
Citation: Che ST, Bie L, Li X, Qi H, Yu P, Zuo L. Parthenolide
inhibits the proliferation and induces the apoptosis of human uveal melanoma
cells. Int J Ophthalmol
2019;12(10):1531-1538. DOI:10.18240/ijo.2019.10.03
·Basic Research·
Parthenolide
inhibits the proliferation and induces the apoptosis of human uveal melanoma
cells
Song-Tian Che1, Li Bie2, Xu Li1,
Hui Qi1, Peng Yu1, Ling Zuo1
1Department of Ocular Fundus Disease,
the Second Hospital of Jilin University, Changchun 130022, Jilin Province,
China
2Department of Neurosurgery, the
First Hospital of Jilin University, Changchun 130022, Jilin Province, China
Co-first authors: Song-Tian Che and Li Bie
Correspondence to: Song-Tian Che. Department of Ocular Fundus
Disease, the Second Hospital of Jilin University, No. 218 Ziqiang Street,
Nanguan District, Changchun 130022, Jilin Province, China.
chesongtian_stche@163.com
Received:
Abstract
AIM: To explore the effect of parthenolide (PTL) on human uveal melanoma (UM)
cells (C918 and SP6.5 cells) and its molecular mechanism.
METHODS: Carboxyfluorescein succinimidyl amino ester (CFSE)
assays and cell counting kit-8 (CCK-8) were performed to detect the cell
viability. Flow cytometry was used to analyze cell cycle and apoptosis.
Quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot
assays were performed to measure proliferation-related and apoptosis-related
factors.
RESULTS: Firstly, PTL decreased the viability of C918 and
SP6.5 cells in a dose-dependent manner, and the effect of PTL on C918 cells was
stronger than on SP6.5; however, it did not affect normal cells. Secondly, PTL
increased the proportion of cell number at cell cycle G1 phase in C918 cells,
and decreased the proportion of cell number at S phase, but the proportion did
not change at G2 phase. In addition, PTL induced the apoptosis of C918 cells,
and decreased the expressions of Cyclin D1, B-cell lymphoma-2 (Bcl-2) and
B-cell lymphoma-extra large (Bcl-XL). Also, PTL increased Cyclin inhibition
protein 1 (P21), Bcl-2-associated X protein (Bax), Cysteinyl aspartate specific
proteinas-3 (Caspase-3) and Caspase-9 expression. However, the expression of
Caspase-8 was not changed.
CONCLUSION: PTL inhibites proliferation and induces apoptosis
in UM cells by arresting G1 phase and regulating mitochondrial pathway,
however, it does not affect normal cells.
KEYWORDS: parthenolide; uveal melanoma;
proliferation; apoptosis; mitochondrial pathway
DOI:10.18240/ijo.2019.10.03
Citation:
Che ST, Bie L, Li X, Qi H, Yu P, Zuo L. Parthenolide inhibits the proliferation
and induces the apoptosis of human uveal melanoma cells. Int J Ophthalmol 2019;12(10):1531-1538
INTRODUCTION
Though uveal melanoma (UM) is rare tumor,
however, it is the most common primary malignant tumor in adults’ eyes[1-2]. Surveillance and epidemiology of
the National Cancer Institute (NCI) reported that the incidence of UM in Caucasians
is higher than in other colored population, thus, the occurrence of the disease
is racially different[1,3].
Meanwhile, the incidence of UM is gender different, and is higher in men than
in women. The incidence of the disease is also positively correlated with age[4-5]. It has been found that the
survival rate of UM was low, as the survival rates of 5, 10, 15, and 20y are
72%, 59%, 53% and 16%, respectively[1]. Moreover,
UM is highly metastatic, and metastasis occurs in more than 50% of the cases.
Liver metastasis is the most common, causing death within 2-4mo[6-7]. Although UM patients are treated
with advanced drugs and technologies, the prognosis remains poor and half of UM
patients die within 25y[8-9]. UM
is characterized by a high malignancy, invasion, metastasis and poor prognosis,
which can seriously affect the quality of life and even life of people[1,7,9-10].
Therefore, it is necessary to find effective drugs and treatment methods to
prevent and treat UM.
