
Figure 1 Ciliprobe and G-probe TCP handpieces Ciliprobe TCP hand-piece (Katalyst Surgical, Chester field, MO, USA) on the left and G-probe TCP hand-piece (IRIDEX Medical Instruments, Mountain View, CA, USA) on the right.
Glaucoma is one of the leading causes of blindness worldwide[1]. All current therapies for glaucoma target intraocular pressure (IOP) lowering either through the use of oral or topical medications, laser, or traditional surgical therapy. Most laser and surgical therapies aim to increase aqueous outflow in order to reduce IOP. Alternatively, other medical and laser treatments lower IOP by targeting reduction of aqueous inflow via the ciliary epithelium, the site of aqueous production[2]. Cyclo-destruction, or destruction of the ciliary body, has primarily been used to treat refractory glaucoma. Since its inception in the 1930s, several different modalities have been used to achieve ciliary ablation in order to reduce, but not eliminate aqueous production[3].Today, the most commonly utilized method is through cyclophotocoagulation (CPC) with a non-invasive trans-scleral diode laser (810 nm). Historically, because of the risk of severe complications including phthisis and hypotony, trans-scleral cyclophotocoagulation (TCP) has been used only for refractory glaucoma with poor visual potential[4-6]. However, more recently, emerging evidence suggests TCP is safe to use earlier in the treatment algorithm[6-7].
TCP is known to cause localized destruction of the pigmented and non-pigmented ciliary epithelium. Previous studies have shown separation of the non-pigmented and pigmented ciliary epithelium, pigment clumping and coagulative necrosis on histological sections of eyes treated with TCP[8-11]. This damage and destruction of the non-pigmented epithelium subsequently leads to decreased aqueous production and the resultant IOP reduction after TCP. Earlier methods for CPC were associated with more damage and destruction to collateral tissues rather than focused effects on the ciliary epithelium[6]. These more severe and widespread histologic changes were likely associated with the higher rate of phthisis encountered with these other techniques. However, it is important to remember that surrounding ocular tissue damage and phthisis still remains a concern for physicians today.
Since its introduction to the market, the G-probe hand-piece(Figure 1, right hand side; IRIDEX Medical Instruments,Mountain View, CA, USA) has made TCP technically easier by eliminating the need to manually measure each laser application spot. The probe is placed with its anterior edge at the corneoscleral limbus so that the laser energy is positioned directly over the ciliary body, approximately 1.2 mm posterior to the limbus. In a traditional, full treatment, 16-24 spots are placed circumferentially around 270-360 degrees of limbus with care taken to avoid the 3 and 9 o’clock positions. Settings vary by surgeon but are most commonly titrated until there is an audible “pop” indicating explosion of a ciliary process at which point the power and/or duration is then decreased again[12-13].

Figure 1 Ciliprobe and G-probe TCP handpieces Ciliprobe TCP hand-piece (Katalyst Surgical, Chester field, MO, USA) on the left and G-probe TCP hand-piece (IRIDEX Medical Instruments, Mountain View, CA, USA) on the right.
Recently a new probe has become commercially available,the Ciliprobe (Figure 1, left hand side; Katalyst Surgical,Chesterfield, MO, USA). The Ciliprobe has comparable dimensions to the G-probe in regards to both the handle and the footplate, however the colors vary between the two. Both footplates are wedge shaped and designed with a thinner edge meant to be placed along the cornea-scleral limbus and a wider posterior edge. The laser fiber protrudes from the footplate of each probe and are each positioned approximately 1-1.2 mm posterior to the anterior edge of the footplate to ensure proper location of laser energy delivery. While the Ciliprobe and G-probes appear nearly structurally identical, no preclinical or clinical data have been published regarding whether differences exist in their treatment effects. This study sought to evaluate whether treatment differences exist histologically in human cadaver eyes between TCP performed with the traditional G-probe and the newer Ciliprobe.
Ethical Approval This study followed the tenets of the Declaration of Helsinki.
TCP was performed on two cadaveric human eyes from the same patient obtained through the Rocky Mountain Lion’s Eye Bank (Aurora, CO, USA). Of note, review of medical records showed the patient had no clinically relevant ocular history in either eye. The vertical meridian was marked with prolene suture placed intra-corneal. Laser application was performed in the usual fashion with the anterior, scalloped edge of the probe aligned with the corneoscleral limbus and the footplate placed firmly against the globe. Seven TCP spots were evenly spaced over 4 clock hours on either side of the pre-marked meridian with the G-probe on one side and the Ciliprobe on the other. The same diode laser unit (OcuLight SLx laser system,IRIDEX, Mountain View, CA, USA) was used for treatment with both probes. The two clock hours spanning each side of the vertical meridian, were spared to ensure no cross over of treatment zones. The side treated with the Ciliprobe was marked with a 10-0 nylon suture placed in the cornea. The first eye was treated in this manner by each probe on its respective side set at 2000ms duration and 2000 mW power. The second eye was treated in the same manner with each probe respectively set at 3000ms duration and 1500 mW power.
