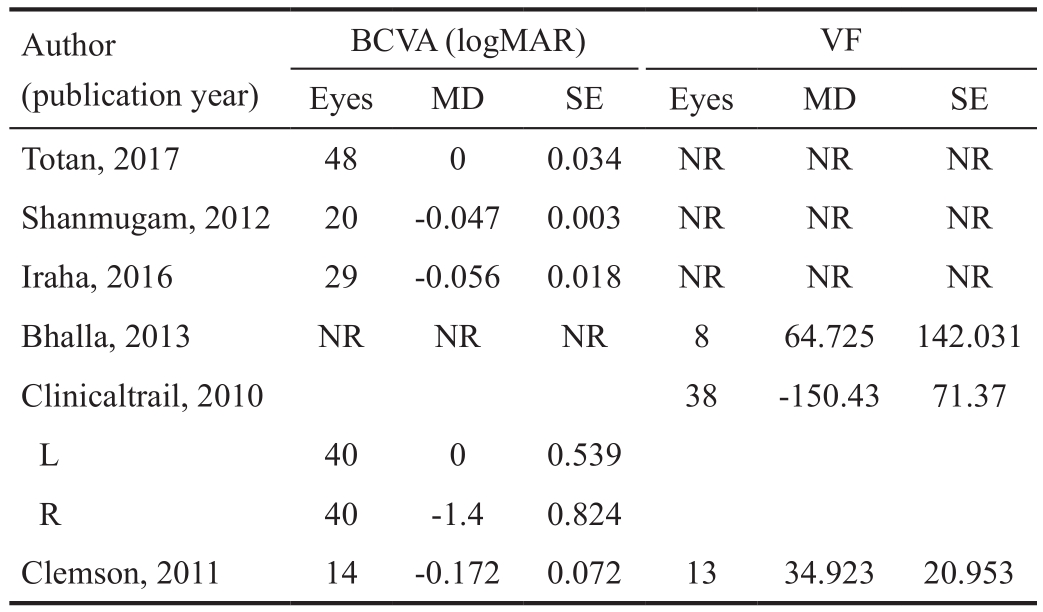
Efficacy Analysis Data on the therapeutic effects of treatment with VPA in RP were available for pooled analysis. Short-term treatment with VPA was 2mo, and long-term treatment was 12mo. The raw data with BCVA and VF of the included studies are presented in Table 2. The combined BCVA results showed a reduction based on the logMAR scores. The pooled mean difference in BCVA was -0.05 (95%CI: -0.05, -0.04, I2=36%)from baseline to the final treatment points (Figure 2), which means a significant improvement in BCVA.
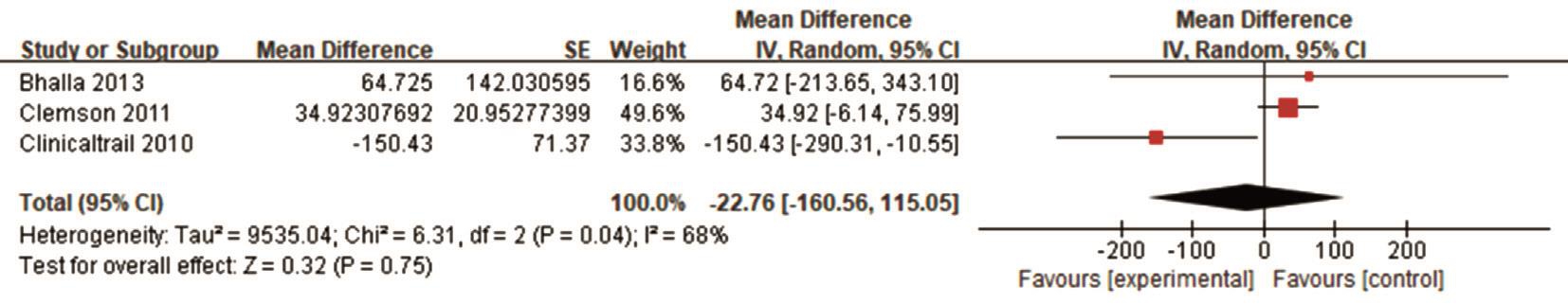
However, when we tried to combine all VF results, there were no statistically differences between scores at the baseline and final treatment points (Figure 3). Altogether, these analyses show that VPA treatment improved BCVA, but the effect on VF was inconsistent across studies.