In recent decades, many Chinese
medicine researchers have carried out studies on anti-tumor screening in
vivo and in vitro. Results show that many Chinese herbal medicines
had anti-cancer effects at different levels[11-12]. Parthenolide (PTL) is the main extract of Chinese
herbal medicine parthenium hysterophorus, which contains the component of
sesquiterpene lactone[13] that is
α-methylene-γ-lactone ring and has epoxide structure[14].
This structure can react with enzymes, which contain mercapto groups and other
functional proteins, to interfere with the many key biological processes of
cells, such as cell signaling pathways, mitochondrial respiration,
proliferation and apoptosis[15]. In the past, PTL
was primarily used to treat migraine, fever and rheumatoid arthritis[16]. In recent years, the studies find that PTL exerted
anti-cancer effect in a variety of tumors, such as breast cancer,
cholangiocarcinoma, pancreatic cancer, bladder cancer, prostate cancer,
leukemia[17-24]. However, as
far as we know, the potential effect of PTL on UM has not been investigated,
and the molecular mechanism of PTL on UM remains to be studied.
PTL may control cell growth and
apoptosis in tumor cells[24-27].
Cell cycle is the most important process of cellular activities. The regulation
of cell cycle is achieved by the specific cell cycle protein in each phase of
cell cycle. As we all known, cyclin D1 and Cyclin inhibition protein 1 (P21)
played key roles in G1 phase[28]. So far, it has
been reported that the Cyclin D1 and P21 genes were amplified or overexpressed
in breast cancers, mammary hyperplasia and carcinoma[28-29]. According to the report, the family of Bcl-2 and
Caspase proteins plays a vital role in the process of tumor apoptosis[30]. The members of Bcl-2 proteins family include, for
example, Bax, Bcl-2, Bcl-XL. Bax is a protein that promotes apoptosis, while
Bcl-2 and Bcl-XL are proteins that suppress apoptosis[31].
The members of Caspase proteins family include, for example, Caspase-3,
Caspase-8 and Caspase-9, which are divided into initiators (Caspase-8,
Caspase-9) and executors (Caspase-3), and the initiator can activate the
executor[32]. Herein, we studied the effect of
PTL on the proliferation of human UM (C918 and SP6.5 cells) and normal cells
[human normal uveal melanocytes, retinal pigment epithelial (RPE)], and
fibroblasts). Furthermore, whether PTL affected the apoptosis of C918 cells was
also determined. We further explored the effect of PTL on the proliferation and
apoptosis of C918 cells by arresting the corresponding stage of cell cycle and
regulating corresponding pathway.
MATERIALS AND METHODS
Cell Lines and Cell Culture Human UM (C918 and SP6.5), human
normal uveal melanocyte, RPE and fibroblast cell lines were all purchased from
American Type Culture Collection (ATCC, USA). C918 and SP6.5 cells were
originated from a UM patient with liver metastasis[33]
and a primary UM patient[34], respectively. C918 cells
were epithelioid in morphology, which have highly an invasive and metastatic
ability[33]. C918 and SP6.5 cells were cultured
in Ham’s F12 nutrient mixture (F12; Gibco, USA) containing 10% fetal bovine
serum (FBS; Invitrogen, USA) and 50 μg/mL gentamicin (Solarbio, Beijin, China).
Human normal uveal melanocytes, RPE, and fibroblasts were cultured in
Dulbecco’s modified Eagle medium (DMEM; Gibco, USA) containing 10% FBS and 50
μg/mL gentamicin. The cells were cultured in a 5% CO2 humidified
incubator with at
Drug Treatment PTL was obtained from Desite
Biotechnology Co., LTD. (Chengdu, China) and dissolved in absolute alcohol to
form a concentration of 50, 100, and 200 μmol/L, respectively. In subsequent
experiments, these different concentrations of PTL were used to treat cells
respectively to explore the effect of PTL on C918 and SP6.5 cells.