Tissue samples were immediately preserved in 4%paraformaldehyde/phosphate-buffered saline overnight at 4℃ and then processed for paraffin histology. Tissue sections were cut and stained with Mayer’s hematoxylin and eosin Y(H&E: Richard-Allan Scientific, Kalamazoo, MI). Bright- field imaging was performed using a Nikon Eclipse 80i microscope(Nikon, Melville, NY, USA) equipped with a Nikon D5-Fi1 color camera and a Nikon CFI 20×/Plan Fluor objective lens.The histopathologic examiner was blinded from knowing which sections were treated with which probe or protocol. The appearance and structure of ciliary body samples were then compared qualitatively to one another.
On histological examination, vacuolization, loss, and separation of the non-pigmented ciliary epithelium was noted in all tissue sections for both probes and both treatment settings. As demonstrated in Figures 2A and 2B, the histologic changes noted to the non-pigmented ciliary epithelium in the eye treated at 3000ms and 1500 mW were similar between the two probes.
In the eye treated at 2000ms and 2000 mW, vacuolization levels were equivalent when comparing multiple sections treated by each probe. However, a somewhat more complete separation of the non-pigmented epithelium was noted in the Ciliprobe treated sections as compared to the G-probe treated sections (Figure 2C, 2D).
TCP is a non-invasive cilio-ablative laser procedure which has been shown to successfully reduce aqueous production and thus IOP in patients with uncontrolled glaucoma[5-7].While complications such as intraocular inflammation, postoperative pain, choroidal detachment, hypotony and phthisis have limited its use to refractory glaucoma with poor visual potential previously, more recent evidence has shown that newer techniques and settings have made it safe to use even in eyes with good visual acuity[5-8]. The procedure is traditionally performed with the G-probe, which allows for consistent, reliable probe placement and laser delivery. The novel Ciliprobe, despite its comparable dimensions and reported equivalence to the G-probe, has not previously been investigated. Our study demonstrates that the Ciliprobe is capable of producing at least equivalent histologic changes to the target tissue, the non-pigmented ciliary epithelium when compared to the G-probe.
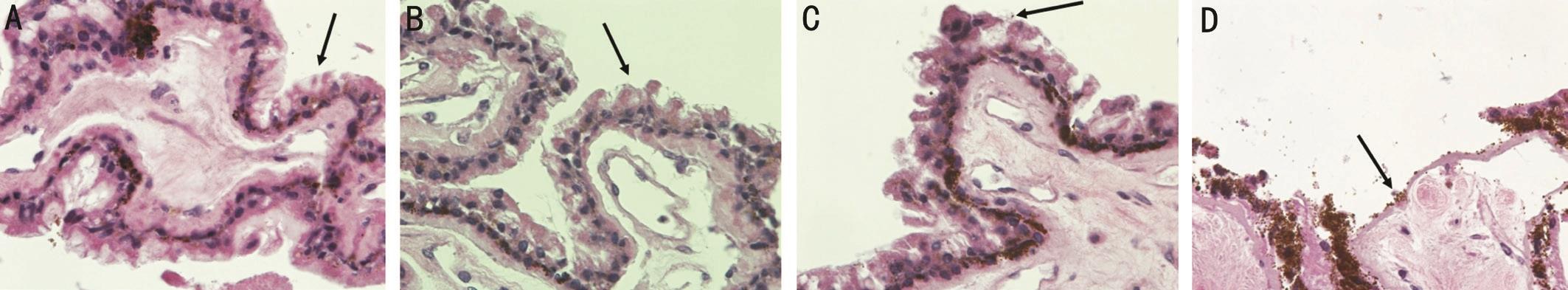
Figure 2 Light microscopy of the ciliary body from a cadaveric eye treated with the following probes and settings A: Cadaveric eye treated with the G-probe set at 3000ms and 1500 mW demonstrates vacuolization and loss of the non-pigmented ciliary epithelium (black arrow) with Mayer’s hematoxylin and eosin (400× magnification) which is equivalent to B; B: Cadaveric eye treated with the Ciliprobe set at 3000ms and 1500 mW shows an equivalent amount of vacuolization and loss of the non-pigmented ciliary epithelium (black arrow) with Mayer’s hematoxylin and eosin (400× magnification) when compared to A; C: Cadaveric eye treated with the G-probe set at 2000 mW and 2000ms demonstrates loss of the non-pigmented ciliary epithelium (black arrow) with Mayer’s hematoxylin and eosin (400× magnification); D:Cadaveric eye treated with the Ciliprobe set at 2000 mW and 2000ms demonstrates greater loss of the non-pigmented ciliary epithelium (black arrow) with Mayer’s hematoxylin and eosin (400× magnification) when compared to C.