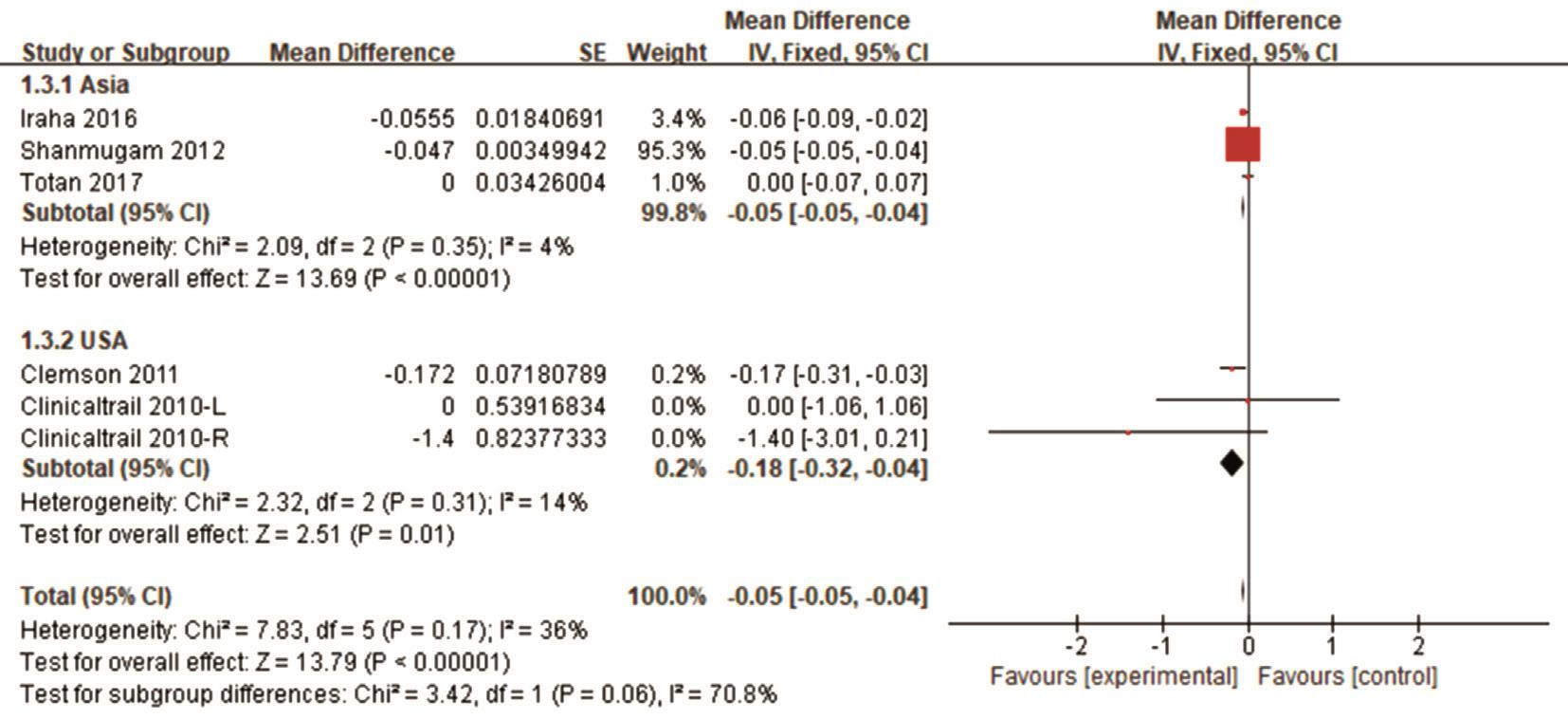
Sensitivity Analyses We conducted a subgroup analysis to explore the source of heterogeneity in BCVA and VF regarding the length of treatment, location, sample size and study design.More BCVA changes were observed in short-term (≤6mo)treatment studies (Figure 4), studies conducted in Asia (Figure 5),studies with 30 patients or fewer (Figure 6) and prospective study (Figure 7).