CCK-8 Assay C918, SP6.5, RPE, fibroblast and
human normal uveal melanocyte cells were seeded in plates (96-well) at a
density of 3×103 cell/well and incubated in 5% CO2 humidified
incubator at
CFSE Assay C918 and SP6.5 cells (2×104 cell/well)
were seeded in plates (24-well) and cultured in 5% CO2 humidified
incubator at
Cell Cycle Assay C918 cells (1×106 cell/well)
were seeded in plates (6-well), and incubated in 5% CO2 humidified
incubator at
Cell Apoptosis Assay C918 cells were seeded in plates
(6-well, 1×106 cell/well) and incubated in 5% CO2 humidified
incubator at
Quantitative Real-time Polymerase
Chain Reaction Assay C918 cells (1×106
cell/well) were seeded in plates (6-well), and cultured in 5% CO2
humidified incubator at
Table 1 Sequences of the primers used for qRT-PCR
|
Primer name |
Forward
sequence ( |
Reverse
sequence ( |
|
Cyclin D1 |
CCCTCGGTGTCCTACTTCAA |
CTTAGAGGCCACGAACATGC |
|
P21 |
ACAAGAGGCCCAGTACTTCC |
AGAAATCTGTCAGGCTGGTCT |
|
Caspase-3 |
TGCCCAAGTGACTGACATCA |
CATCCCCATTGACTGTGCAG |
|
Caspase-8 |
TTTGGCTGGCATCATCTGTG |
CATCCACATGTGTCCCGTTC |
|
Caspase-9 |
ATGCTCCGTGTCCATTGAGA |
AGTCACTGTCCAAGGTCCTG |
|
Bcl-XL |
ATGCTCCGTGTCCATTGAGA |
AGTCACTGTCCAAGGTCCTG |
|
Bax |
GACCCGGTGCCTCAGGATGC |
AGGTCAGCTCATCATGCTTG |
|
Bcl-2 |
GTGGAGGAGCTCTTCAGGGA |
GTCTGTGTCCACGGCGGCAA |
|
GAPDH |
CACCCACTCCTCCACCTTTG |
CCACCACCCTGTTGCTGTAG |
qRT-PCR: Quantitative real-time
polymerase chain reaction.
Western Blot Assay C918 cells (1×106 cell/well)
were seeded in plates (6-well), and cultured in 5% CO2 humidified
incubator at
Statistical Analysis All data were presented as mean±SD,
all analysis was conducted using GraphPad Prism 6.0. The Student’s t-test
was used to assess difference between the experimental groups. The statistical
difference was considered significant if P<0.05. Each experiment was
implemented in triplicate.
Results
Effect of PTL on the Viability of
Human Uveal Melanoma Cells We explored how PTL affected the
viability of human UM (C918 and SP6.5), human normal uveal melanocyte, RPE and
fibroblast cells by CCK-8 and CFSE analysis, respectively. The viabilities of
RPE, human normal uveal melanocytes and fibroblasts did not changed when cells
were treated with PTL (Figure
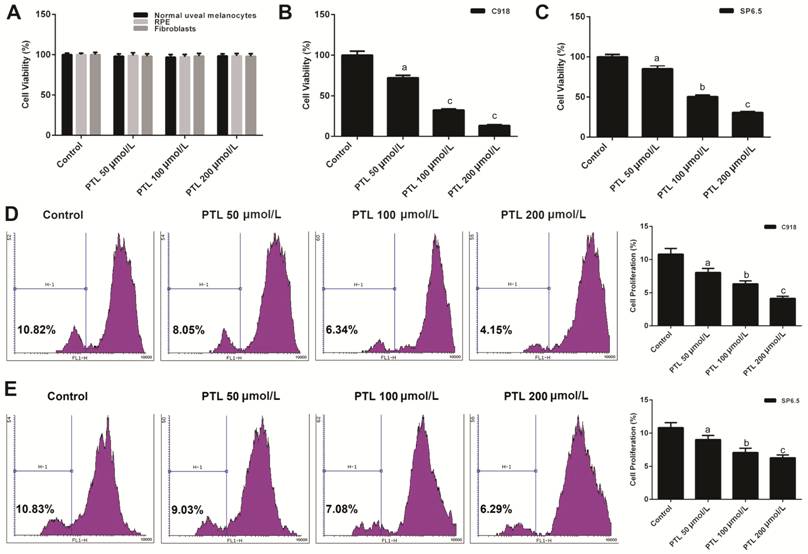
Figure 1 PTL decreased the viability
of human uveal melanoma cells (C918 and SP6.5) A: The cell viabilities of human
normal uveal melanocytes, fibroblasts and RPE treated with different
concentration of PTL was detected by CCK-8 assay. B, C: CCK-8 assay was applied
to test the viabilities of C918 and SP6.5 cells treat with different
concentration of PTL. D, E: The viabilities of C918 and SP6.5 cells were
further detected by CFSE assay. aP<0.05, bP<0.01,
cP<0.001, compared with control.