TCP has been shown histologically to cause localized tissue destruction. This includes damage to both the pigmented and non-pigmented ciliary epithelium. Previous studies have shown separation of the non-pigmented and pigmented ciliary epithelium, pigment clumping and coagulative necrosis on histological sections[8-11]. As the site of aqueous production,the destruction of the non-pigmented epithelium subsequently leads to decreased aqueous in flow and reduced IOP[2]. In this study, both probes produced separation, vacuolization, and loss of the non-pigmented ciliary epithelium at two different TCP settings. At a lower power, but longer duration, the histologic changes were equivalent between the two sections. The only detectable difference occurred at higher power setting with shorter duration where the Ciliprobe produced a more complete separation of the non-pigmented epithelium when compared to the G-probe.
The advent of a commercially available probe alternative to the G-probe is particularly timely. Recently, production of the original G-probes for older 810 nm laser systems has been discontinued by the manufacturer in favor of newer laser systems that require an updated version of the G-probe. As a result, older laser systems were potentially rendered obsolete as newer G-probes are incompatible with these systems. The Ciliprobe now provides an alternative probe with comparable tissue effects that is still compatible with these older laser systems, allowing surgeons the option to continue with existing equipment.
Due to the non-clinical nature of this study, it is unclear if the minor histological difference noted at the 2000 mW/2000ms setting would result in a clinically significant difference in treatment efficacy or safety. Further, this study is limited by sample size as only one eye was treated with each setting.Despite this however, it can be extrapolated that it should produce at least equivalent IOP lowering efficacy when used clinically since the Ciliprobe produced at least equivocal histologic changes. Future human studies with a larger sample size should be performed to confirm these findings and to compare clinical outcomes with each probe.
Conflicts of Interest: Capitena Young CE, None; Kahook MY, None; Ammar DA, None; Seibold LK, None.
1 Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040. Ophthalmology 2014;121(11):2081-2090.
2 Goel M, Picciani RG, Lee RK, Bhattacharya SK. Aqueous humor dynamics: a review. Open Ophthalmol J 2010;4:52-59.
3 Weve H. Die Zyklodiatermie das Corpus ciliare bei Glaukom. Zentralbl Ophthalmol 1933;29:562-569.
4 Bhartiya S, Ichhpujani P, Angmo D. Transscleral diode cyclophotocoagulation.In: International Society of Glaucoma Surgery Textbook of Glaucoma Surgery. Jaypee Brothers Medical Publishers Ltd; 2014:65-68.
5 Pantcheva MB, Schuman JS. Cyclodestruction. In: Chandler and Grant’s Glaucoma, Fifth Edition. SLACK Incorporated;2013:511-519.
6 Ndulue JK, Rahmatnejad K, Sanvicente C, Wizov SS, Moster MR.Evolution of cyclophotocoagulation. J Ophthalmic Vis Res 2018;13(1):55-61.
7 Levinson JD, Giangiacomo AL, Beck AD, Pruett PB, Superak HM,Lynn MJ, Costarides AP. A comparison of sequential glaucoma drainage device implantation versus cyclophotocoagulation following failure of a primary drainage device. J Glaucoma 2017;26(4):311-314.
8 Pantcheva MB, Kahook MY, Schuman JS, Noecker RJ. Comparison of acute structural and histopathological changes in human autopsy eyes after endoscopic cyclophotocoagulation and trans-scleral cyclophotocoagulation. Br J Ophthalmol 2007;91(2):248-252.
9 Schuman JS, Noecker RJ, Puliafito CA, Jacobson JJ, Shepps GJ,Wang N. Energy levels and probe placement in contact transscleral semiconductor diode laser cyclophotocoagulation in human cadaver eyes.Arch Ophthalmol 1991;109(11):1534-1538.
10 Feldman RM, El-Harazi SM, Lorusso FJ, McCash C, Lloyd WC III, Warner PA. Histopathologic findings following contact transscleral semiconductor diode laser cyclophotocoagulation in a human eye. J Glaucoma 1997;6(2):139-140.
11 McKelvie PA. Pathology of cyclodiode laser: a series of nine enucleated eyes. Br J Ophthalmol 2002;86(4):381-386.
12 Chang SHL, Chen YC, Li CY, Wu SC. Contact diode laser transscleral cyclophotocoagulation for refractory glaucoma: comparison of two treatment protocols. Can J Ophthalmol 2004;39(5):511-516.
13 Pastor SA, Singh K, Lee DA, Juzych MS, Lin SC, Netland PA,Nguyen NT. Cyclophotocoagulation: a report by the American Academy of Ophthalmology. Ophthalmology 2001;108(11):2130-2138.