Table 2 The raw data with BCVA and VF of the included studies
MD: Mean difference; SE: Standard error; NR: Not reported; BCVA:Best-corrected visual acuity; VF: Visual field.
BCVA (logMAR) VF Eyes MD SE Eyes MD SE Totan, 2017 48 0 0.034 NR NR NR Shanmugam, 2012 20 -0.047 0.003 NR NR NR Iraha, 2016 29 -0.056 0.018 NR NR NR Bhalla, 2013 NR NR NR 8 64.725 142.031 Clinicaltrail, 2010 38 -150.43 71.37 L 40 0 0.539 R 40 -1.4 0.824 Clemson, 2011 14 -0.172 0.072 13 34.923 20.953 Author(publication year)
DISCUSSION
This Meta-analysis analyzed the associations between VPA and RP, with the purpose to draw a conclusion of great significance. As far as we know, it is the first time for the Meta-analysis method to be used for comprehensively discussing how VPA impacts RP. According to the combined BCVA results in this Meta-analysis, logMAR scores declined to a large extent, which implied BCVA improvement in RP patients. However, for VF, difference presented no statistical significance from baseline to final treatment points, and VPA’s effect on VF was inconsistent across studies. The clinicaltrials.gov[17] data suggested that VF decreased after a period of VPA intake. Nevertheless, Clemson et al[9] and Bhalla et al[10] found VF improved significantly with treatment.
The exact mechanism of how VPA improved BCVA in patients suffering RP remains unclear. The VPA functions as an emotional and anticonvulsant stabilizer. It is able to impact the GABA level by decarboxylating glutamic acid and modulating GABA transaminase, which mediate its effect on abovementioned capacities. According to some evidences,due to the strong neuroprotective properties, VPA can well protect cell death and mediate in flammation[20-21]. In addition,VPA can serve as an inhibitor in flammatory response pathway associated with photoreceptors in virtue of the microglial cell apoptosis[7-8,22]. Besides, it can effectively subdue HDAC[6,20,23].VPA has a special property, which has been reported in recent days, that is the special ability to overthrow damage to photoreceptor. Cells can achieve differentiation in one culture under the induced by VPA[6]. Also, glial cells can achieve differentiation into the photoreceptor-type cells under the simulation of VPA[24], and VPA downregulates complement proteins as well as enhances diverse neurotrophic factors.Together, these VPA properties possibly result in the rescue of some of the borderline photoreceptors (i.e. slightly damaged or yet to be damaged), thereby improving visual function.
According to some studies, VPA is able to lead to side effects like the hepatotoxicity as well as the neurological and mitochondrial toxicity[25]. VPA negatively influence some mitochondrial events such as inhibiting and lowering activity of mitochondrial complexes I and IV, curbing oxygen consumption as well as adenosine triphosphate synthesis,sequestrating coenzyme A, damaged structural organization of inner mitochondrial membrane, decreased hepatic cytochrome aa3, damaged oxidative phosphorylation, the subduing mitochondrial β-oxidation, and fragmenting vascular[11,25-26].However, these adverse effects were often described in patients at higher dosages used for other indications such as anticonvulsant activity (25-40 mg/kg·day), and it would be reduced when administered at doses which is extremely lower compared with the dose of anticonvulsants.