Inhibitory Effect of PTL on the
Viability of C918 Cells by Arresting G1 Phase To analyze which phase of cell cycle
was arrested after the viability of C918 cells was decreased by MTE, cell cycle
was examined by quantitating the content cells’ DNA using flow cytometry. The
treatment of C918 cells with different concentration of PTLs decreased the
percentage of cell number at S phase, and increased accumulation of the cell
percentage at G1 phase, however, the percentage of cell number did not change
at G2 phase (P<0.05; Figure
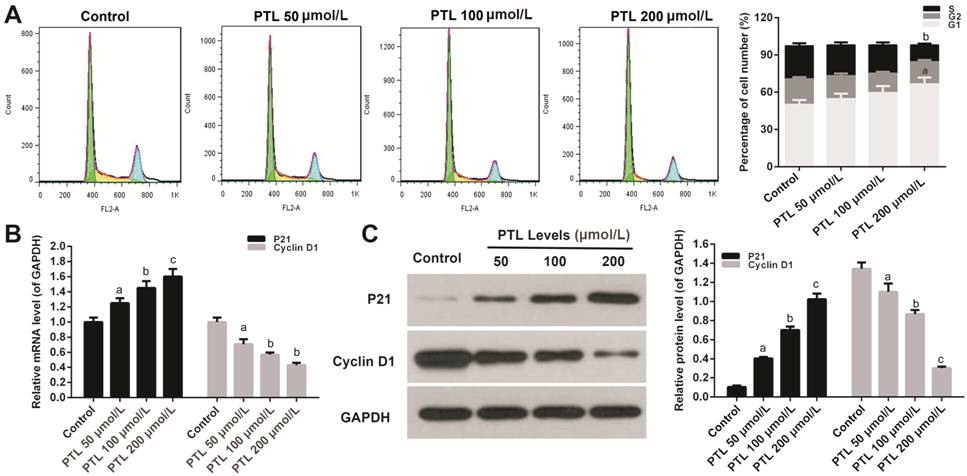
Figure 2 PTL decreased the viability
of C918 cells by arresting G1 phase A: PI staining kit was used to measure
the percentage of cell number at cell cycle S, G1, and G2 phase in C918 cells
treat with different concentration of PTL. B, C: The relative mRNAs and protein
expressions of P21 and Cyclin D1 were detected by qRT-PCR (B) and Western blot
(C) assays, respectively. GAPDH served as an internal control. Quality one was
applied to measure and count the gray value. aP<0.05, bP<0.01,
cP<0.001, compared with control.
Cyclin D1 and P21 were measured by
qRT-PCR and Western blot assay, respectively. PTL significantly stimulated the
mRNA expression of Cyclin D
Promotive Effect of PTL on the
Apoptosis of C918 Cells Annexin V-FITC apoptosis detection
kit was applied to study the effect of PTL on the apoptosis of C918 cells. Our
data showed that PTL increased apoptosis of C918 cells in a dose-dependent
manner. When C918 cells were treated with different concentrations of PTL, the
apoptosis rate increased by 2.60-folds, 3.80-folds, 6.50-folds, respectively (P<0.05;
Figure 3).