The work suffers many limitations, which should be taken into account carefully. All the study results are largely dependent on individual studies which covered samples at small size and exhibited some differences involving intervention time,population, routes of administration, as well as followup length. Meanwhile, patients did not exhibit a genetic characteristic and therapeutic effect of VPA is varying due to RP genetic variation. As there are insufficient randomized control trial (RCTs), the review focused on assessing singlearm studies. In addition, since we recruit 3 retrospective studies in this Meta-analysis, there may have some impact on reliability of the data collected and analyzed. The clinical trial included in the Meta-analysis only reported the BCVA scores’mean and standard deviation for right and left eyes in each patient, respectively. Therefore, the data cannot be combined to obtain a total result without the original data, and the inclusion of left and right eye data separately in this paper may also be a source of heterogeneity.
To sum up, according to the Meta-analysis adopted in the paper, treating RP patients with VPA significantly improves BCVA, yet many studies hold controversial opinions about its curing effect on VF. On that account, it is the most proper to adopt prospective trial, multi-center trial and RCT to effectively exam the clinical effect of VF on patients suffering RP.
ACKNOWLEDGEMENTS
We would like to thank Fu-Sheng Tang and Sheng-Yao Chen for their help on statistical methods.
Authors’ contributions: Conceptualization: Chen WJ, Ma L, Ma X; Data curation: Chen WJ, Li MS; Formal Analysis:Chen WJ, Li MS, Ma L; Funding Acquisition: Ma X;Investigation: Chen WJ, Li MS; Methodology: Chen WJ, Ma L; Project administration: Chen WJ, Ma L; Resources: Ma X;Software: Chen WJ, Ma L, Li MS; Supervision: Chen WJ, Li MS; Validation: Ma L, Ma X; Visualization: Ma X; Writing -Original Draft Preparation: Chen WJ, Li MS; Writing-Review& Editing: Ma L, Ma X.
Foundations: Supported by the National Natural Science Foundation of China (No.81770970, No.81271022).
Confiicts of Interest: Chen WJ, None; Ma L, None; Li MS,None; Ma X, None.
REFERENCES
1 Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet 2006;368(9549):1795-1809.
2 Berson EL. Retinitis pigmentosa. The friedenwald lecture. Invest Ophthalmol Vis Sci 1993;34(5):1659-1676.
3 Daiger SP, Sullivan LS, Bowne SJ. Genes and mutations causing retinitis pigmentosa. Clin Genet 2013;84(2):132-141.
4 Dias MF, Joo K, Kemp JA, Fialho SL, da Silva Cunha A Jr, Woo SJ, Kwon YJ. Molecular genetics and emerging therapies for retinitis pigmentosa: Basic research and clinical perspectives. Prog Retin Eye Res 2018;63:107-131.
5 Henry TR. The history of valproate in clinical neuroscience.Psychopharmacol Bull 2003;37(Suppl 2):5-16.
6 Göttlicher M, Minucci S, Zhu P, Krämer OH, Schimpf A, Giavara S,Sleeman JP, Lo Coco F, Nervi C, Pelicci PG, Heinzel T. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J 2001;20(24):6969-6978.
7 Chen PS, Wang CC, Bortner CD, Peng GS, Wu X, Pang H, Lu RB,Gean PW, Chuang DM, Hong JS. Valproic acid and other histone deacetylase inhibitors induce microglial apoptosis and attenuate lipopolysaccharide-induced dopaminergic neurotoxicity. Neuroscience 2007;149(1):203-212.
8 Dragunow M, Greenwood JM, Cameron RE, Narayan PJ, O’carroll SJ, Pearson AG, Gibbons HM. Valproic acid induces caspase 3-mediated apoptosis in microglial cells. Neuroscience 2006;140(4):1149-1156.
9 Clemson CM, Tzekov R, Krebs M, Checchi JM, Bigelow C, Kaushal S. Therapeutic potential of valproic acid for retinitis pigmentosa. Br J Ophthalmol 2011;95(1):89-93.