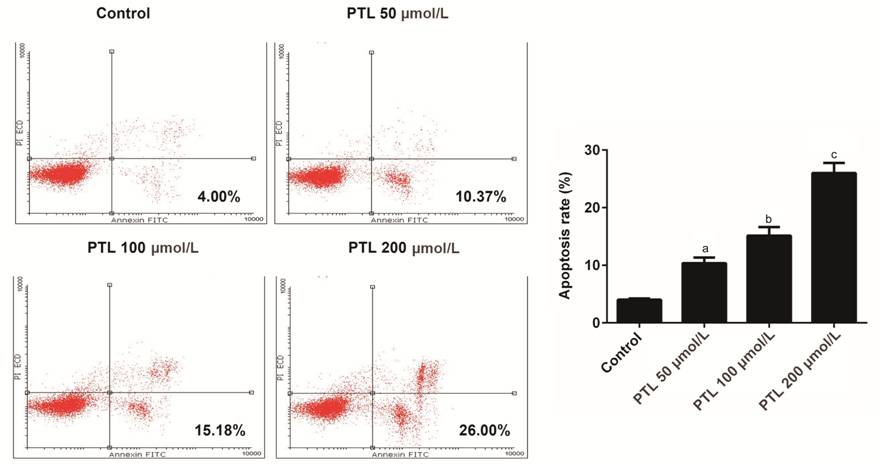
Figure 3 PTL promoted the apoptosis
of C918 cells Annexin V-FITC apoptosis detection
kit assay was used to detect the apoptosis rate of C918 cells treated with
different concentration of PTL. aP<0.05, bP<0.01,
cP<0.001, compared with control.
Effect of PTL on the Expression of
Bcl-2 Family Members in C918 Cells qRT-PCR and Western blot assays were
performed to further explore whether PTL promoted apoptosis in C918 cells. PTL
obviously enhanced the expressions of Bax in C918 cells in a dose-dependent
manner (P<0.05; Figure 4). By contrast, the expression levels of
Bcl-XL and Bcl-2 were decreased in C918 cells treat with PTL.

Figure 4 PTL regulated the members
of Bcl-2 family expression in C918 cells
A: qRT-PCR
assay was applied to detect the mRNA expression levels of Bax, Bcl-2, and
Bcl-XL in C918 cells. B: The protein expressions of Bax, Bcl-2, and Bcl-XL were
detected by Western blot assays in C918 cells. Quality one was applied to
detect count the gray value. aP<0.05, bP<0.01,
cP<0.001, compared with control.
Effect of PTL on the Expression of
Caspase Family Members in C918 Cells
qRT-PCR and
Western blot analysis were used to explore the pathways of PTL-mediated C918
cells apoptosis. The results demonstrated that the mRNA expressions of
Caspase-3 and Caspase-9 were obviously increased in C918 cells treat with
different concentrations of PTL. PTL did not affect the mRNA expression level
of Caspase-8 (P<0.05; Figure
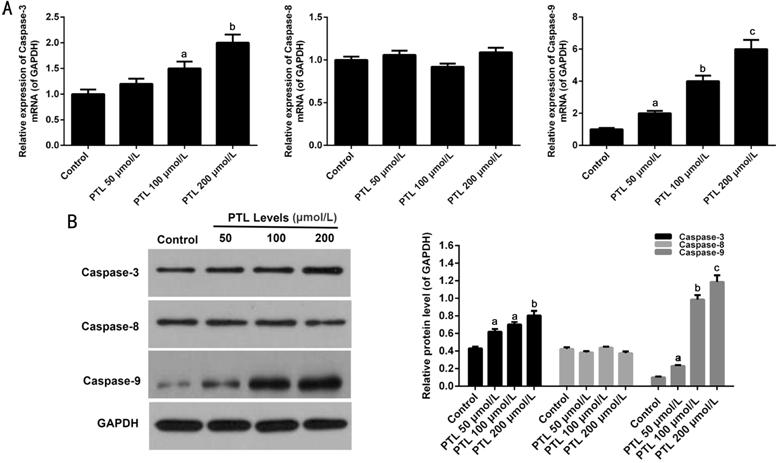
Figure 5 PTL regulated the members
of Caspase family expression in C918 cells A: The mRNA expression levels of
Caspase-3, Caspase-8, and Caspase-9 were surveyed by qRT-PCR assay in C918
cells. B: Western blot assay was used to survey the protein expressions of Caspase-3,
Caspase-8, and Caspase
DISCUSSION
Recently, the extraction of new anti-tumor
drugs from plants has drawn much research attention. Especially, studies have
been increasingly carried out on the extraction of anticancer substances from
Compositae Plants (Chrysanthemum Parthenium). PTL is one of the most
important active ingredients in Chrysanthemum Parthenium, and it belongs
to sesquiterpene lactone compounds[13]. In
addition to immunomodulatory effects, PTL has been widely used to treat
different kinds of tumors. Studies have shown that PTL has the effect of
inhibiting proliferation and inducing apoptosis of tumor cells[24-27]. However, as far as we know,
the effect of PTL on UM cells still remains unknown.
We explored the relationship between
PTL and human UM cells (C918 and SP6.5) and normal cells (human normal uveal
melanocytes, RPE, and fibroblasts). The results revealed that PTL decreased the
viabilities of C918 and SP6.5 cells in a concentration-dependent manner.
Therefore, the cytotoxic effect of PTL in C918 cells was stronger than in SP6.5
cells. However, the viability of human normal uveal melanocytes, RPE, and
fibroblasts were not affected by PTL. So, it was suggested that PTL had a
significant anti-tumor effect on human UM cells.
PTL inhibits anti-tumor activity
through various molecular mechanisms[35]. It has
been found that the cell cycle and apoptosis change partly made of anti-tumor
mechanisms, and the cell cycle and apoptosis change may cause the corresponding
protein change[18,36]. Cell
cycle is accomplished by the combination of Cyclin-dependent kinases (CDK) and
Cyclins. Cyclin D1 is a member of Cyclins family, which affects G1 phase and
has been recognized as a proto-oncogene. Overexpression of Cyclin D1 is closely
related to the development of cancer, and it plays a key role in cell cycle
regulation[37-39]. Besides, P21
is CDK inhibitor, and P21 and P53 are composed of the check point of cell cycle
G1 phase[40]. Many researches demonstrated that
anti-tumor drugs induced cell cycle by arresting G1 phase to up-regulate P21
expression in tumor cells[41-43].
Similar to previous studies, our data showed that PTL arrested cell cycle G1
phase to up-regulate P21 expression and down-regulate Cyclin D1 expression in
C918 cells.
Apoptosis is a complex process in which multiple
signaling proteins are transmitted via several pathways[31]. At present, it is clear that there are two
characteristic pathways via which activated Caspase cascade regulate apoptosis,
one is a death receptor pathway (external pathway), another is the
mitochondrial pathway (internal pathway). Under certain circumstances, the two
apoptotic pathways may cross each other in specific cases. External pathway
activates death receptor to combine with corresponding ligands. Subsequently,
it can further stimulate Caspase-8 to cause downstream events, including
Caspase cleavage and apoptosis. The internal pathway is mediated by Bcl-2
family proteins (Bax, Bcl-2, etc.). The number of pro-apoptotic protein
(Bax) is positively correlated with the mitochondrial membrane permeability.
Bax can promote the mitochondrial membrane permeability by activating Caspase-3
and Caspase-9, eventually leading to apoptosis[31-32,44]. It has been reported that
Bcl-2 was up-regulated in 70% UMs, however, the anti-tumor drugs down-regulate
Bcl-2 expression in tumor cells[45]. It has been
proved that application of arsenic and other drugs can increase the expressions
of Caspase-3 and Caspase-9 to promote tumor cells apoptosis[46-47]. Similar to previous studies, we found that PTL
induced the apoptosis of C918 cells, therefore, the expressions of Bcl-2 and
Bcl-XL were decreased and Bax, Caspase-3, and Caspase-9 expression were
increased in C918 cells in a dose-dependent manner. Therefore, it was explained
that PTL induced the apoptosis of C918 cells by regulating mitochondrial
pathway.
In conclusion, PTL reduced the
proliferation of human UM cells (C918 and SP6.5), and the reduction was more
noticeable in C918 cells than in SP6.5 cells, however, PTL did not affect normal
cells. PTL inhibited proliferation and induced apoptosis of C918 cells by
arresting G1 phase and regulating mitochondrial pathway. Note that this
conclusion still requires further investigation in vivo.
ACKNOWLEDGEMENTS
Foundation: Supported by the Health Special
Project of Jilin Province Department of Finance (No.3D5177883429).
Conflicts of Interest: Che ST, None; Bie L, None; Li X,
None; Qi H, None; Yu P, None; Zuo L, None.
REFERENCES