10 Bhalla S, Joshi D, Bhullar S, Kasuga D, Park Y, Kay CN. Long-term follow-up for efficacy and safety of treatment of retinitis pigmentosa with valproic acid. Br J Ophthalmol 2013;97(7):895-899.
11 Iraha S, Hirami Y, Ota S, Sunagawa GA, Mandai M, Tanihara H, Takahashi M, Kurimoto Y. Efficacy of valproic acid for retinitis pigmentosa patients: a pilot study. Clin Ophthalmol 2016;10:1375-1384.
12 Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17(1):1-12.
13 Wells GA, Shea BJ, O’Connell D, Peterson J, Welch V, Losos M,Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies in Meta-analysis. Applied Engineering in Agriculture 2014;18(6):727-734.
14 Chang JW, Kim JH, Kim SJ, Yu YS. Congenital aniridia: longterm clinical course, visual outcome, and prognostic factors. Korean J Ophthalmol 2014;28(6):479-485.
15 Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities “hand motion” and “counting fingers” can be quantified with the freiburg visual acuity test. Invest Ophthalmol Vis Sci 2006;47(3):1236-1240.
16 Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I² index?Psychological Methods 2006;11(2):193-206.
17 ClinicalTrials.gov. Trial of Oral Valproic Acid for Retinitis Pigmentosa.2010-2017. NCT01233609.
18 Shanmugam PM, Minija CK, Ramanjulu R, Tekwani P, Saxena M.Effect of short-term oral valproic acid on vision and visual field in retinitis pigmentosa. Ophthalmology and Therapy 2012;1:6.
19 Totan Y, Güler E, Yüce A, Dervişogulları MS. The adverse effects of valproic acid on visual functions in the treatment of retinitis pigmentosa.Indian J Ophthalmol 2017;65(10):984-988.
20 Leng Y, Liang MH, Ren M, Marinova Z, Leeds P, Chuang DM.Synergistic neuroprotective effects of lithium and valproic acid or other histone deacetylase inhibitors in neurons: roles of glycogen synthase kinase-3 inhibition. J Neurosci 2008;28(10):2576-2588.
21 Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol Psychiatry 2009;14(1):51-59.
22 Kim HJ, Rowe M, Ren M, Hong JS, Chen PS, Chuang DM. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol Exp Ther 2007;321(3):892-901.
23 Kanai H, Sawa A, Chen RW, Leeds P, Chuang DM. Valproic acid inhibits histone deacetylase activity and suppresses excitotoxicityinduced GAPDH nuclear accumulation and apoptotic death in neurons.Pharmacogenomics J 2004;4(5):336-344.
24 Kubota A, Nishida K, Nakashima K, Tano Y. Conversion of mammalian Müller glial cells into a neuronal lineage by in vitro aggregateculture. Biochemical and Biophysical Research Communications 2006;351(2):514-520.
25 Nanau RM, Neuman MG. Adverse drug reactions induced by valproic acid. Clin Biochem 2013;46(15):1323-1338.
26 Finsterer J, Zarrouk Mahjoub S. Mitochondrial toxicity of antiepileptic drugs and their tolerability in mitochondrial disorders. Expert Opin Drug Metab Toxicol 2012;8(1):71-79.
Citation: Chen WJ, Ma L, Li MS, Ma X. Valproic acid’s effects on visual acuity in retinitis pigmentosa: a systemic review and Metaanalysis. Int J Ophthalmol 2019;12(1):129-134
DOl:10.18240/ijo.2019.01.20
● KEYWORDS: retinitis pigmentosa; valproic acid; visual acuity; Meta-analysis
Received: 2018-06-02 Accepted: 2018-09-18
Correspondence to: Xiang Ma. The First Affiliated Hospital of Dalian Medical University, No. 222 Zhongshan Road,Xigang District, Dalian 116011, Liaoning Province, China.xma9467@vip.sina.com